Comparative Characterization of Vaginal and Gut Microbiota in Late-Pregnancy Women with or Without Group B Streptococcus Colonization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Study Population and Inclusion/Exclusion Criteria
2.3. Sample Collection and Microbiome/GBS Analyses
2.4. Statistical Analysis of Baseline Characteristics
2.5. Microbiota Data Analysis
2.5.1. Sequencing and Preprocessing
2.5.2. Microbiota Community Composition, Diversity, and Visualization
2.5.3. Differential Abundance and Association Analysis
2.5.4. Vaginal-Gut Microbiota Correlation Analysis
3. Results
3.1. Maternal Baseline Characteristics and Delivery Outcomes Across GBS Status Groups
3.2. Vaginal Microbiota
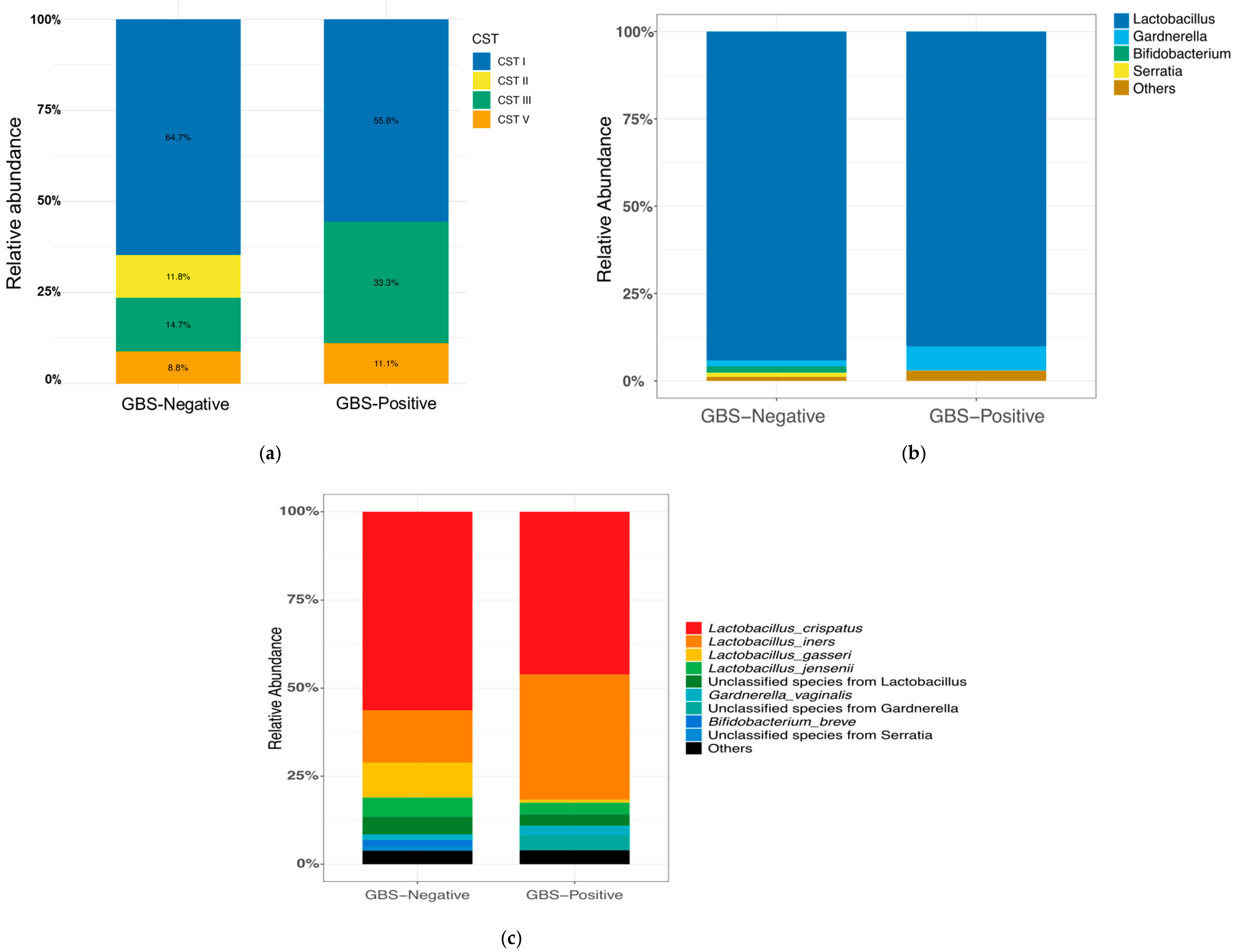
3.2.1. CST Composition
3.2.2. Vaginal Microbial Community Composition
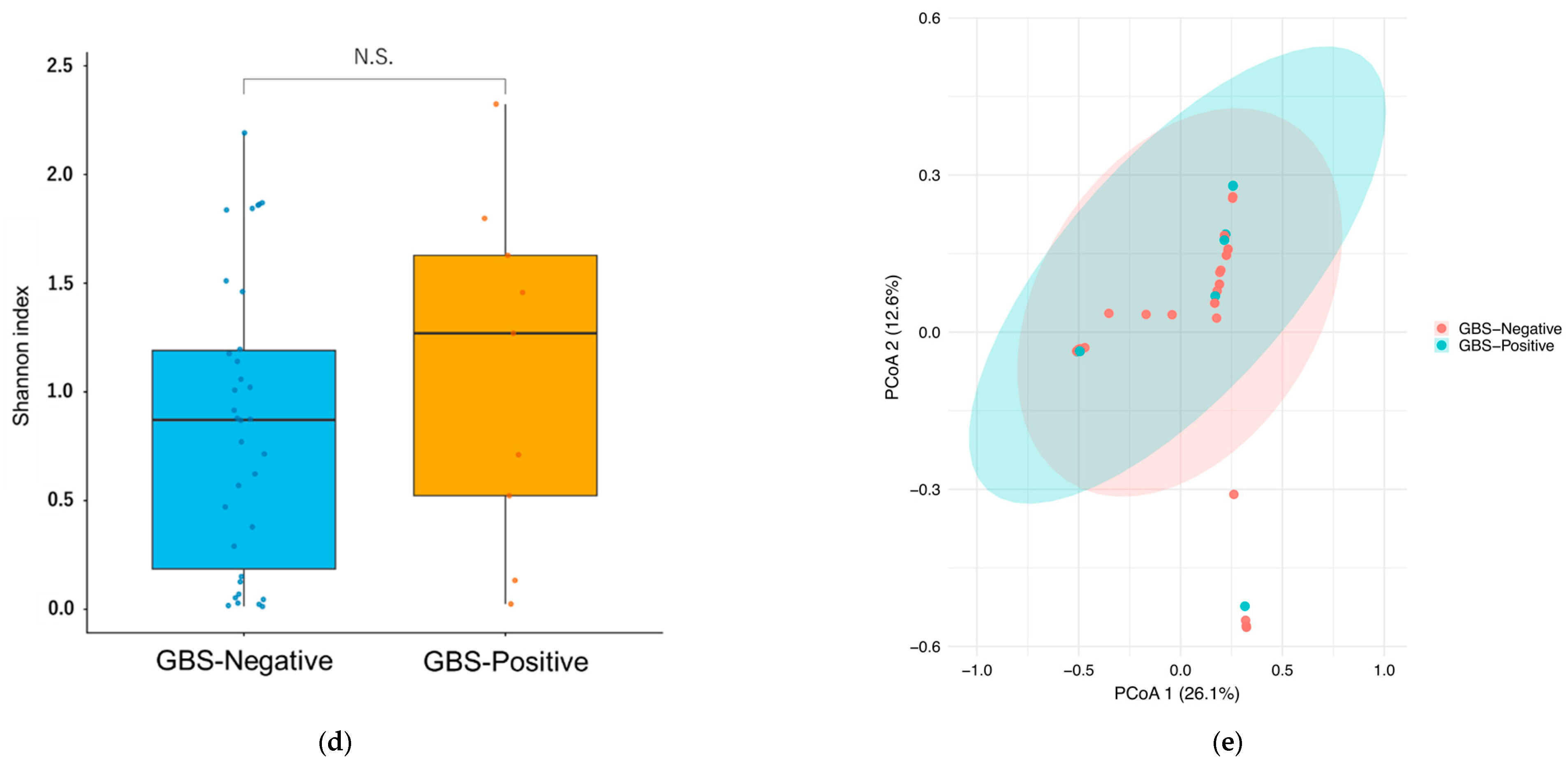
3.2.3. Vaginal Microbiota Diversity
3.3. Gut Microbiota
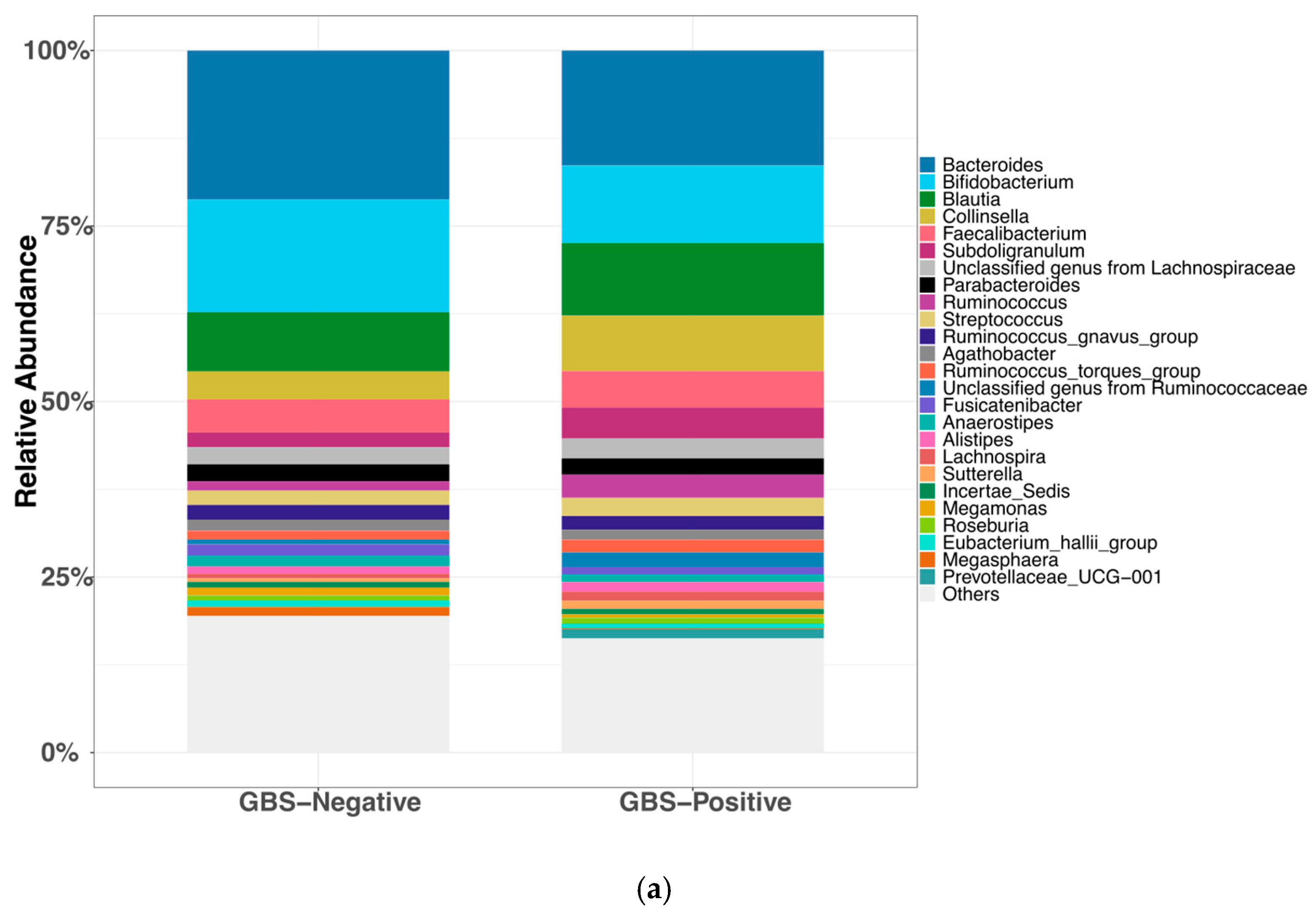
3.3.1. Gut Microbial Community Composition
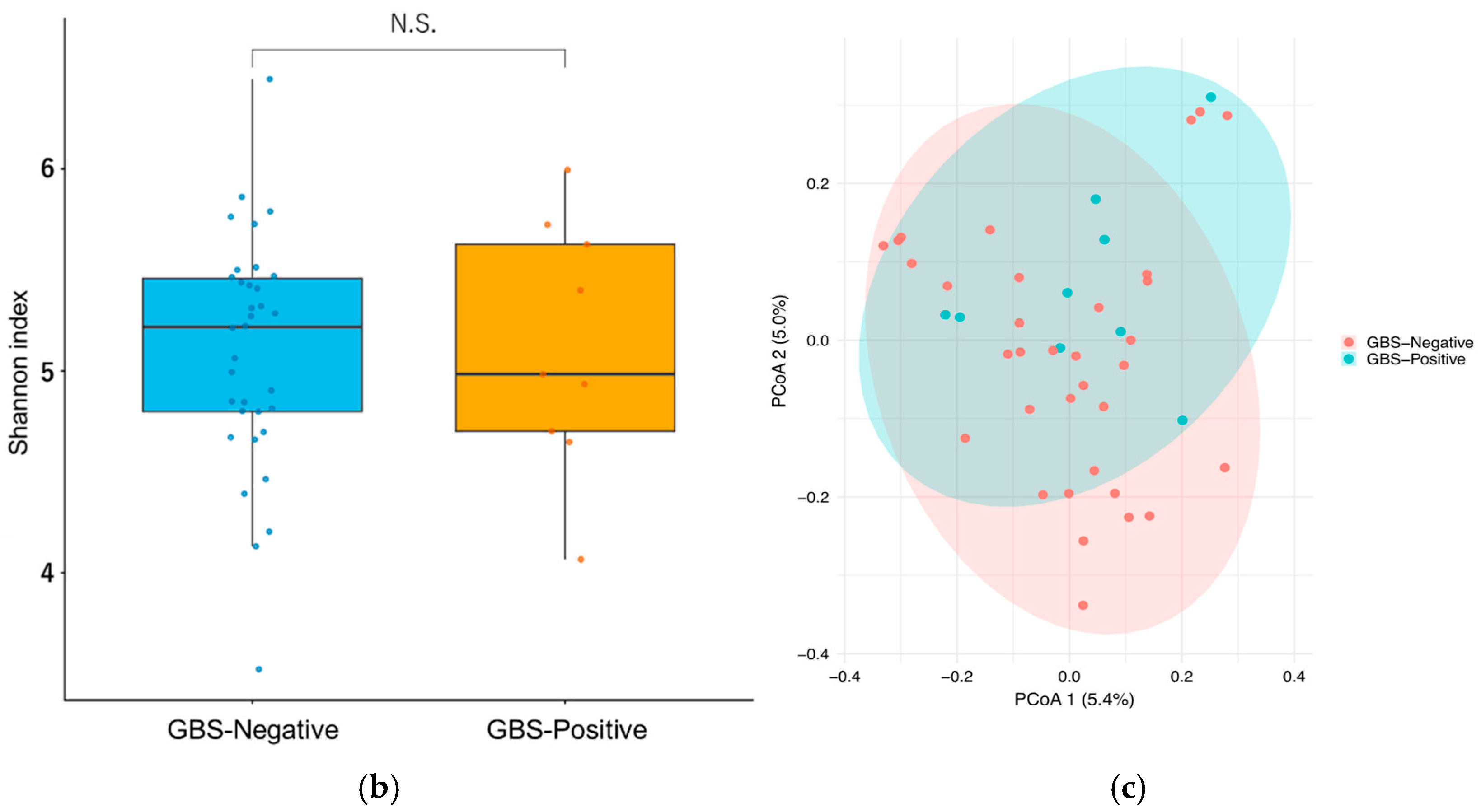
3.3.2. Diversity Analysis of Gut Microbiota
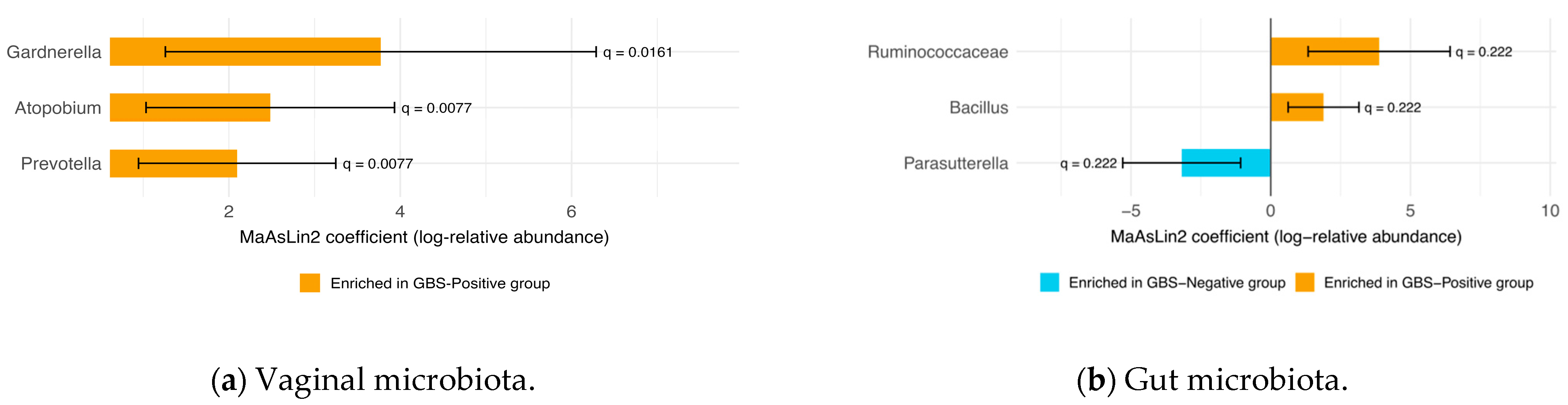
3.4. Differential Abundance of Vaginal and Gut Microbiota
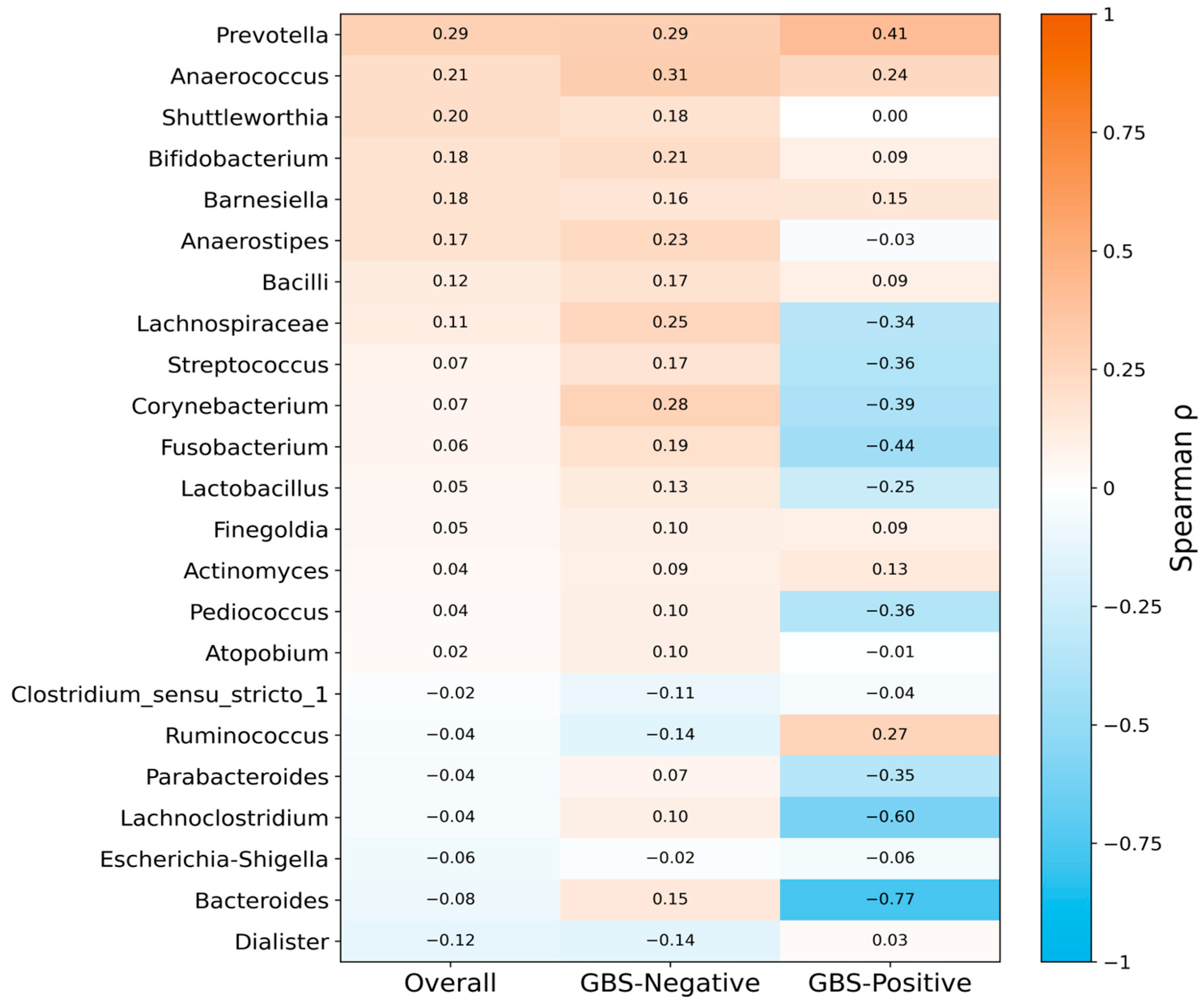
3.5. Spearman Correlation Analysis of Shared Vaginal and Gut Genera
