Antioxidant and Microbiota Characteristics Across Different Mucosal Sites of Rice Flower Carp (Procypris merus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurement of Antioxidant Indicators
2.3. Genomic DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.4. 16S rRNA Gene Amplicon Sequencing Data Processing
2.5. Bioinformatics Analysis
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Antioxidant Characteristics Across Different Mucosal Sites of Rice Flower Carp
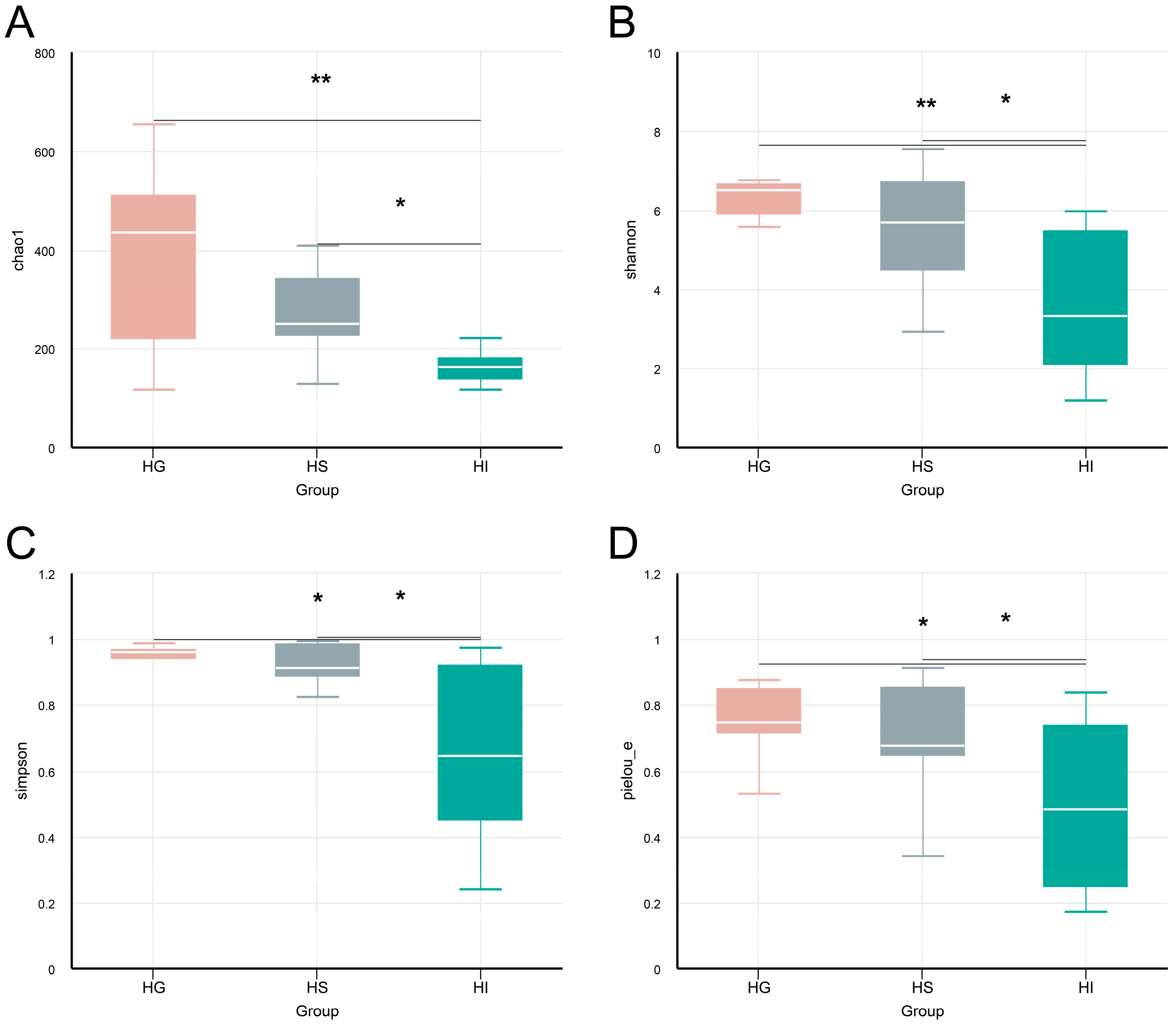
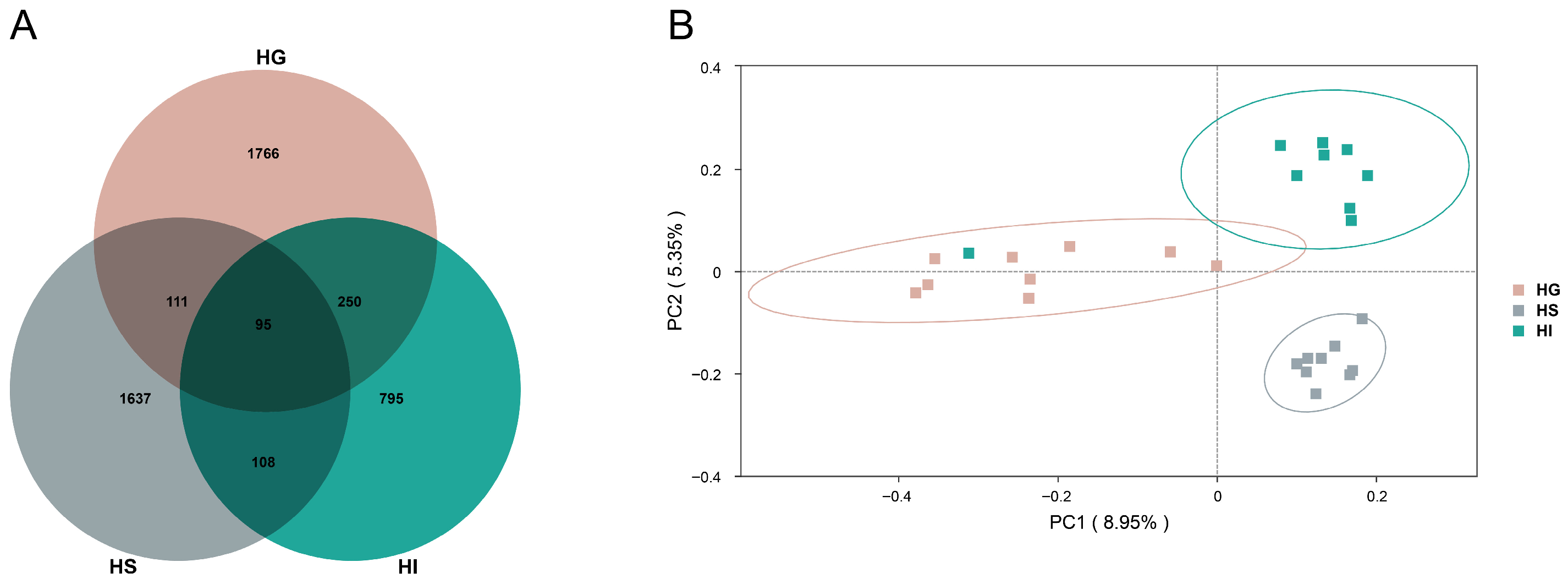
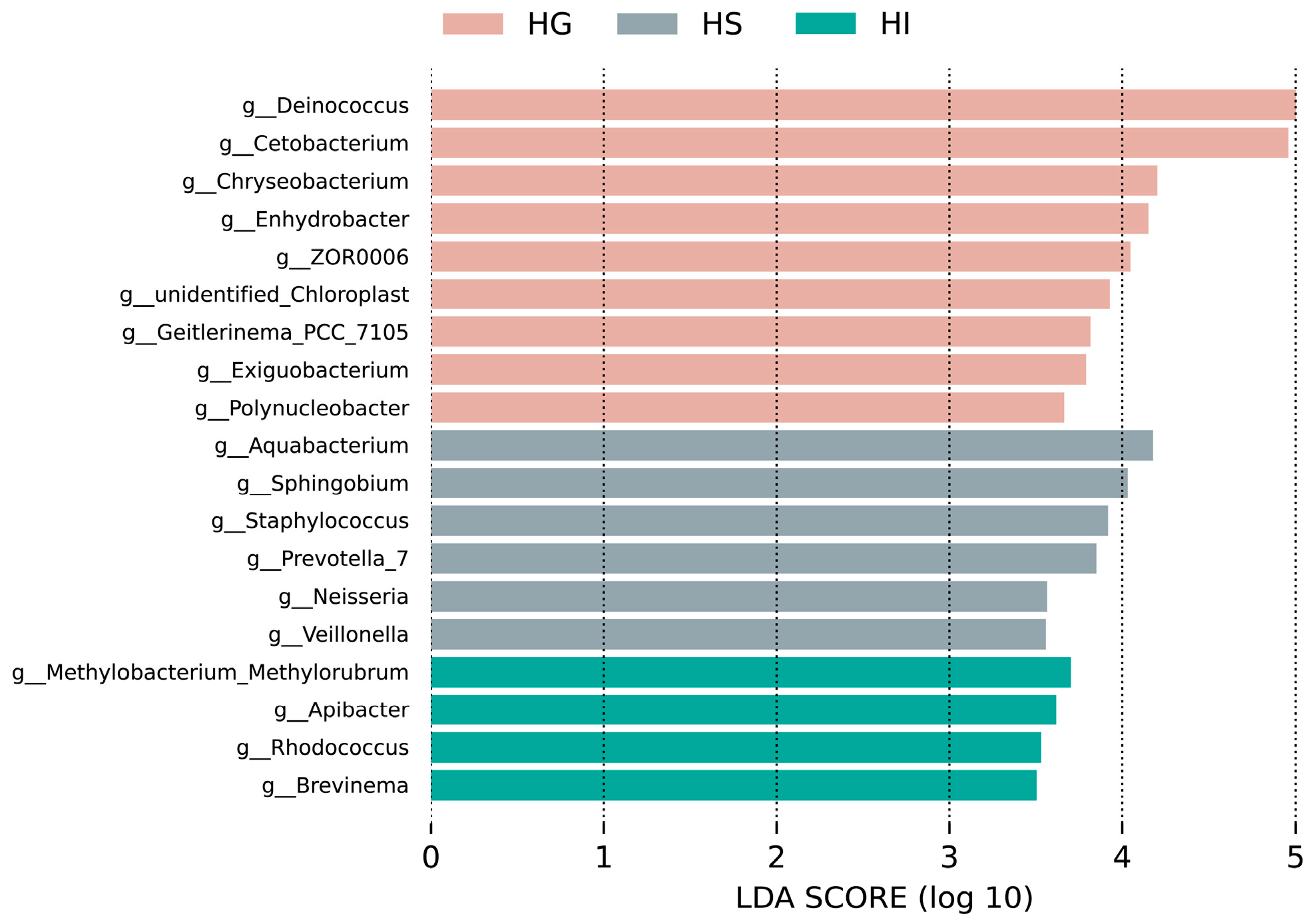
3.2. Microbiota Characteristics Across Different Mucosal Sites of Rice Flower Carp
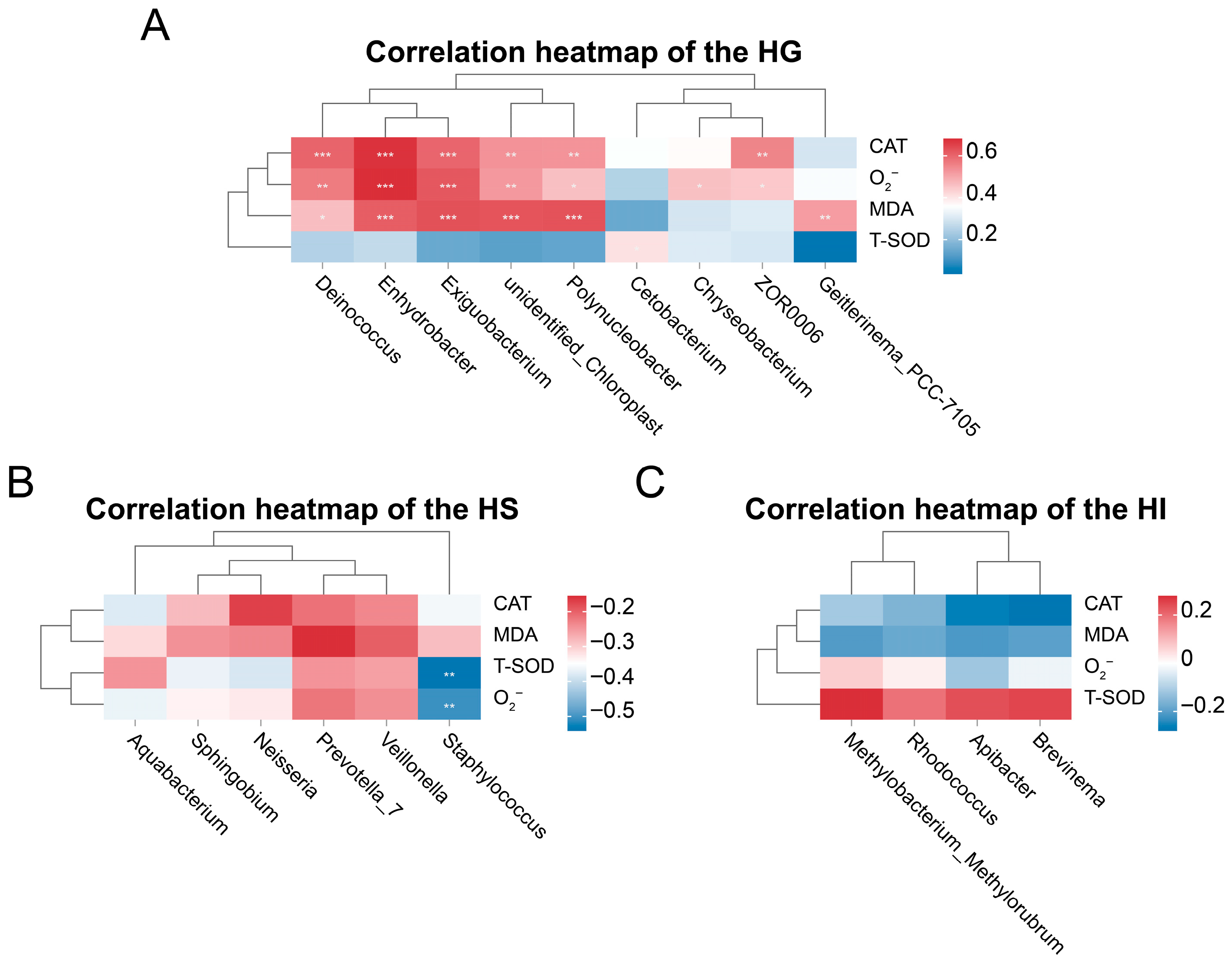
3.3. Correlation Analysis Between Differentially Abundant Genera and Antioxidant Indicators Across Different Mucosal Sites of Rice Flower Carp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- Ramasubburayan, R.; Prakash, S.; Immanuel, G.; Mubarakali, D.; Rajakumar, G.; Thirumurugan, D.; Palavesam, A. The transformative role of prebiotics, probiotics, and microbiomes in biofloc systems for sustainable aquaculture: A comprehensive review. Rev. Aquac. 2025, 17, e13000. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquacult. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Dabrowski, K.; Izquierdo, M.; Hematyar, N.; Imentai, A.; Steinbach, C.; Policar, T. Effects of vitamin c and e supplementation on growth, fatty acid composition, innate immunity, and antioxidant capacity of rainbow trout (Oncorhynchus mykiss) fed oxidized fish oil. Front. Mar. Sci. 2021, 8, 760587. [Google Scholar] [CrossRef]
- Gadhiya, A.; Katariya, S.; Khapandi, K.; Chhatrodiya, D. Probiotics as a sustainable alternative to antibiotics in aquaculture: A review of the current state of knowledge. Microbe 2025, 8, 100426. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial manipulations to improve fish health and production–a mediterranean perspective. Fish Shellfish Immunol. 2011, 30, 1–16. [Google Scholar] [CrossRef]
- Lazado, C.C.; Caipang, C.M.A. Atlantic cod in the dynamic probiotics research in aquaculture. Aquaculture 2014, 424–425, 53–62. [Google Scholar] [CrossRef]
- Picchietti, S.; Fausto, A.M.; Randelli, E.; Carnevali, O.; Taddei, A.R.; Buonocore, F.; Scapigliati, G.; Abelli, L. Early treatment with Lactobacillus delbrueckii strain induces an increase in intestinal t-cells and granulocytes and modulates immune-related genes of larval Dicentrarchus labrax (L.). Fish Shellfish Immunol. 2009, 26, 368–376. [Google Scholar] [CrossRef]
