A New Gilliam Genotypic Variant of Orientia tsutsugamushi in Human Scrub Typhus Cases from South India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Molecular Detection and Genotyping of O. tsutsugamushi
2.3.1. Genomic DNA Extraction and GroEL Gene Amplification
2.3.2. Amplification of the 56-kDa Type-Specific Antigen Gene
2.3.3. Sequence Editing, BLAST Analysis, and Submission
2.3.4. Phylogenetic Analysis
2.3.5. Similarity and Bootscan Analysis of 56-kDa Sequences
3. Results
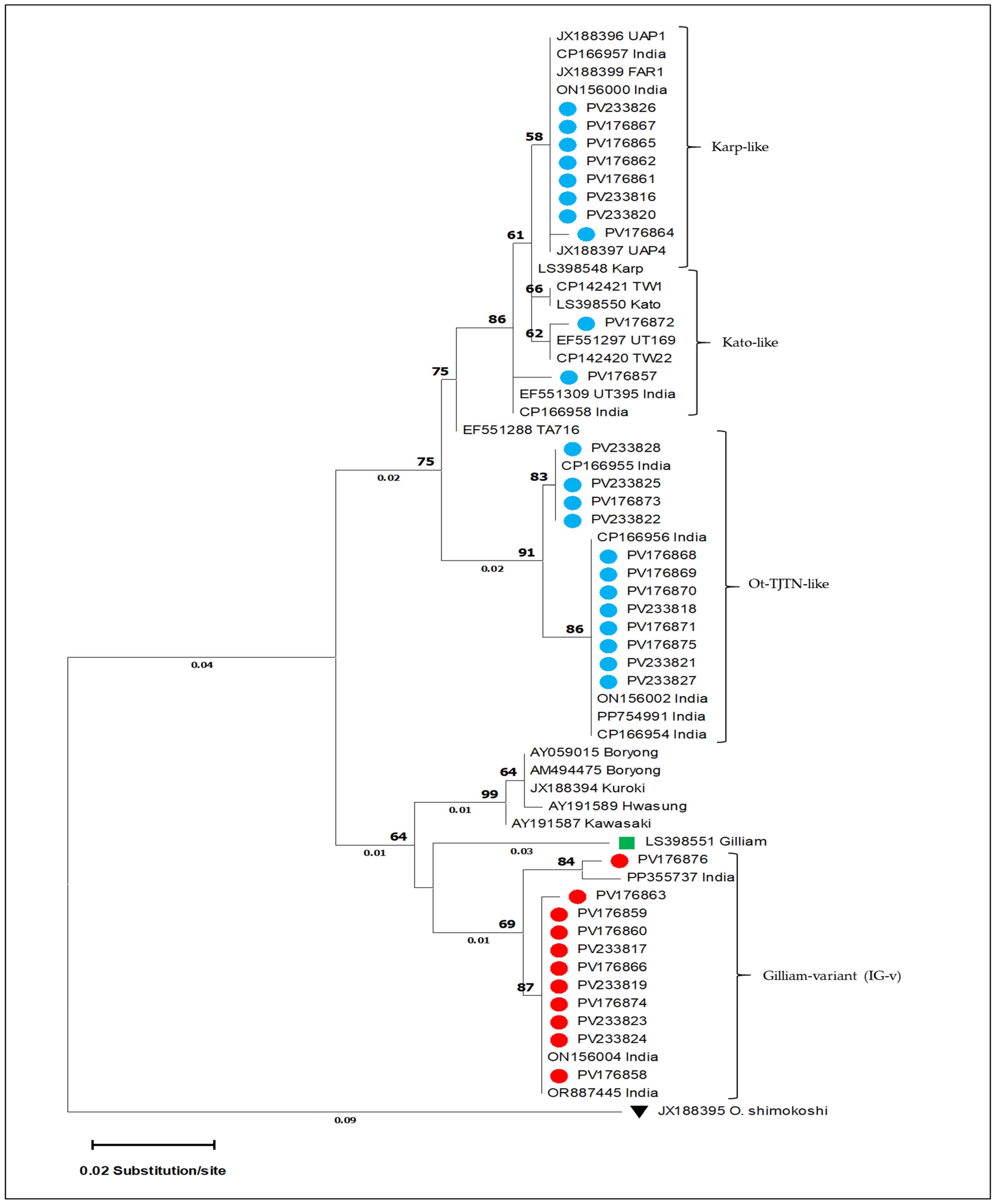
3.1. Molecular Detection and GroEL Gene Sequence Analysis
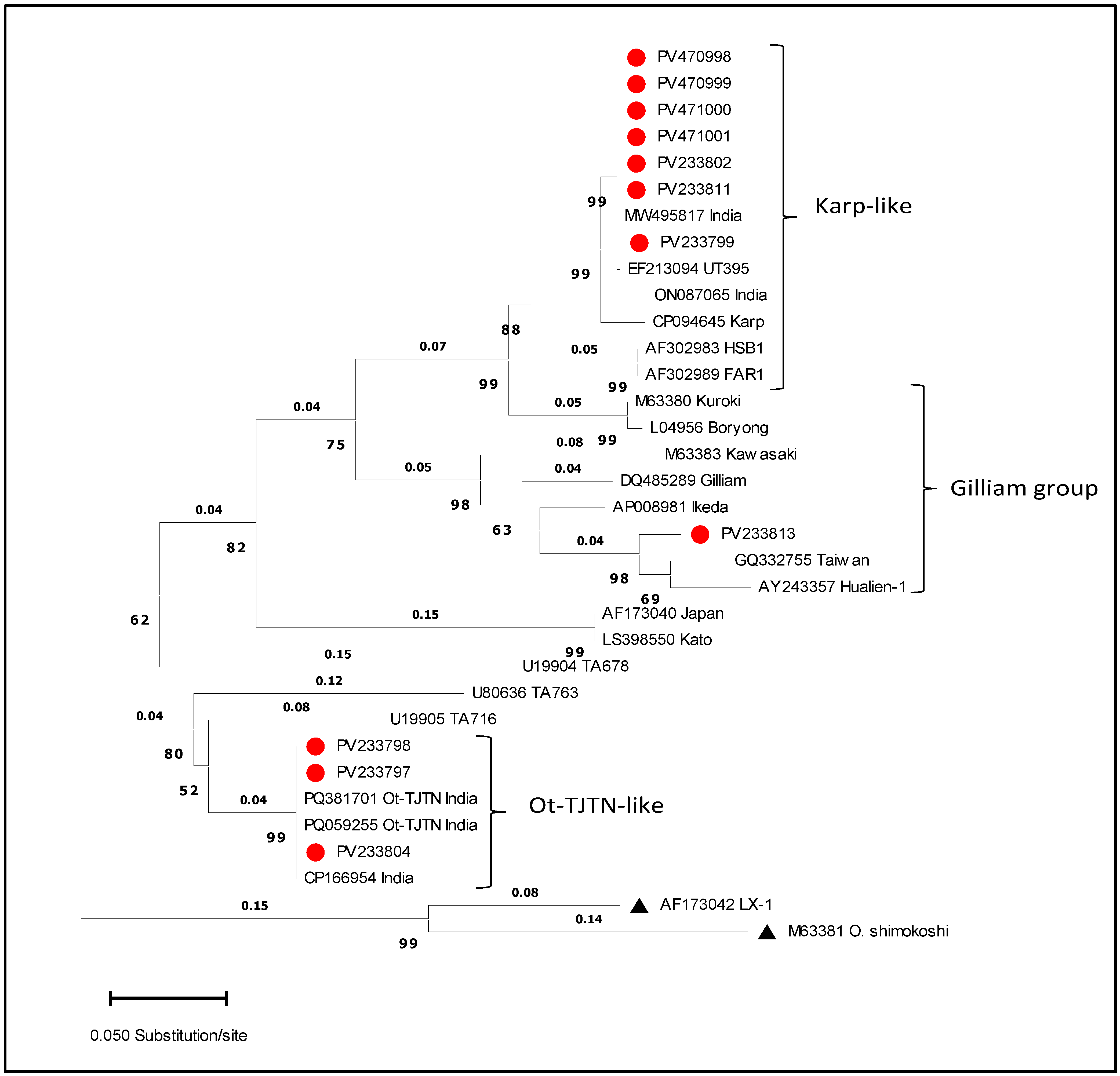
3.2. Sequence Analysis of the 56-kDa Gene
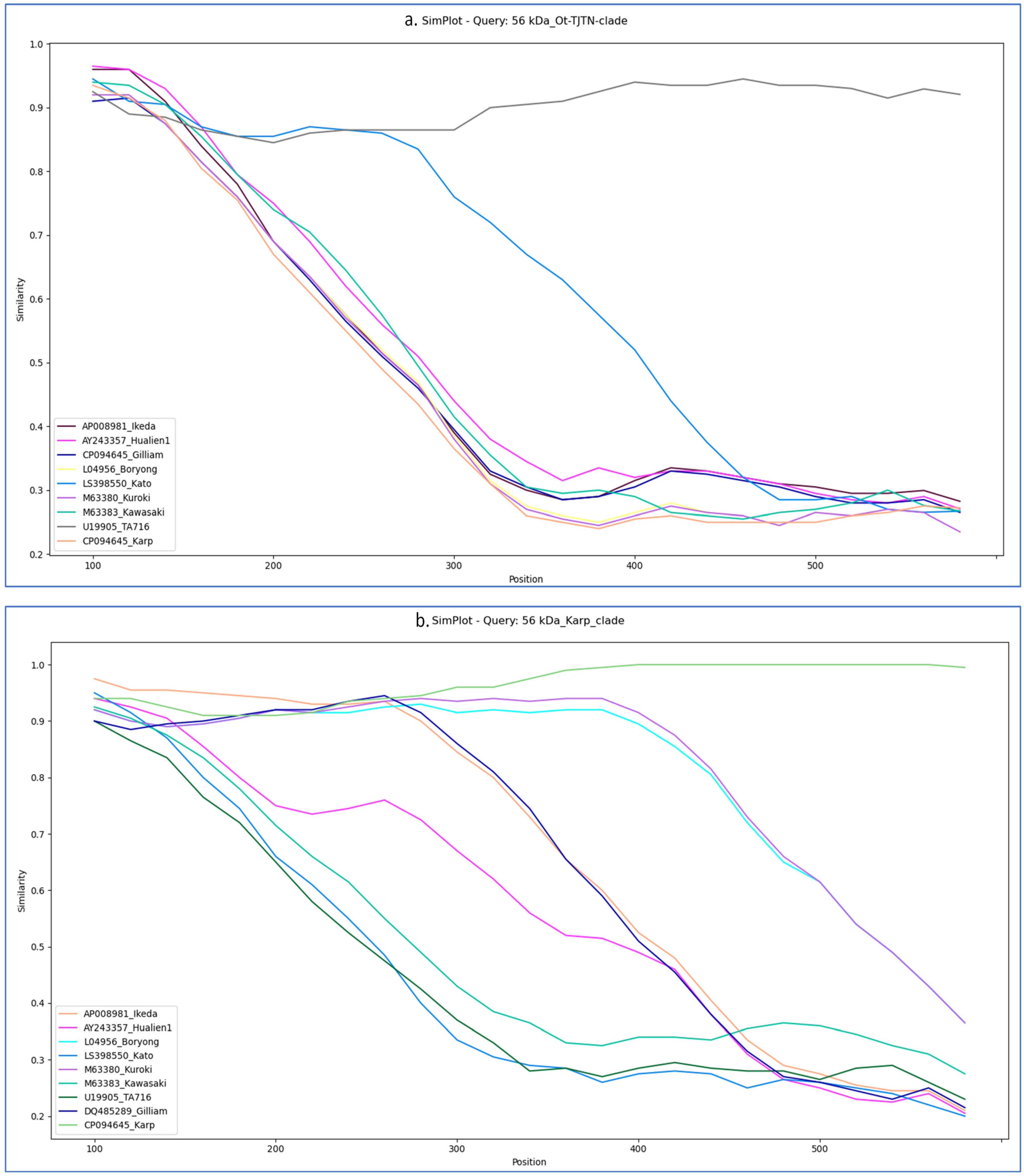
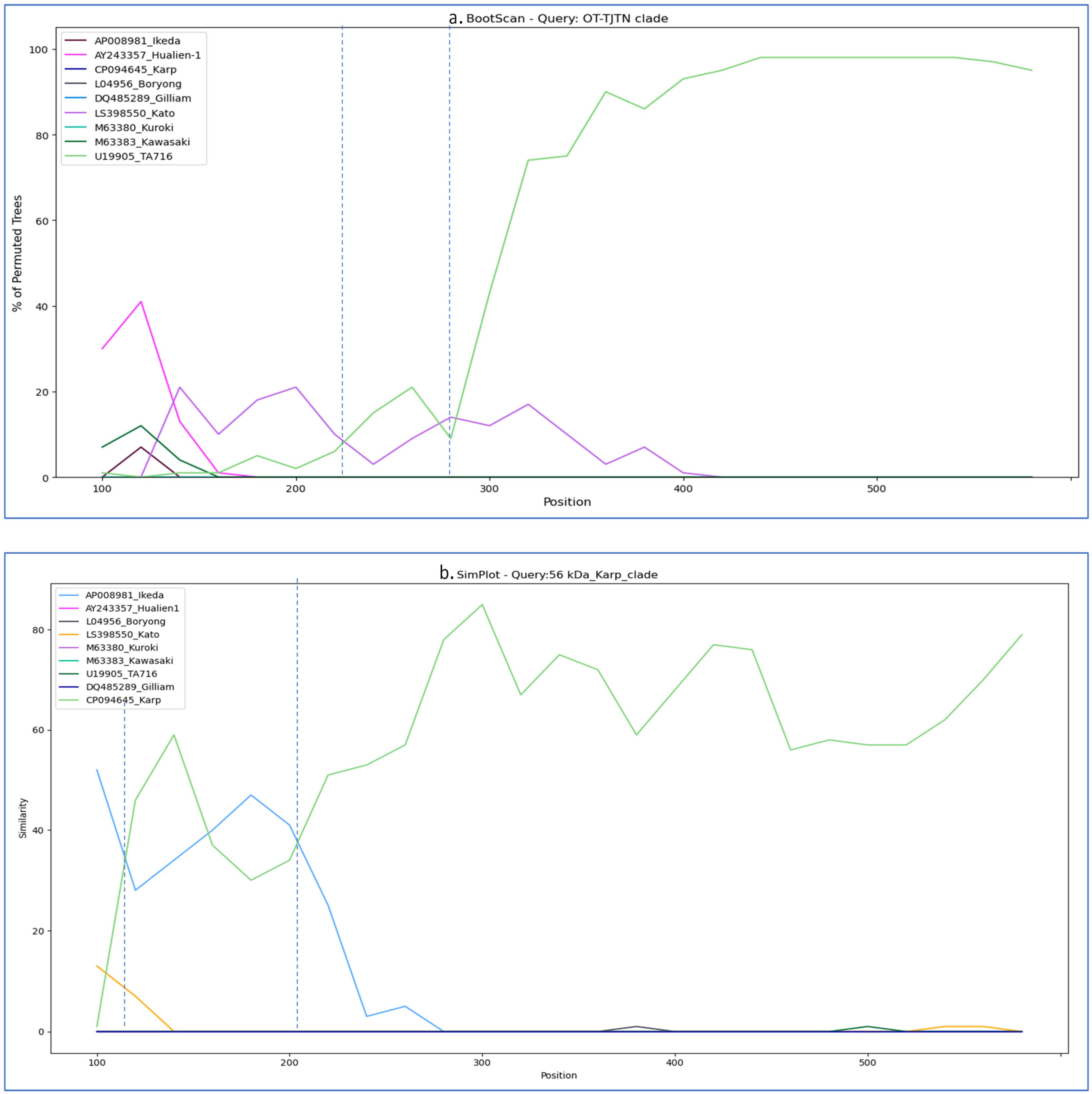
3.3. Similarity Plot and Recombination Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A Review of the Global Epidemiology of Scrub Typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef] [PubMed]
