Identification and Characterization of Three Novel B-Cell Epitopes in African Swine Fever Virus p22 Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Serum and Reagents
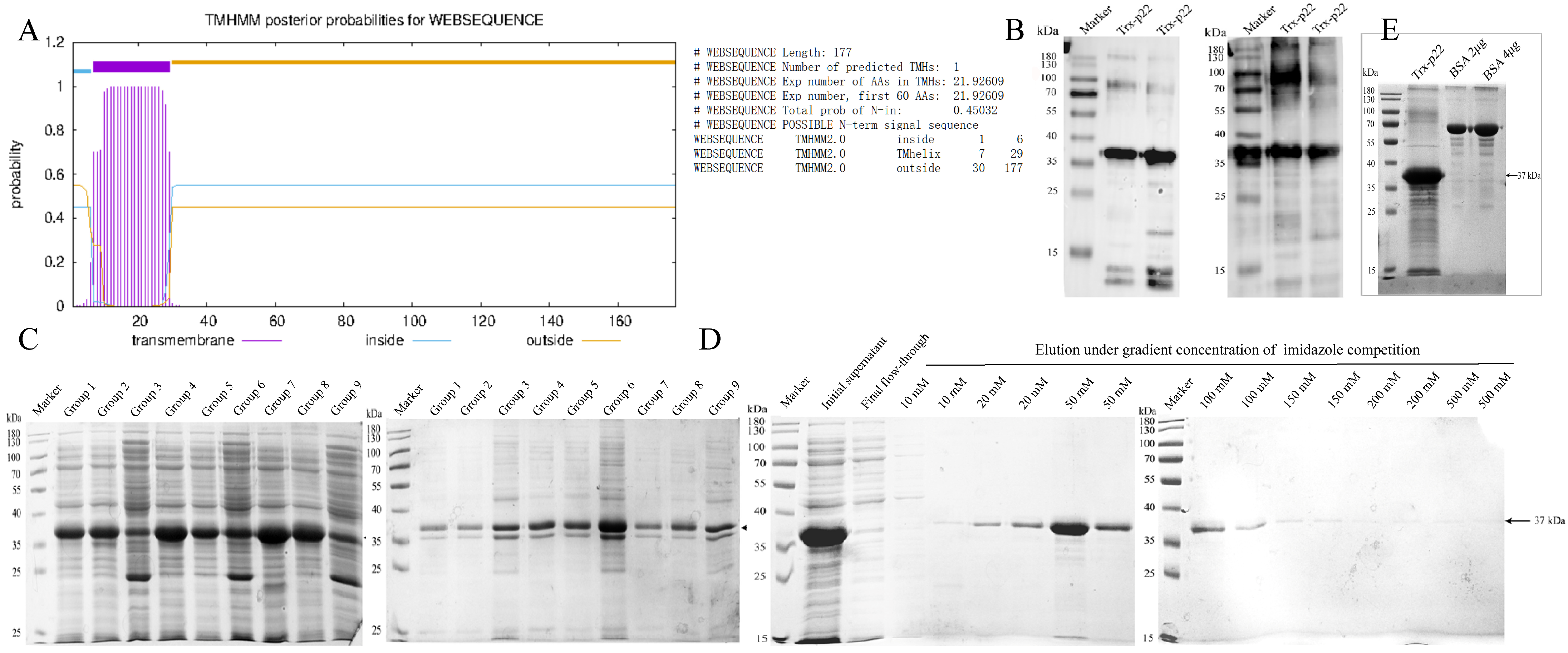
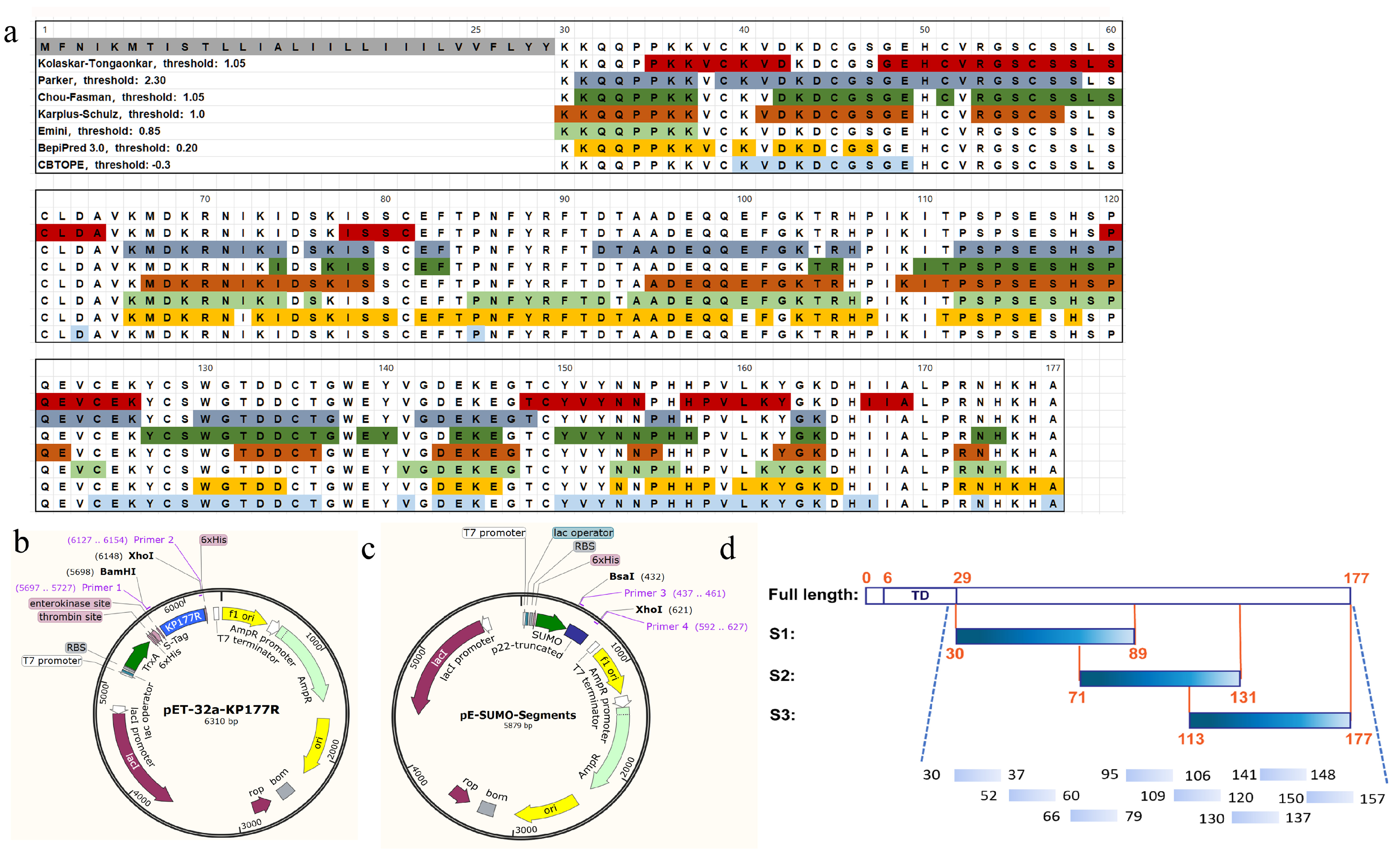
2.2. Bioinformatical Analysis
2.3. Preparation of Recombinant p22 Protein
2.4. Western Blot
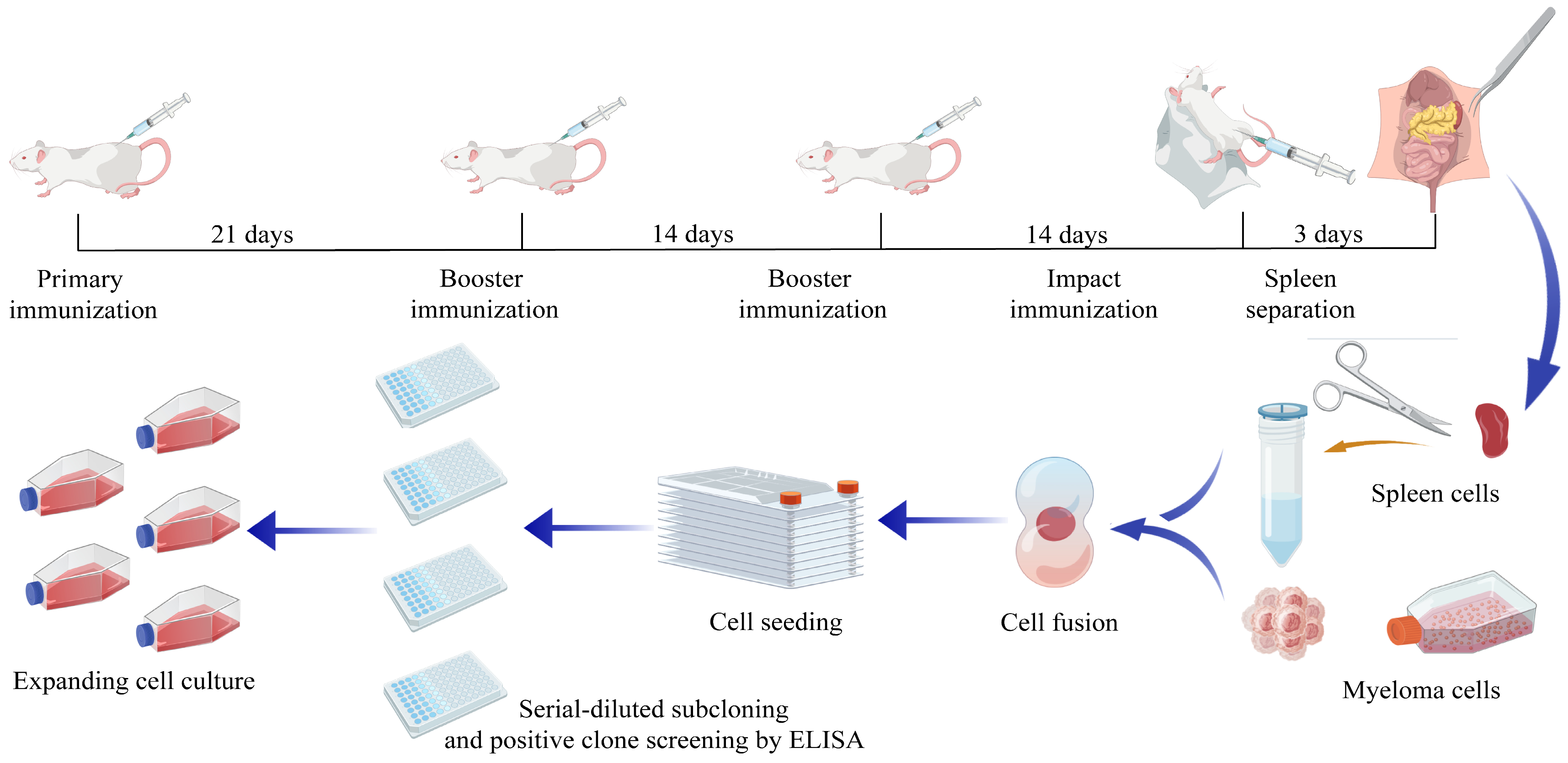