- Picchietti, S.; Mazzini, M.; Taddei, A.R.; Renna, R.; Fausto, A.M.; Mulero, V.; Carnevali, O.; Cresci, A.; Abelli, L. Effects of administration of probiotic strains on galt of larval gilthead seabream: Immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2007, 22, 57–67. [Google Scholar] [CrossRef]
- Hissen, K.L.; He, W.; Wu, G.; Criscitiello, M.F. Immunonutrition: Facilitating mucosal immune response in teleost intestine with amino acids through oxidant-antioxidant balance. Front. Immunol. 2023, 14, 1241615. [Google Scholar] [CrossRef] [PubMed]
- Rohmah, M.K.; Salahdin, O.D.; Gupta, R.; Muzammil, K.; Qasim, M.T.; Al-qaim, Z.H.; Abbas, N.F.; Jawad, M.A.; Yasin, G.; Mustafa, Y.F.; et al. Modulatory role of dietary curcumin and resveratrol on growth performance, serum immunity responses, mucus enzymes activity, antioxidant capacity and serum and mucus biochemicals in the common carp, Cyprinus carpio exposed to abamectin. Fish Shellfish Immunol. 2022, 129, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Xu, W.; Jiang, G.; Lu, K.; Wang, L.; Liu, W. Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminalis). Fish Shellfish. Immunol. 2013, 35, 1380–1386. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Xing, K.; Jiang, P.; Wang, J. Dietary cinnamaldehyde and Bacillus subtilis improve growth performance, digestive enzyme activity, and antioxidant capability and shape intestinal microbiota in tongue sole, Cynoglossus semilaevis. Aquaculture 2021, 531, 735798. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Chu, G.; Liu, H.; Shan, X.; Wang, G.; Han, G. The positive effects of single or conjoint administration of lactic acid bacteria on Channa argus: Digestive enzyme activity, antioxidant capacity, intestinal microbiota and morphology. Aquaculture 2021, 531, 735852. [Google Scholar] [CrossRef]
- Wang, L.; Ge, C.; Wang, J.; Dai, J.; Zhang, P.; Li, Y. Effects of different combinations of Bacillus on immunity and antioxidant activities in common carp. Aquac. Int. 2017, 25, 2091–2099. [Google Scholar] [CrossRef]
- Andersson, T.; Ertürk Bergdahl, G.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Z.; Liu, M.; Sun, B.; Tang, L.; Chen, L. Shift in skin microbiota and immune functions of zebrafish after combined exposure to perfluorobutanesulfonate and probiotic Lactobacillus rhamnosus. Ecotoxicol. Environ. Saf. 2021, 218, 112310. [Google Scholar] [CrossRef]
- Mougin, J.; Joyce, A. Fish disease prevention via microbial dysbiosis-associated biomarkers in aquaculture. Rev. Aquac. 2023, 15, 579–594. [Google Scholar] [CrossRef]