- Lerdthusnee, K.; Khlaimanee, N.; Monkanna, T.; Sangjun, N.; Mungviriya, S.; Linthicum, K.J.; Frances, S.P.; Kollars, T.M.; Coleman, R.E. Efficiency of Leptotrombidium Chiggers (Acari: Trombiculidae) at Transmitting Orientia tsutsugamushi to Laboratory Mice. J. Med. Entomol. 2002, 39, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Varghese, G.M.; Trowbridge, P.; Janardhanan, J.; Thomas, K.; Peter, J.V.; Mathews, P.; Abraham, O.C.; Kavitha, M.L. Clinical Profile and Improving Mortality Trend of Scrub Typhus in South India. Int. J. Infect. Dis. 2014, 23, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Sonthayanon, P.; Peacock, S.J.; Chierakul, W.; Wuthiekanun, V.; Blacksell, S.D.; Holden, M.T.G.; Bentley, S.D.; Feil, E.J.; Day, N.P.J. High Rates of Homologous Recombination in the Mite Endosymbiont and Opportunistic Human Pathogen Orientia tsutsugamushi. PLoS Negl. Trop. Dis. 2010, 4, e752. [Google Scholar] [CrossRef]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.J.; et al. Isolation of a Novel Orientia Species (O. Chuto Sp. Nov.) from a Patient Infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef]
- Paris, D.H.; Shelite, T.R.; Day, N.P.; Walker, D.H. Unresolved Problems Related to Scrub Typhus: A Seriously Neglected Life-Threatening Disease. Am. Soc. Trop. Med. Hyg. 2013, 89, 301–307. [Google Scholar] [CrossRef]