2.5. Preparation of p22-Specific Monoclonal Antibodies
2.6. Indirect ELISA
2.7. Immunofluorescence Assay
2.8. Construction and Expression of Truncated p22 Segments
2.9. Dot-Blot
2.10. Peptide Design and Synthesis
2.11. Statistical Analysis
3. Results
3.1. Expression, Identification and Purification of Recombinant p22 Protein
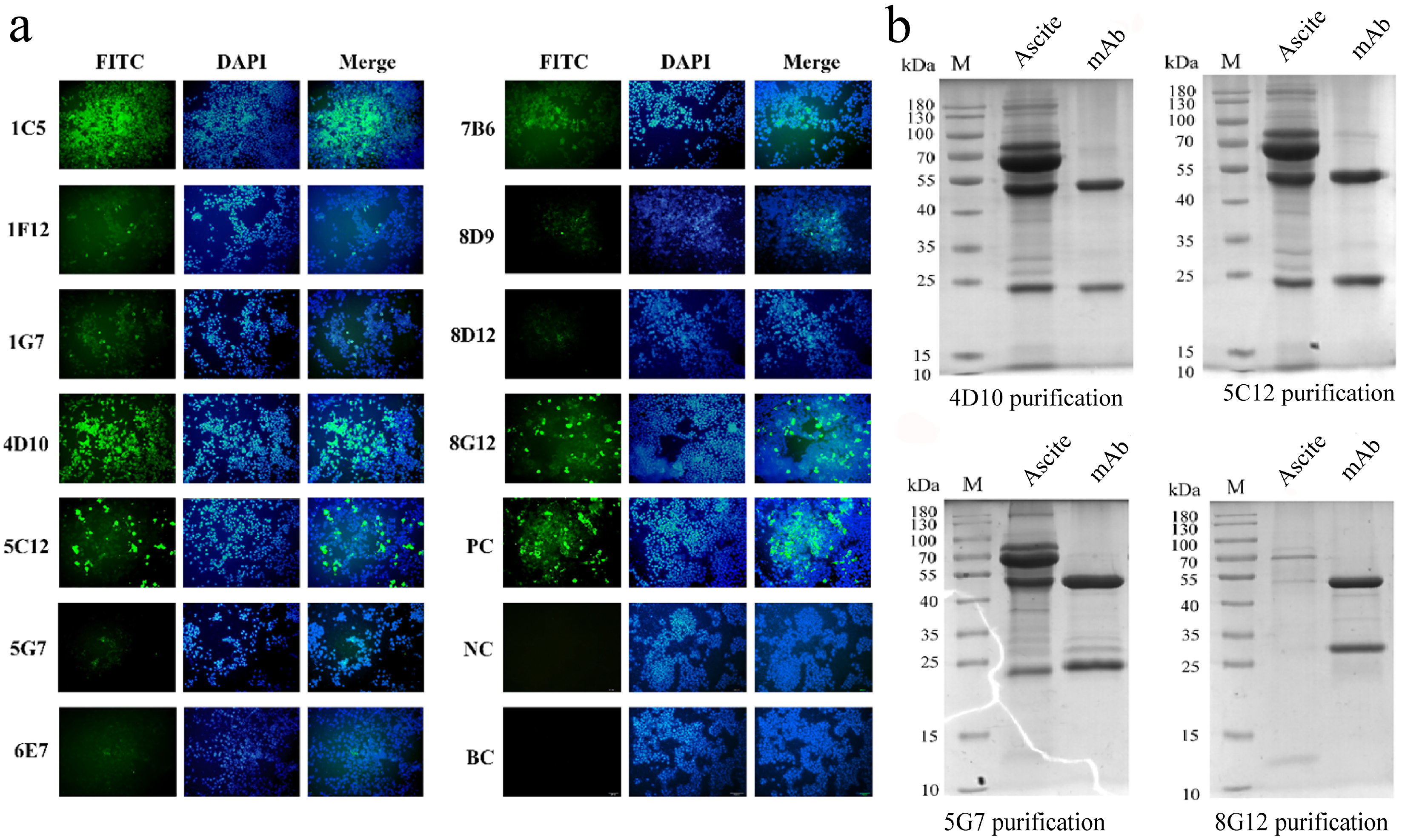