- Beghin, M.; Ambroise, V.; Lambert, J.; Garigliany, M.; Cornet, V.; Kestemont, P. Environmental exposure to single and combined ZnO and TiO2 nanoparticles: Implications for rainbow trout gill immune functions and microbiota. Chemosphere 2025, 373, 144148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tian, W.; Wei, J.; Li, L.; Tan, H.; Wu, Z.; Jiang, J. Response of rice flower carp (Cyprinus carpio var.) to high-temperature stress: Mechanisms and limits of tolerance. J. Therm. Biol. 2025, 132, 104244. [Google Scholar] [CrossRef]
- Li, Z.; Du, X.; Wen, L.; Li, Y.; Qin, J.; Chen, Z.; Huang, Y.; Wu, X.; Luo, H.; Lin, Y.; et al. Transcriptome analysis reveals the involvement of ubiquitin-proteasome pathway in the regulation of muscle growth of rice flower carp. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100948, Erratum in Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100987. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zeng, D.; He, P.; Wei, P.; Hui, W.; Wu, T.; Zhuo, X.; Lin, Y. mRNA and microRNA transcriptomics analyses in intermuscular bones of two carp species, rice flower carp (Cyprinus carpio var. Quanzhounensis) and Jian carp (Cyprinus carpio var. Jian). Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 71–80. [Google Scholar] [CrossRef]
- Cheng, C.; Tian, W.; Wu, Y.; Wei, J.; Yang, L.; Wei, Y.; Jiang, J. Microplastics have additive effects on cadmium accumulation and toxicity in rice flower carp (Procypris merus). Sci. Total Environ. 2024, 930, 172679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; You, Y.; Peng, F.; Tang, X.; Zhou, Y.; Liu, J.; Lin, D.; Zhou, Y. Interaction of microbiota between fish and the environment of an in-pond raceway system in a lake. Microorganisms 2022, 10, 1143. [Google Scholar] [CrossRef]
- Zhao, N.; Guo, J.; Zhang, B.; Liu, K.; Liu, Y.; Shen, Y.; Li, J. Heterogeneity of the tissue-specific mucosal microbiome of normal grass carp (Ctenopharyngodon idella). Mar. Biotechnol. 2022, 24, 366–379. [Google Scholar] [CrossRef]
- Lyons, P.P.; Turnbull, J.F.; Dawson, K.A.; Crumlish, M. Exploring the microbial diversity of the distal intestinal lumen and mucosa of farmed rainbow trout Oncorhynchus mykiss (Walbaum) using next generation sequencing (NGS). Aquac. Res. 2017, 48, 77–91. [Google Scholar] [CrossRef]
- Coelho, S.; Oliveira, R.; Pereira, S.; Musso, C.; Domingues, I.; Bhujel, R.C.; Soares, A.M.V.M.; Nogueira, A.J.A. Assessing lethal and sub-lethal effects of trichlorfon on different trophic levels. Aquat. Toxicol. 2011, 103, 191–198. [Google Scholar] [CrossRef]