- Valbuena, G.; Walker, D.H. Approaches to Vaccines against Orientia tsutsugamushi. Front. Cell. Infect. Microbiol. 2013, 2, 170. [Google Scholar] [CrossRef]
- Kelly, D.J.; Fuerst, P.A.; Ching, W.; Richards, A.L. Scrub Typhus: The Geographic Distribution of Phenotypic and Genotypic Variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009, 48, S203–S230. [Google Scholar] [CrossRef]
- Varghese, G.M.; Janardhanan, J.; Mahajan, S.K.; Tariang, D.; Trowbridge, P.; Prakash, J.A.J.; David, T.; Sathendra, S.; Abraham, O.C. Molecular Epidemiology and Genetic Diversity of Orientia tsutsugamushi from Patients with Scrub Typhus in 3 Regions of India. Emerg. Infect. Dis. 2015, 21, 64–69. [Google Scholar] [CrossRef]
- Devamani, C.S.; Prakash, J.A.J.; Alexander, N.; Stenos, J.; Schmidt, W.-P. The Incidence of Orientia tsutsugamushi Infection in Rural South India. Epidemiol. Infect. 2022, 150, e132. [Google Scholar] [CrossRef]
- Khan, S.A.; Bora, T.; Laskar, B.; Khan, A.M.; Dutta, P. Scrub Typhus Leading to Acute Encephalitis Syndrome, Assam, India. Emerg. Infect. Dis. 2017, 23, 148–150. [Google Scholar] [CrossRef]