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verani, J.R.; McGee, L.; Schrag, S.J.; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of Perinatal Group B Streptococcal Disease—Revised Guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–36. [Google Scholar]
- Shabayek, S.; Spellerberg, B. Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Starc, M.; Lučovnik, M.; Eržen Vrlič, P.; Jeverica, S. Protective Effect of Lactobacillus crispatus against Vaginal Colonization with Group B Streptococci in the Third Trimester of Pregnancy. Pathogens 2022, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obs. Gynecol. 2020, 135, e51–e72. [Google Scholar] [CrossRef] [PubMed]
- Nishio, E.; Ishitani, K.; Arimoto, T.; Igarashi, T.; Ishikawa, T.; Iwase, A.; Ogawa, M.; Ozawa, N.; Kajiyama, H.; Kawasaki, K.; et al. Guideline for Gynecological Practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists 2023 Edition. J. Obstet. Gynaecol. Res. 2024, 50, 1073–1094. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The Vaginal Microbiome and Preterm Birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- Gryaznova, M.; Kozarenko, O.; Smirnova, Y.; Burakova, I.; Syromyatnikov, M.; Maslov, A.; Lebedeva, O. Cervical and Vaginal Microbiomes in Early Miscarriages and Ongoing Pregnancy with and without Dydrogesterone Usage. Int. J. Mol. Sci. 2023, 24, 13836. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-Gut Microbiome Axis: Physiological and Clinical Implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Takada, K.; Melnikov, V.G.; Kobayashi, R.; Komine-Aizawa, S.; Tsuji, N.M.; Hayakawa, S. Female Reproductive Tract-Organ Axes. Front. Immunol. 2023, 14, 1110001. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile Acids as Modulators of Gut Microbiota Composition and Function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Gilbert, N.M.; Foster, L.R.; Cao, B.; Yin, Y.; Mysorekar, I.U.; Lewis, A.L. Gardnerella vaginalis Promotes Group B Streptococcus Vaginal Colonization, Enabling Ascending Uteroplacental Infection in Pregnant Mice. Am. J. Obs. Gynecol. 2021, 224, e1–e530. [Google Scholar] [CrossRef]
- Li, M.; Zeng, Z.; Wang, X.; Liu, Y.; Wei, H.; Liu, J.; Zhu, S.; Jiang, Q.; Zhang, K.; Wu, Y.; et al. Mechanisms of S. Agalactiae Promoting G. vaginalis Biofilm Formation Leading to Recurrence of BV. npj Biofilms Microbiomes 2024, 10, 138. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857, Erratum in Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Ogasawara, O.; Kodama, Y.; Mashima, J.; Kosuge, T.; Fujisawa, T. DDBJ Database Updates and Computational Infrastructure Enhancement. Nucleic Acids Res. 2020, 48, D45–D50. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The Role of Lactic Acid Production by Probiotic Lactobacillus Species in Vaginal Health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Carter, K.A.; Fischer, M.D.; Petrova, M.I.; Balkus, J.E. Epidemiologic Evidence on the Role of Lactobacillus iners in Sexually Transmitted Infections and Bacterial Vaginosis: A Series of Systematic Reviews and Meta-Analyses. Sex. Transm. Dis. 2023, 50, 224–235. [Google Scholar] [CrossRef]
- Buchta, V.; Nekvindová, J.; Leško, D.; Vrbacký, F.; Veščičík, P.; Uhlířová, Z.; Andrýs, C.; Bolehovská, R.; Kacerovský, M.; Špaček, J.; et al. Vaginal Microbiota: Different Roles of Lactobacilli and Community Instability in Chronic Vulvovaginal Discomfort. Front. Cell. Infect. Microbiol. 2025, 15, 1636873. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Savicheva, A.M.; Krysanova, A.A.; Budilovskaya, O.V.; Spasibova, E.V.; Khusnutdinova, T.A.; Shalepo, K.V.; Beliaeva, N.R.; Safarian, G.K.; Sapozhnikov, K.V.; Tapilskaya, N.I.; et al. Vaginal Microbiota Molecular Profiling in Women with Bacterial Vaginosis: A Novel Diagnostic Tool. Int. J. Mol. Sci. 2023, 24, 15880. [Google Scholar] [CrossRef]