- Gatta, P.P.; Pirini, M.; Testi, S.; Vignola, G.; Monetti, P.G. The influence of different levels of dietary vitamin e on sea bass Dicentrarchus labrax flesh quality. Aquac. Nutr. 2000, 6, 47–52. [Google Scholar] [CrossRef]
- Arockiaraj, J.; Palanisamy, R.; Bhatt, P.; Kumaresan, V.; Gnanam, A.J.; Pasupuleti, M.; Kasi, M. A novel murrel Channa striatus mitochondrial manganese superoxide dismutase: Gene silencing, sod activity, superoxide anion production and expression. Fish Physiol. Biochem. 2014, 40, 1937–1955. [Google Scholar] [CrossRef]
- Melo, N.; de Souza, S.P.; Konig, I.; de Jesus Paula, D.A.; Ferreira, I.S.; Luz, R.K.; Murgas, L.D.S. Sensitivity of different organs and tissues as biomarkers of oxidative stress in juvenile tambaqui (Colossoma macropomum) submitted to fasting. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 291, 111595. [Google Scholar] [CrossRef]
- Huang, S.; Li, C.; Li, Z.; Duanzhi, D.; Liu, Y.; Ran, F.; Ding, J.; Yan, W.; Jia, C.; Zhang, Z.; et al. Short-term exposure to 5‰ and 15‰ salinity causes the dynamic changes of thenka gene, enzyme activities and morphological characteristics in fish tissues of Gymnocypris przewalskii. Aquac. Res. 2022, 53, 6389–6398. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Alkafafy, M.; Sewilam, H. The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol. Biochem. 2022, 48, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Ángeles Esteban, M. An overview of the immunological defenses in fish skin. ISRN Immunol. 2012, 2012, 853470. [Google Scholar] [CrossRef]
- Djordjevic, B.; Morales Lange, B.; Øverland, M.; Mercado, L.; Lagos, L. Immune and proteomic responses to the soybean meal diet in skin and intestine mucus of Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2021, 27, 929–940. [Google Scholar] [CrossRef]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019, 5, 366–372. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Abdelghany, M.F.; Khames, D.K.; Abd El-hameed, S.A.A.; Mansour, E.M.G.; El-Nadi, A.S.M.; Shoukry, A.A. Administration of some probiotic strains in the rearing water enhances the water quality, performance, body chemical analysis, antioxidant and immune responses of Nile tilapia, Oreochromis niloticus. Appl. Water Sci. 2022, 12, 209. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.; Zhao, R.; Lu, T.; Li, S.; Wang, D. Effects of dietary supplementation with endogenous probiotics Bacillus subtilis on growth performance, immune response and intestinal histomorphology of juvenile Rainbow trout (Oncorhynchus mykiss). Fishes 2024, 9, 229. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Tong, X.; Gao, Y.; Liang, R.; Liu, C.; Zhao, K. Microbial communities associated with the skin, gill, and gut of large yellow croaker (Larimichthys crocea). BMC Microbiol. 2025, 25, 16. [Google Scholar] [CrossRef]