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crump, J.A.; Paris, D.H. Estimating the Burden of Scrub Typhus: A Systematic Review. PLoS Negl. Trop. Dis. 2017, 11, e0005838. [Google Scholar] [CrossRef]
- Nallan, K.; Kalidoss, B.C.; Jacob, E.S.; Mahadevan, S.K.; Joseph, S.; Ramalingam, R.; Renu, G.; Thirupathi, B.; Ramasamy, B.; Gupta, B.; et al. A Novel Genotype of Orientia tsutsugamushi in Human Cases of Scrub Typhus from Southeastern India. Microorganisms 2025, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Ruang-areerate, T.; Jeamwattanalert, P.; Rodkvamtook, W.; Richards, A.L.; Sunyakumthorn, P.; Gaywee, J. Genotype Diversity and Distribution of Orientia tsutsugamushi Causing Scrub Typhus in Thailand. J. Clin. Microbiol. 2011, 49, 2584–2589. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Tabara, K.; Yamamoto, N.; Fujita, H.; Itagaki, A.; Kon, M.; Satoh, H.; Araki, K.; Tanaka-Taya, K.; Takada, N.; et al. Molecular Phylogenetic Analysis of Orientia tsutsugamushi Based on the GroES and GroEL Genes. Vector-Borne Zoonotic Dis. 