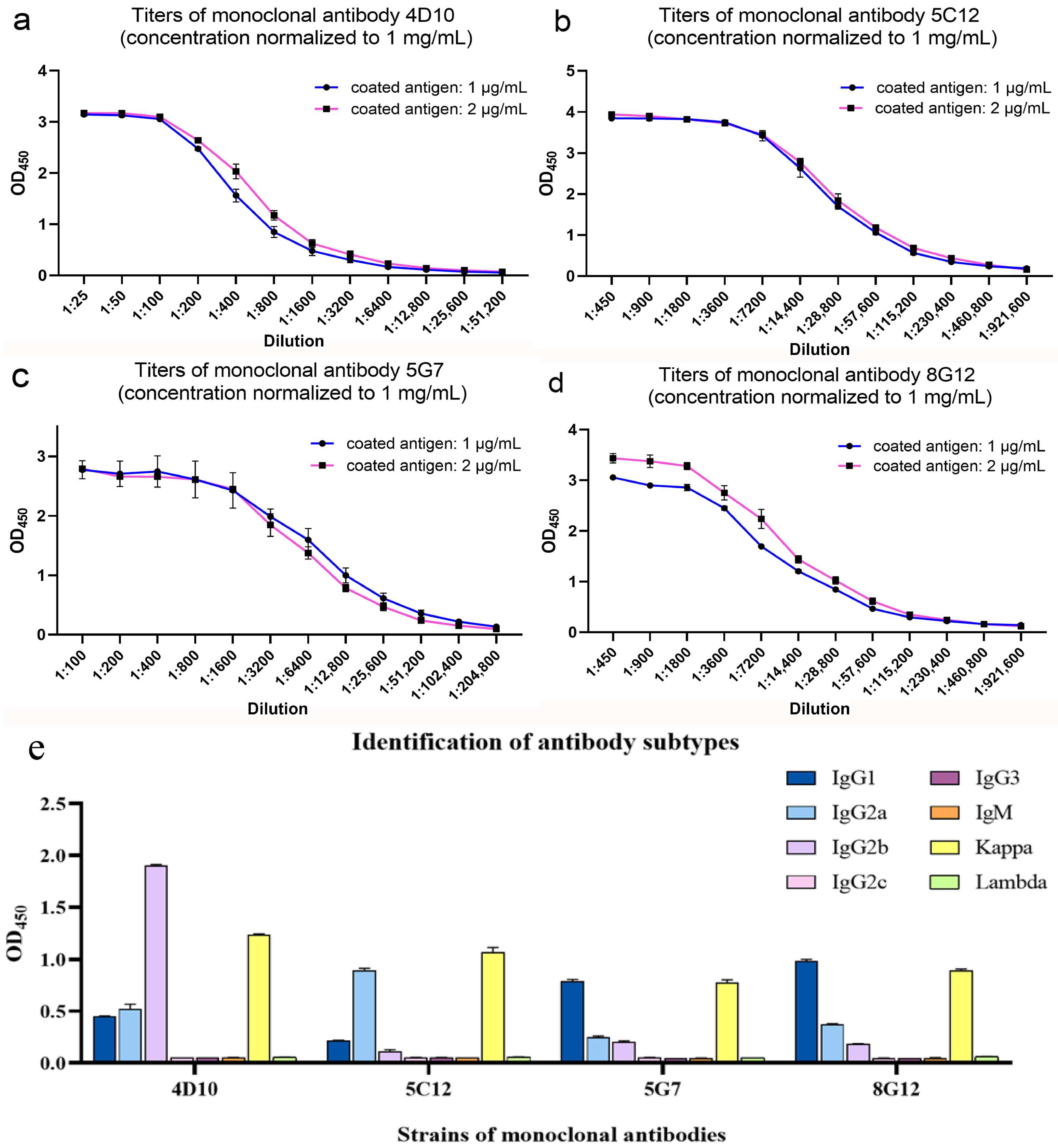
3.2. Preparation and Characterization of p22-Specific Monoclonal Antibodies
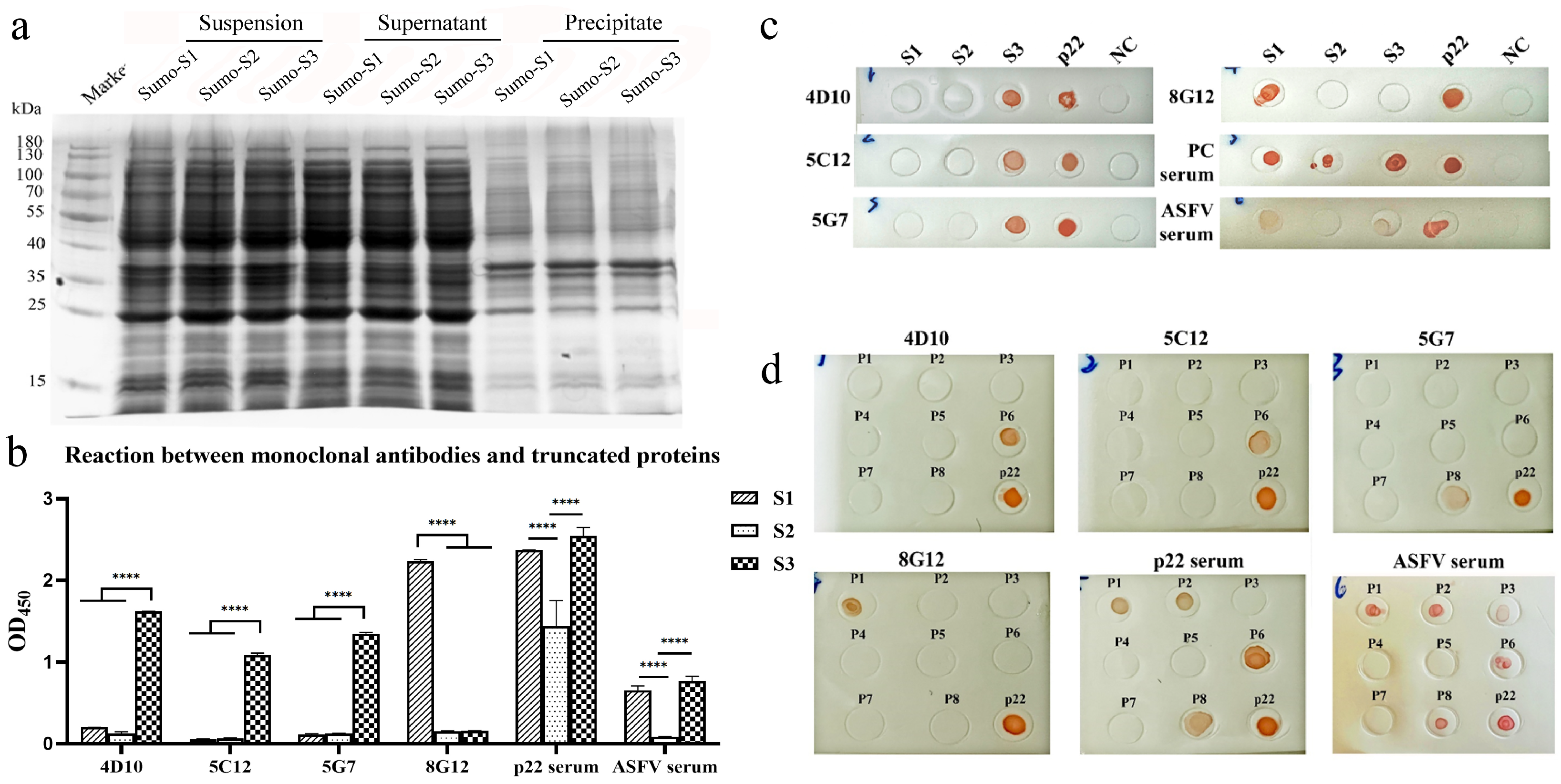
3.3. Construction and Expression of Truncated p22 Protein
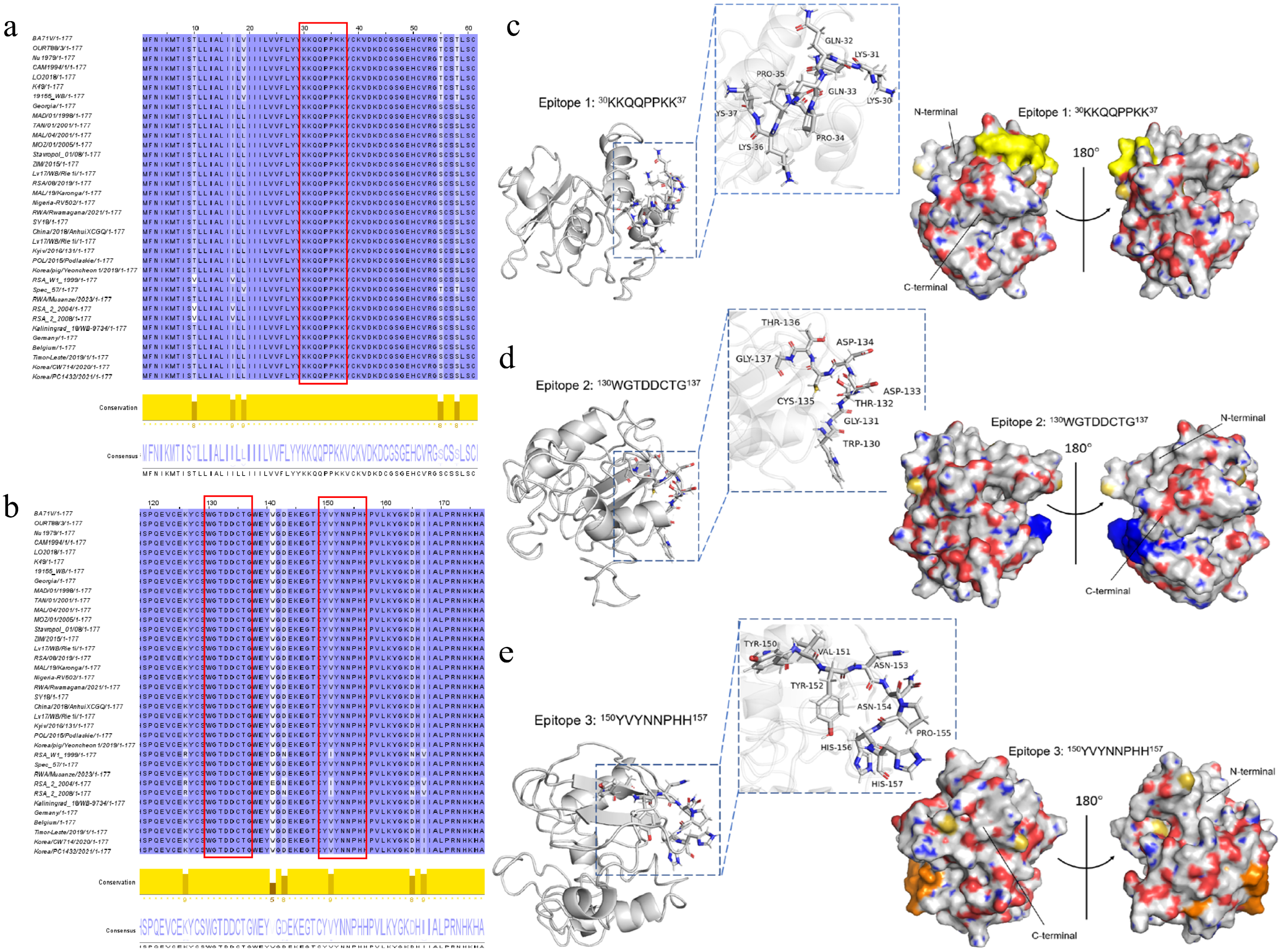
3.4. Mapping of Linear B-Cell Epitopes on p22 Protein
3.5. Conservation Analysis and Presentation of Linear B-Cell Epitopes on p22 Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| DIVA | differentiation of infected from vaccinated animals |
| DMSO | dimethylsulfoxide |
| eECL | enhanced Electrochemiluminescent |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HRP | horseradish Peroxidase |
| IFA | Immunofluorescence Assay |
| IPTG | isopropyl β-D-thiogalactoside |
| PEG | polyethylene Glycol |
| PVDF | polyvinylidene fluoride |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SMCC | N-succinimidyl 4-(maleimidome)cyclo-hexanecarboxylate |
References
- African Swine Fever. Available online: https://www.woah.org/en/disease/african-swine-fever/ (accessed on 20 August 2024).
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef]
- Chenais, E.; Stahl, K.; Guberti, V.; Depner, K. Identification of Wild Boar-Habitat Epidemiologic Cycle in African Swine Fever Epizootic. Emerg. Infect. Dis. 2018, 24, 810–812. [Google Scholar] [CrossRef] [PubMed]
- The Terrestrial Animal Health Code. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_oie_listed_disease.htm (accessed on 20 August 2024).