- Liu, J.; Pan, Y.; Jin, S.; Zheng, Y.; Xu, J.; Fan, H.; Khalid, M.; Wang, Y.; Hu, M. Effects of Citrobacter freundii on sturgeon: Insights from skin mucosal immunology and microbiota. Fish Shellfish Immunol. 2024, 149, 109527. [Google Scholar] [CrossRef]
- Guivier, E.; Pech, N.; Chappaz, R.; Gilles, A. Microbiota associated with the skin, gills, and gut of the fish Parachondrostoma toxostoma from the rhône basin. Freshw. Biol. 2020, 65, 446–459. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Xu, W.; Tang, R.; Li, L. Microbiome of co-cultured fish exhibits host selection and niche differentiation at the organ scale. Front. Microbiol. 2019, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Minich, J.J.; Härer, A.; Vechinski, J.; Frable, B.W.; Skelton, Z.R.; Kunselman, E.; Shane, M.A.; Perry, D.S.; Gonzalez, A.; McDonald, D.; et al. Host biology, ecology and the environment influence microbial biomass and diversity in 101 marine fish species. Nat. Commun. 2022, 13, 6978. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Tao, L.; Chai, J.; Liu, H.; Huang, W.; Zou, Y.; Wu, M.; Peng, B.; Wang, Q.; Tang, K. Characterization and dynamics of the gut microbiota in rice fishes at different developmental stages in rice-fish coculture systems. Microorganisms 2022, 10, 2373. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Y.; Ye, Q.; Yao, Y.; Li, L.; Guo, Z.; Yang, L.; Tian, W.; Jiang, J. Individual and combined effects of microplastics and cadmium on intestinal histology and microflora of Procypris merus. Aquacult. Rep. 2023, 31, 101659. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Q.; Lin, Y.; Hao, J.; Wang, S.; Zhang, J.; Li, A. Taxonomic and functional characteristics of the gill and gastrointestinal microbiota and its correlation with intestinal metabolites in new gift strain of farmed adult Nile tilapia (Oreochromis niloticus). Microorganisms 2021, 9, 617. [Google Scholar] [CrossRef]
- Minich, J.J.; Petrus, S.; Michael, J.D.; Michael, T.P.; Knight, R.; Allen, E.E. Temporal, environmental, and biological drivers of the mucosal microbiome in a wild marine fish, Scomber japonicus. mSphere 2020, 5, e401–e420. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Jiang, Y.; Ji, T.; Bai, H.; Zhao, H.; Yang, H. Addition of Bdellovibrio to aquaculture water can significantly alter the distribution of microbial community on the gills and enhance the survival rate of Carassius auratus gibelio. Aquaculture 2023, 576, 739820. [Google Scholar] [CrossRef]