2013, 13, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, A.; Koralur, M.C.; Dasch, G.A. Complexity of Type-Specific 56-kDa Antigen CD4 T-Cell Epitopes of Orientia tsutsugamushi Strains Causing Scrub Typhus in India. PLoS ONE 2018, 13, e0196240. [Google Scholar] [CrossRef]
- Government of Tamil Nadu. Trend of Scrub Typhus in Tamil Nadu—2021–2023 (Based on IHIP Data); Directorate of Public Health and Preventive Medicine: Chennai, India, 2024. [Google Scholar]
- Tilak, R.; Anand, V.; Gupte, M.D.; Devarakonda, R.; Yadav, R.S. Re-Emergence of Scrub Typhus as a Public Health Problem in India: Its Spatial and Temporal Distribution Based on Analysis of 15-Year Data of the National Integrated Disease Surveillance Programme. J. Commun. Dis. 2024, 56, 2. [Google Scholar] [CrossRef]
- The New Indian Express. Dindigul Reports Five Scrub Typhus Cases; Health Officials Assure Situation Under Control. 25 December 2024. Available online: https://www.newindianexpress.com/states/tamil-nadu/2024/Dec/25/dindigul-reports-five-scrub-typhus-cases-health-officials-assure-situation-under-control (accessed on 15 April 2025).