- Dong, W.; Wang, S.; Wang, X.; Xu, G.; Liu, Q.; Li, Z.; Lv, N.; Pan, Y.; Xiong, Q.; Liu, D.; et al. Characteristics of Vaginal Microbiota of Women of Reproductive Age with Infections. Microorganisms 2024, 12, 1030. [Google Scholar] [CrossRef]
- Maidment, T.I.; Pelzer, E.S.; Borg, D.J.; Cheung, E.; Begun, J.; Nitert, M.D.; Rae, K.M.; Clifton, V.L.; Carey, A.J. Group B Streptococcus Vaginal Colonisation throughout Pregnancy Is Associated with Decreased Lactobacillus crispatus and Increased Lactobacillus iners Abundance in the Vaginal Microbial Community. Front. Cell Infect. Microbiol. 2024, 14, 1435745. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent Biofilms in Bacterial Vaginosis. Obs. Gynecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef]
- Hardy, L.; Jespers, V.; Abdellati, S.; De Baetselier, I.; Mwambarangwe, L.; Musengamana, V.; van de Wijgert, J.; Vaneechoutte, M.; Crucitti, T. A Fruitful Alliance: The Synergy between Atopobium vaginae and Gardnerella vaginalis in Bacterial Vaginosis-Associated Biofilm. Sex. Transm. Infect. 