- Meng, K.; Ding, L.; Wu, S.; Wu, Z.; Cheng, G.; Zhai, X.; Sun, R.; Xu, Z. Interactions between commensal microbiota and mucosal immunity in teleost fish during viral infection with svcv. Front. Immunol. 2021, 12, 654758. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, Y.; Ou, W.; Dai, J.; Ai, Q.; Zhang, W.; Mai, K.; Zhang, Y. The protective role of daidzein in intestinal health of turbot (Scophthalmus maximus L.) Fed soybean meal-based diets. Sci. Rep. 2021, 11, 3352. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, W.; Xie, Y.; Li, Y.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Ran, C.; Zhou, Z. Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 2021, 543, 736943. [Google Scholar] [CrossRef]
- Bernardet, J.F.; Vancanneyt, M.; Matte-Tailliez, O.; Grisez, L.; Tailliez, P.; Bizet, C.; Nowakowski, M.; Kerouault, B.; Swings, J. Polyphasic study of Chryseobacterium strains isolated from diseased aquatic animals. Syst. Appl. Microbiol. 2005, 28, 640–660. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Duman, M.; Altun, S. Genome analysis and antimicrobial resistance characteristics of Chryseobacterium aquaticum isolated from farmed salmonids. Aquaculture 2021, 535, 736364. [Google Scholar] [CrossRef]
- Guo, J.; Lin, J.; Li, X.; Wang, L.; Song, K.; Lu, K.; Zhang, C. Enhanced intestinal microflora composition and phosphorus-transportation efficiency in fast-growing spotted seabass (Lateolabrax maculatus) fed a low-phosphorus diet. Aquaculture 2023, 577, 739916. [Google Scholar] [CrossRef]
- Hirose, S.; Tank, M.; Hara, E.; Tamaki, H.; Mori, K.; Takaichi, S.; Haruta, S.; Hanada, S. Aquabacterium pictum sp. Nov., the first aerobic bacteriochlorophyll a-containing fresh water bacterium in the genus Aquabacterium of the class betaproteobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 596–603. [Google Scholar] [CrossRef]
- Kalmbach, S.; Manz, W.; Bendinger, B.; Szewzyk, U. In situ probing reveals Aquabacterium commune as a widespread and highly abundant bacterial species in drinking water biofilms. Water Res. 2000, 34, 575–581. [Google Scholar] [CrossRef]
- Musharrafieh, R.; Tacchi, L.; Trujeque, J.; LaPatra, S.; Salinas, I. Staphylococcus warneri, a resident skin commensal of rainbow trout (Oncorhynchus mykiss) with pathobiont characteristics. Vet. Microbiol. 2014, 169, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Itay, P.; Shemesh, E.; Ofek-Lalzar, M.; Davidovich, N.; Kroin, Y.; Zrihan, S.; Stern, N.; Diamant, A.; Wosnick, N.; Meron, D.; et al. An insight into gill microbiome of eastern mediterranean wild fish by applying next generation sequencing. Front. Mar. Sci. 2022, 9, 1008103. [Google Scholar] [CrossRef]