- Karalyan, Z.; Zakaryan, H.; Arzumanyan, H.; Sargsyan, K.; Voskanyan, H.; Hakobyan, L.; Abroyan, L.; Avetisyan, A.; Karalova, E. Pathology of porcine peripheral white blood cells during infection with African swine fever virus. BMC Vet. Res. 2012, 8, 18. [Google Scholar] [CrossRef]
- Salguero, F.J. Comparative Pathology and Pathogenesis of African Swine Fever Infection in Swine. Front. Vet. Sci. 2020, 7, 282. [Google Scholar] [CrossRef]
- Gomez-Villamandos, J.C.; Carrasco, L.; Bautista, M.J.; Sierra, M.A.; Quezada, M.; Hervas, J.; Chacon Mde, L.; Ruiz-Villamor, E.; Salguero, F.J.; Sonchez-Cordon, P.J.; et al. African swine fever and classical swine fever: A review of the pathogenesis. Dtsch. Tierarztl. Wochenschr. 2003, 110, 165–169. [Google Scholar]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef]
- Sanchez-Vizcaino, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W.; Jori, F.; Bastos, A.D. African swine fever virus eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African swine fever virus isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef]
- Cadenas-Fernandez, E.; Ito, S.; Aguilar-Vega, C.; Sanchez-Vizcaino, J.M.; Bosch, J. The Role of the Wild Boar Spreading African Swine Fever Virus in Asia: Another Underestimated Problem. Front. Vet. Sci. 2022, 9, e844209. [Google Scholar] [CrossRef] [PubMed]
- Vuono, E.A.; Ramirez-Medina, E.; Pruitt, S.; Rai, A.; Espinoza, N.; Velazquez-Salinas, L.; Gladue, D.P.; Borca, M.V. Evaluation of the Function of the ASFV KP177R Gene, Encoding for Structural Protein p22, in the Process of Virus Replication and in Swine Virulence. Viruses 2021, 13, 986. [Google Scholar] [CrossRef]
- Camacho, A.; Vinuela, E. Protein P22 of African Swine Fever Virus-an Early Structural Protein That Is Incorporated into the Membrane of Infected-Cells. Virology 1991, 181, 251–257. [Google Scholar] [CrossRef]
- Andrés, G. African Swine Fever Virus Gets Undressed: New Insights on the Entry Pathway. J. Virol. 2017, 91, e01906. [Google Scholar] [CrossRef]
- Hernaez, B.; Diaz-Gil, G.; Garcia-Gallo, M.; Ignacio Quetglas, J.; Rodriguez-Crespo, I.; Dixon, L.; Escribano, J.M.; Alonso, C. The African swine fever virus dynein-binding protein p54 induces infected cell apoptosis. FEBS Lett. 2004, 569, 224–228. [Google Scholar] [CrossRef]
- Hernáez, B.; Guerra, M.; Salas, M.L.; Andrés, G. African Swine Fever Virus Undergoes Outer Envelope Disruption, Capsid Disassembly and Inner Envelope Fusion before Core Release from Multivesicular Endosomes. PLoS Pathog. 2016, 12, e1005595. [Google Scholar] [CrossRef]
- Matamoros, T.; Alejo, A.; Rodríguez, J.M.; Hernáez, B.; Guerra, M.; Fraile-Ramos, A.; Andrés, G. African Swine Fever Virus Protein pE199L Mediates Virus Entry by Enabling Membrane Fusion and Core Penetration. mBio 2020, 11, e00789-20. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Y.; Li, J.; Wang, A.; Meng, X.; Chen, L.; Wei, J.; Tong, W.; Kong, N.; Yu, L.; et al. Screening and identification of the dominant antigens of the African swine fever virus. Front. Vet. Sci. 2023, 10, 1175701. [Google Scholar] [CrossRef] [PubMed]
- Nah, J.J.; Kwon, O.K.; Choi, J.D.; Jang, S.H.; Lee, H.J.; Ahn, D.G.; Lee, K.; Kang, B.; Hae-Eun, K.; Shin, Y.K. Development of an indirect ELISA against African swine fever virus using two recombinant antigens, partial p22 and p30. J. Virol. Methods 2022, 309, 114611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiao, J.; Liu, N.; Ren, S.; Zeng, H.; Peng, J.; Zhang, Y.; Guo, L.; Liu, F.; Lv, T.; et al. Novel p22 and p30 dual-proteins combination based indirect ELISA for detecting antibodies against African swine fever virus. Front. Vet. Sci. 2023, 10, 1093440. [Google Scholar] [CrossRef]
- Tsegay, G.; Tesfagaber, W.; Zhu, Y.M.; He, X.J.; Wang, W.; Zhang, Z.J.; Sun, E.C.; Zhang, J.Y.; Guan, Y.T.; Li, F.; et al. Novel P22-monoclonal antibody based blocking ELISA for the detection of African swine fever virus antibodies in serum. Biosaf. Health 2022, 4, 234–243. [Google Scholar] [CrossRef]
- Zhang, G.; Li, N.; Chen, Y.; Zhou, J.; Liu, H.; Qi, Y.; Liu, Y.; Peng, Y.; Sun, H.; Wang, A. Identification of the B-cell epitopes on N protein of type 2 porcine reproductive and respiratory syndrome virus, using monoclonal antibodies. Int. J. Biol. Macromol. 2019, 130, 300–306. [Google Scholar] [CrossRef]
- Jiang, M.; Guo, J.; Zhang, G.; Jin, Q.; Liu, Y.; Jia, R.; Wang, A. Fine mapping of linear B cell epitopes on capsid protein of porcine circovirus 3. Appl. Microbiol. Biotechnol. 2020, 104, 6223–6234. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Y.; Guo, J.; Qi, S.; Li, D.; Zhang, Q.; Ma, J.; Chang, H.; Liu, Z.; Liu, X.; et al. Development and validation of a 3ABC indirect ELISA for differentiation of foot-and-mouth disease virus infected from vaccinated animals. Vet. Microbiol. 2007, 125, 157–169. [Google Scholar] [CrossRef]
- Shang, S.B.; Jin, Y.L.; Jiang, X.T.; Zhou, J.Y.; Zhang, X.; Xing, G.; He, J.L.; Yan, Y. Fine mapping of antigenic epitopes on capsid proteins of porcine circovirus, and antigenic phenotype of porcine circovirus type 2. Mol. Immunol. 2009, 46, 327–334. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.S.; Pan, F.G.; Zhang, Y.Y.; Lu, S.Y.; Ren, H.L.; Li, Z.H.; Liu, Z.S.; Zhang, J.H. Development of a new monoclonal antibody based direct competitive enzyme-linked immunosorbent assay for detection of brevetoxins in food samples. Food Chem. 2010, 118, 467–471. [Google Scholar] [CrossRef]
- Control Measures to Halt ASF Spread. Available online: https://www.woah.org/en/disease/african-swine-fever/#ui-id-3 (accessed on 20 August 2024).