2016, 92, 487–491. [Google Scholar] [CrossRef]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Mu, X.; Zhao, C.; Yang, J.; Wei, X.; Zhang, J.; Liang, C.; Gai, Z.; Zhang, C.; Zhu, D.; Wang, Y.; et al. Group B Streptococcus Colonization Induces Prevotella and Megasphaera Abundance-Featured Vaginal Microbiome Compositional Change in Non-Pregnant Women. PeerJ 2019, 7, e7474. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Hu, F.; Sun, X.; Su, Y.; Huang, M. The Dynamic Changes in the Composition and Diversity of Vaginal Microbiota in Women of Different Pregnancy Periods. Microorganisms 2023, 11, 2686. [Google Scholar] [CrossRef]
- Pierański, M.K.; Kosiński, J.G.; Szymczak, K.; Sadowski, P.; Grinholc, M. Antimicrobial Photodynamic Inactivation: An Alternative for Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Antioxidants 2023, 12, 847. [Google Scholar] [CrossRef]
- Hanson, L.; VandeVusse, L.; Malloy, E.; Garnier-Villarreal, M.; Watson, L.; Fial, A.; Forgie, M.; Nardini, K.; Safdar, N. Probiotic Interventions to Reduce Antepartum Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Midwifery 2022, 105, 103208. [Google Scholar] [CrossRef]
- Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-Specific Probiotic Strain. Nutrients 2019, 11, 810. [Google Scholar] [CrossRef]