- Araújo, W.L.; Santos, D.S.; Dini-Andreote, F.; Salgueiro-Londoño, J.K.; Camargo-Neves, A.A.; Andreote, F.D.; Dourado, M.N. Genes related to antioxidant metabolism are involved in Methylobacterium mesophilicum-soybean interaction. Antonie. Van. Leeuwenhoek. 2015, 108, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bijlani, S.; Singh, N.K.; Eedara, V.V.R.; Podile, A.R.; Mason, C.E.; Wang, C.C.C.; Venkateswaran, K. Methylobacterium ajmalii sp. Nov., Isolated from the international space station. Front. Microbiol. 2021, 12, 639396. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Su, Q.; Tang, M.; Zheng, H.; Zhou, X. Genomic features underlying the evolutionary transitions of Apibacter to honey bee gut symbionts. Insect Sci. 2022, 29, 259–275. [Google Scholar] [CrossRef]
- Han, L.; Chang, Z.M.; Ren, C.S.; Chen, X.S.; Smagghe, G.; Yuan, Y.G.; Long, J.K. Colony performance of three native bumblebee species from south china and association with their gut microbiome. Insect Sci. 2024, 31, 1960–1983. [Google Scholar] [CrossRef]
- Khan, A.; Mandal, S.; Samanta, D.; Chatterjee, S.; Ghosh, K. Phytase-producing Rhodococcus sp. (Mtcc 9508) from fish gut: A preliminary study. Proc. Zool. Soc. 2011, 64, 29–34. [Google Scholar] [CrossRef]
- Ringø, E.; Li, X.; Doan, H.V.; Ghosh, K. Interesting probiotic bacteria other than the more widely used lactic acid bacteria and bacilli in finfish. Front. Mar. Sci. 2022, 9, 848037. [Google Scholar] [CrossRef]
- Paimeeka, S.; Tangsongcharoen, C.; Lertwanakarn, T.; Setthawong, P.; Bunkhean, A.; Tangwattanachuleeporn, M.; Surachetpong, W. Tilapia lake virus infection disrupts the gut microbiota of red hybrid tilapia (Oreochromis spp.). Aquaculture 2024, 586, 740752. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Xu, Z.; Yang, Z.; Mu, Q.; Li, Z.; Liu, P.; Hu, J.; Bao, Z. Unveiling the characteristics of microbiota in different mucosal layers of leopard coral grouper (Plectropomus leopardus). Mar. Biotechnol. 2025, 27, 86. [Google Scholar] [CrossRef]
- Zaidi, S.; Ali, K.; Khan, A.U. It’s all relative: Analyzing microbiome compositions, its significance, pathogenesis and microbiota derived biofilms: Challenges and opportunities for disease intervention. Arch. Microbiol. 2023, 205, 257. [Google Scholar] [CrossRef] [PubMed]