- Nallan, K.; Rajan, G.; Sivathanu, L.; Devaraju, P.; Thiruppathi, B.; Kumar, A.; Rajaiah, P. Molecular Detection of Multiple Genotypes of Orientia tsutsugamushi Causing Scrub Typhus in Febrile Patients from Theni District, South India. Trop. Med. Infect. Dis. 2023, 8, 174. [Google Scholar] [CrossRef]
- Li, W.; Dou, X.; Zhang, L.; Lyu, Y.; Du, Z.; Tian, L.; Zhang, X.; Sun, Y.; Guan, Z.; Chen, L.; et al. Laboratory Diagnosis and Genotype Identification of Scrub Typhus from Pinggu District, Beijing, 2008 and 2010. Am. J. Trop. Med. Hyg. 2013, 89, 123–129. [Google Scholar] [CrossRef]
- Chromas; Version 2.6.6; Technelysium Pty Ltd.: South Brisbane, QLD, Australia, 2018; Available online: https://technelysium.com.au/wp/chromas/ (accessed on 15 April 2025).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Samson, S.; Lord, É.; Makarenkov, V. SimPlot++: A Python Application for Representing Sequence Similarity and Detecting Recombination. Bioinformatics 2022, 38, 3118–3120. [Google Scholar] [CrossRef] [PubMed]
- Phetsouvanh, R.; Sonthayanon, P.; Pukrittayakamee, S.; Paris, D.H.; Newton, P.N.; Feil, E.J.; Day, N.P.J. The Diversity and Geographical Structure of Orientia tsutsugamushi Strains from Scrub Typhus Patients in Laos. PLoS Negl. Trop. Dis. 2015, 9, e0004024. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.; Blassdell, K.; May, T.T.X.; Sreyrath, L.; Gavotte, L.; Morand, S.; Frutos, R.; Buchy, P. Diversity of Orientia tsutsugamushi Clinical Isolates in Cambodia Reveals Active Selection and Recombination Process. Infect. Genet. Evol. 2013, 15, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.R.; Tsai, H.P.; Tsui, P.Y.; Weng, M.H.; Kuo, M.D.; Lin, H.C.; Chen, K.C.; Ji, D.D.; Chu, D.M.; Liu, W.T. Genetic Typing, Based on the 56-Kilodalton Type-Specific Antigen Gene, of Orientia tsutsugamushi Strains Isolated from Chiggers Collected from Wild-Caught Rodents in Taiwan. Appl. Environ. Microbiol. 2011, 77, 3398–3405. [Google Scholar] [CrossRef]
- Kelly, D.J.; Fuerst, P.A.; Richards, A.L. Origins, Importance and Genetic Stability of the Prototype Strains Gilliam, Karp and Kato of Orientia tsutsugamushi. Trop. Med. Infect. Dis. 2019, 4, 75. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.R. Determinants of the Rate of Protein Sequence Evolution. Nat. Rev. Genet. 2015, 16, 409–420. [Google Scholar] [CrossRef]
- Paris, D.H.; Aukkanit, N.; Jenjaroen, K.; Blacksell, S.D.; Day, N.P.J. A Highly Sensitive Quantitative Real-Time PCR Assay Based on the GroEL Gene of Contemporary Thai Strains of Orientia tsutsugamushi. Clin. Microbiol. Infect. 2009, 15, 488–495. [Google Scholar] [CrossRef]