- Ho, M.; Chang, Y.-Y.; Chang, W.-C.; Lin, H.-C.; Wang, M.-H.; Lin, W.-C.; Chiu, T.-H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to Reduce Group B Streptococcus Colonization in Pregnant Women: A Randomized Controlled Trial. Taiwan. J. Obs. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef]
| Variable | Overall (n= 43) | Negative (n = 34) | Positive (n = 9) | p-Value |
|---|---|---|---|---|
| Maternal age (years) | 36.00 [32.00, 38.50] | 37.00 [33.00, 38.75] | 30.00 [29.00, 38.00] | 0.192 a |
| Pre-pregnancy BMI (kg/m2) | 21.63 [19.39, 26.76] | 21.60 [19.37, 26.80] | 21.99 [19.52, 24.65] | 0.846 a |
| BMI at delivery (kg/m2) | 26.71 ± 4.19 | 26.55 ± 4.37 | 27.34 ± 3.59 | 0.586 b |
| Gestational age at delivery (weeks) | 39.00 [38.00, 40.00] | 39.00 [38.00, 40.00] | 39.00 [38.00, 40.00] | 0.725 a |
| Infant Birth weight (g) | 3039.72 ± 394.10 | 2998.85 ± 404.00 | 3194.11 ± 328.95 | 0.153 b |
| Parity, n (%) | 1 c | |||
| —Multiparous/Primiparous | 26 (60.5%) | 21 (61.8%) | 5 (55.6%) | |
| —Nulliparous | 17 (39.5%) | 13 (38.2%) | 4 (44.4%) | |
| Infertility Treatment (non-spontaneous conception), n (%) | ||||
| Yes | 23 (53.5%) | 19 (55.9%) | 4 (44.4%) | 0.711 c |
| No | 20 (46.5%) | 15 (44.1%) | 5 (55.6%) | |
| Mode of delivery, n (%) | ||||
| Vaginal | 33 (76.7%) | 26 (76.5%) | 7 (77.8%) | 1 c |