- Gnanagobal, H.; Santander, J. Host–pathogen interactions of marine gram-positive bacteria. Biology 2022, 11, 1316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Ding, J.; Zhang, Y.; Hou, C.; Shen, W.; Wu, X.; Zhu, J. Effects of hypoxia stress on oxidative stress, apoptosis and microorganisms in the intestine of large yellow croaker (Larimichthys crocea). Aquaculture 2024, 581, 740444. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, X.; Zhang, Z.; Jin, Z.; Wang, G.; Ling, F. Effects of dietary Cetobacterium somerae on the intestinal health, immune parameters and resistance against Nocardia seriolae of largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2023, 135, 108693. [Google Scholar] [CrossRef]
- Sachse, F.; Becker, K.; von Eiff, C.; Metze, D.; Rudack, C. Staphylococcus aureus invades the epithelium in nasal polyposis and induces il-6 in nasal epithelial cells in vitro. Allergy 2010, 65, 1430–1437. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Ding, L.G.; Huang, Z.Y.; Xu, H.Y.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]

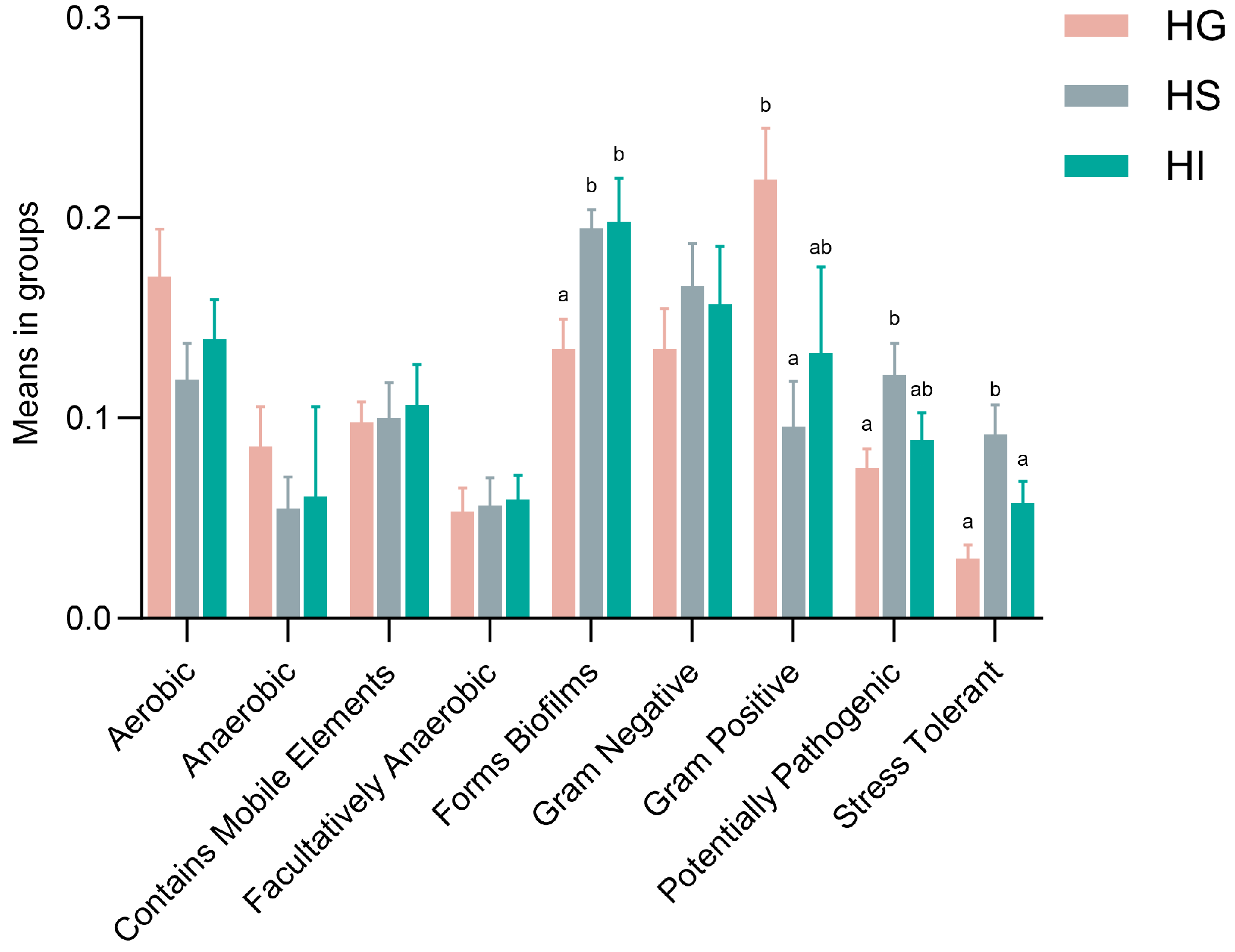
| HG | HS | HI | |
|---|---|---|---|
| CAT (U/mg prot) | 56.12 ± 1.16 c | 26.90 ± 0.98 a | 31.52 ± 1.43 b |
| T-SOD (U/mg prot) | 14.95 ± 0.81 b | 3.42 ± 0.63 a | 17.57 ± 0.61 c |
| MDA (nmol/mg prot) | 23.78 ± 2.45 b | 4.47 ± 0.71 a | 4.69 ± 0.09 a |
| O2− (nmol/mg prot) | 319.68 ± 7.23 c | 65.92 ± 7.33 a | 176.61 ± 8.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Tang, Y.; Du, J.; Xu, Z.; Peng, X.; Qian, Y.; Guo, Z.; Qin, C.; Li, S.; Huang, S.; et al. Antioxidant and Microbiota Characteristics Across Different Mucosal Sites of Rice Flower Carp (Procypris merus). Microorganisms 2025, 13, 2673. https://doi.org/10.3390/microorganisms13122673
Ren H, Tang Y, Du J, Xu Z, Peng X, Qian Y, Guo Z, Qin C, Li S, Huang S, et al. Antioxidant and Microbiota Characteristics Across Different Mucosal Sites of Rice Flower Carp (Procypris merus). Microorganisms. 2025; 13(12):2673. https://doi.org/10.3390/microorganisms13122673
Chicago/Turabian StyleRen, Huige, Yutu Tang, Jingyi Du, Zihao Xu, Xiao Peng, Ye Qian, Zihe Guo, Chanxia Qin, Shihao Li, Sikai Huang, and et al. 2025. "Antioxidant and Microbiota Characteristics Across Different Mucosal Sites of Rice Flower Carp (Procypris merus)" Microorganisms 13, no. 12: 2673. https://doi.org/10.3390/microorganisms13122673
APA StyleRen, H., Tang, Y., Du, J., Xu, Z., Peng, X., Qian, Y., Guo, Z., Qin, C., Li, S., Huang, S., Mo, Y., Huang, C., & Ou, W. (2025). Antioxidant and Microbiota Characteristics Across Different Mucosal Sites of Rice Flower Carp (Procypris merus). Microorganisms, 13(12), 2673. https://doi.org/10.3390/microorganisms13122673