- Ohashi, N.; Nashimoto, H.; Ikeda, H.; Tamura, A. Diversity of Immunodominant 56-KDa Type-Specific Antigen (TSA) of Rickettsia tsutsugamushi. Sequence and Comparative Analyses of the Genes Encoding TSA Homologues from Four Antigenic Variants. J. Biol. Chem. 1992, 267, 12728–12735. [Google Scholar] [CrossRef]
- Tshokey, T.; Stenos, J.; Tadepalli, M.; Nguyen, C.; Graves, S.R. Genetic Characterization of Orientia tsutsugamushi, Bhutan, 2015. Emerg. Infect. Dis. 2025, 31, 1820–1823. [Google Scholar] [CrossRef]
- Yang, H.-H.; Huang, I.-T.; Lin, C.-H.; Chen, T.-Y.; Chen, L.-K. New Genotypes of Orientia tsutsugamushi Isolated from Humans in Eastern Taiwan. PLoS ONE 2012, 7, e46997. [Google Scholar] [CrossRef]
- Chunduru, K.; A R, M.; Poornima, S.; Hande, H.M.; Devaki, R.; Varghese, G.M.; Saravu, K. Clinical, Laboratory Profile and Molecular Characterization of Orientia tsutsugamushi among Fatal Scrub Typhus Patients from Karnataka, India. Infect. Dis. 2024, 56, 220–229. [Google Scholar] [CrossRef]
- Takhampunya, R.; Korkusol, A.; Promsathaporn, S.; Tippayachai, B.; Leepitakrat, S.; Richards, A.L.; Davidson, S.A. Heterogeneity of Orientia tsutsugamushi Genotypes in Field-Collected Trombiculid Mites from Wild-Caught Small Mammals in Thailand. PLoS Negl. Trop. Dis. 2018, 12, e0006632. [Google Scholar] [CrossRef]
- Frances, S.P.; Watcharapichat, P.; Phulsuksombati, D.; Tanskul, P. Transmission of Orientia tsutsugamushi, the Aetiological Agent for Scrub Typhus, to Co-Feeding Mites. Parasitology 2000, 120, 601–607. [Google Scholar] [CrossRef]
- Govindarajan, R.; Rajamannar, V.; Krishnamoorthi, R.; Kumar, A.; Samuel, P.P. Distribution Pattern of Chigger Mites in South Tamil Nadu, India. Entomon 2021, 46, 247–254. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, C.K.; Kwak, Y.G.; Moon, C.; Kim, B.-N.; Kim, E.S.; Kang, J.M.; Lee, C.-S. Antigenic Drift of Orientia tsutsugamushi in South Korea as Identified by the Sequence Analysis of a 56-KDa Protein-Encoding Gene. Am. Soc. Trop. Med. Hyg. 2010, 83, 930–935. [Google Scholar] [CrossRef]
- Kim, G.; Ha, N.-Y.; Min, C.-K.; Kim, H.-I.; Yen, N.T.H.; Lee, K.-H.; Oh, I.; Kang, J.-S.; Choi, M.-S.; Kim, I.-S.; et al. Diversification of Orientia tsutsugamushi Genotypes by Intragenic Recombination and Their Potential Expansion in Endemic Areas. PLoS Negl. Trop. Dis. 2017, 11, e0005408. [Google Scholar] [CrossRef]
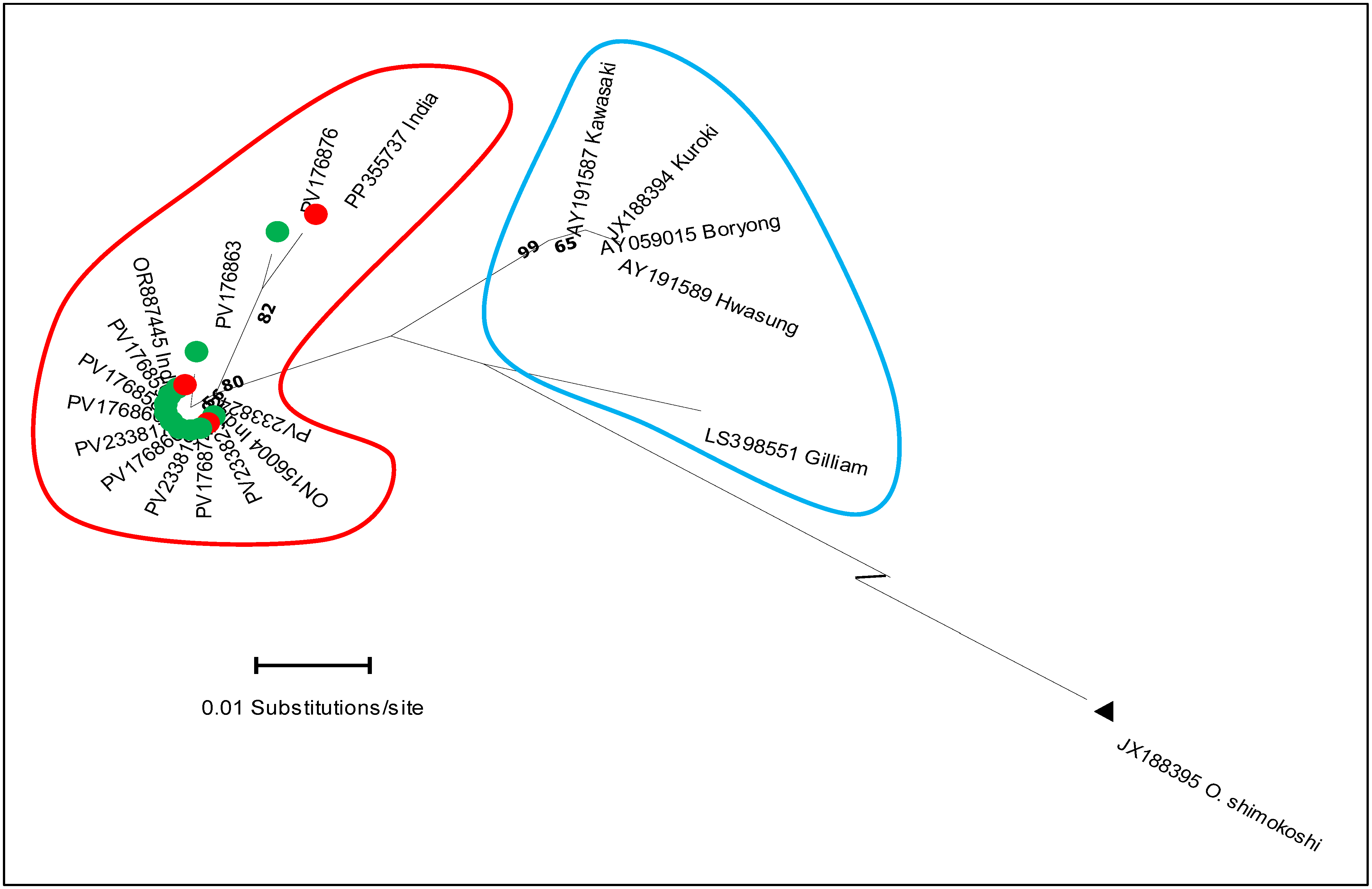
| Genotype | Gilliam Variant (IG-v) | Hwasung | Kawasaki | Gilliam | Kuroki | Boryong |