| Cesarean | 10 (23.3%) | 8 (23.5%) | 2 (22.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Iino, K.; Nakamura, M.; Sato, M.; Oishi, M.; Ito, A.; Yokoyama, Y. Comparative Characterization of Vaginal and Gut Microbiota in Late-Pregnancy Women with or Without Group B Streptococcus Colonization. Microorganisms 2025, 13, 2671. https://doi.org/10.3390/microorganisms13122671
Song S, Iino K, Nakamura M, Sato M, Oishi M, Ito A, Yokoyama Y. Comparative Characterization of Vaginal and Gut Microbiota in Late-Pregnancy Women with or Without Group B Streptococcus Colonization. Microorganisms. 2025; 13(12):2671. https://doi.org/10.3390/microorganisms13122671
Chicago/Turabian StyleSong, Shuang, Kaori Iino, Mako Nakamura, Maki Sato, Maika Oishi, Asami Ito, and Yoshihito Yokoyama. 2025. "Comparative Characterization of Vaginal and Gut Microbiota in Late-Pregnancy Women with or Without Group B Streptococcus Colonization" Microorganisms 13, no. 12: 2671. https://doi.org/10.3390/microorganisms13122671
APA StyleSong, S., Iino, K., Nakamura, M., Sato, M., Oishi, M., Ito, A., & Yokoyama, Y. (2025). Comparative Characterization of Vaginal and Gut Microbiota in Late-Pregnancy Women with or Without Group B Streptococcus Colonization. Microorganisms, 13(12), 2671. https://doi.org/10.3390/microorganisms13122671