|---|---|---|---|---|---|---|
| Gilliam variant (IG-v) | — | |||||
| Hwasung | 0.039 | — | ||||
| Kawasaki | 0.032 | 0.006 | — | |||
| Gilliam | 0.039 | 0.047 | 0.041 | — | ||
| Kuroki | 0.035 | 0.003 | 0.003 | 0.044 | — | |
| Boryong | 0.035 | 0.003 | 0.003 | 0.044 | 0.001 | — |
| S. No. | GroEL | 56-kDa | ||
|---|---|---|---|---|
| Accession Number | Genotype | Accession Number | Genotype | |
| 1. | PV176860 | Gilliam-variant | PV233799 | Karp-like |
| 2. | PV176863 | Gilliam-variant | PV233802 | Karp-like |
| 3. | PV233817 | Gilliam-variant | PV470998 | Karp-like |
| 4. | PV233819 | Gilliam-variant | PV470999 | Karp-like |
| 5. | PV176874 | Gilliam-variant | PV233811 | Karp-like |
| 6. | PV233823 | Gilliam-variant | PV471000 | Karp-like |
| 7. | PV233824 | Gilliam-variant | PV471001 | Karp-like |
| 8. | PV176858 | Gilliam-variant | PV233797 | Ot-TJTN |
| 9. | PV176859 | Gilliam-variant | PV233798 | Ot-TJTN |
| 10. | PV176866 | Gilliam-variant | PV233804 | Ot-TJTN |
| 11. | PV176876 | Gilliam-variant | PV233813 | Gilliam |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, S.V.; Nallan, K.; Rajan, G.; Murugesan, A.; Govindarajan, R.; Sivadoss, R.; Ramalingam, R.; Madhumitha, R.K.; Ganesan, S.T.; Jayakumar, S.K.; et al. A New Gilliam Genotypic Variant of Orientia tsutsugamushi in Human Scrub Typhus Cases from South India. Microorganisms 2025, 13, 2670. https://doi.org/10.3390/microorganisms13122670
Joseph SV, Nallan K, Rajan G, Murugesan A, Govindarajan R, Sivadoss R, Ramalingam R, Madhumitha RK, Ganesan ST, Jayakumar SK, et al. A New Gilliam Genotypic Variant of Orientia tsutsugamushi in Human Scrub Typhus Cases from South India. Microorganisms. 2025; 13(12):2670. https://doi.org/10.3390/microorganisms13122670
Chicago/Turabian StyleJoseph, Steny Vallomkottu, Krishnamoorthy Nallan, Gopinathan Rajan, Amudhan Murugesan, Renu Govindarajan, Raju Sivadoss, Ramkumar Ramalingam, Rajarathinam Kannan Madhumitha, Sucila Thangam Ganesan, Suria Kumar Jayakumar, and et al. 2025. "A New Gilliam Genotypic Variant of Orientia tsutsugamushi in Human Scrub Typhus Cases from South India" Microorganisms 13, no. 12: 2670. https://doi.org/10.3390/microorganisms13122670
APA StyleJoseph, S. V., Nallan, K., Rajan, G., Murugesan, A., Govindarajan, R., Sivadoss, R., Ramalingam, R., Madhumitha, R. K., Ganesan, S. T., Jayakumar, S. K., Rahi, M., & Rajaiah, P. (2025). A New Gilliam Genotypic Variant of Orientia tsutsugamushi in Human Scrub Typhus Cases from South India. Microorganisms, 13(12), 2670. https://doi.org/10.3390/microorganisms13122670