- Danzetta, M.L.; Marenzoni, M.L.; Iannetti, S.; Tizzani, P.; Calistri, P.; Feliziani, F. African Swine Fever: Lessons to Learn From Past Eradication Experiences. A Systematic Review. Front. Vet. Sci. 2020, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fan, B.; Zhou, J.; Wang, D.; Fan, H.; Li, B. A High-Throughput Method to Analyze the Interaction Proteins With p22 Protein of African Swine Fever Virus In Vitro. Front. Vet. Sci. 2021, 8, 719859. [Google Scholar] [CrossRef] [PubMed]
- Spangfort, M.D.; Mirza, O.; Ipsen, H.; Van Neerven, R.J.; Gajhede, M.; Larsen, J.N. Dominating IgE-binding epitope of Bet v 1, the major allergen of birch pollen, characterized by X-ray crystallography and site-directed mutagenesis. J. Immunol. 2003, 171, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Beghetto, E.; Pucci, A.; Minenkova, O.; Spadoni, A.; Bruno, L.; Buffolano, W.; Soldati, D.; Felici, F.; Gargano, N. Identification of a human immunodominant B-cell epitope within the GRA1 antigen of by phage display of cDNA libraries. Int. J. Parasitol. 2001, 31, 1659–1668. [Google Scholar] [CrossRef]
- Basu, R.; Zhai, L.; Contreras, A.; Tumban, E. Immunization with phage virus-like particles displaying Zika virus potential B-cell epitopes neutralizes Zika virus infection of monkey kidney cells. Vaccine 2018, 36, 1256–1264. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization ofSARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Heimerman, M.E.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R. Linear epitopes in African swine fever virus p72 recognized by monoclonal antibodies prepared against baculovirus-expressed antigen. J. Vet. Diagn. Investig. 2018, 30, 406–412. [Google Scholar] [CrossRef]
- Duan, X.; Liu, Y.; Chen, Z.; Xie, Z.; Tian, C.; Li, Y.; Lv, L.; Wang, R.; Liu, J.; Chen, H. Identification of monoclonal antibody targeting epitope on p72 trimeric spike of African swine fever virus. Virus Genes 2023, 59, 582–590. [Google Scholar] [CrossRef]
- Petrovan, V.; Murgia, M.V.; Wu, P.; Lowe, A.D.; Jia, W.; Rowland, R.R.R. Epitope mapping of African swine fever virus (ASFV) structural protein, p54. Virus Res. 2020, 279, 197871. [Google Scholar] [CrossRef]
- Wu, P.; Lowe, A.D.; Rodriguez, Y.Y.; Murgia, M.V.; Dodd, K.A.; Rowland, R.R.; Jia, W. Antigenic regions of African swine fever virus phosphoprotein P30. Transbound. Emerg. Dis. 2020, 67, 1942–1953. [Google Scholar] [CrossRef]
- Zhou, J.; Ni, Y.; Wang, D.; Fan, B.; Zhu, X.; Zhou, J.; Hu, Y.; Li, L.; Li, B. Development of a Competitive Enzyme-Linked Immunosorbent Assay Targeting the-p30 Protein for Detection of Antibodies against African Swine Fever Virus. Viruses 2023, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shao, J.; Liu, W.; Gao, S.; Zhou, G.; Ning, X.; Huang, H.; Liu, Y.; Chang, H. Screening and identification of linear B-cell epitopes on structural proteins of African Swine Fever Virus. Virus Res. 2024, 350, 199465. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Cao, Q.; Liu, A.; Zhang, J.; Han, H.; Jiao, J.; He, P.; Sun, Z.; Xu, Z.; Zheng, W.; et al. Identification of a New Conserved Antigenic Epitope by Specific Monoclonal Antibodies Targeting the African Swine Fever Virus Capsid Protein p17. Vet. Sci. 2024, 11, 650. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, D.; Li, L.; Wang, J.; Wang, P.; Shi, X.; Zhao, Q.; Liu, B.; Ji, P.; Zhang, G. Identification of Two Novel Linear B Cell Epitopes on the CD2v Protein of African Swine Fever Virus Using Monoclonal Antibodies. Viruses 2022, 15, 131. [Google Scholar] [CrossRef]
- Song, J.; Wang, M.; Zhou, L.; Tian, P.; Sun, J.; Sun, Z.; Guo, C.; Wu, Y.; Zhang, G. A novel conserved B-cell epitope in pB602L of African swine fever virus. Appl. Microbiol. Biotechnol. 2024, 108, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Zhu, X.; Jin, J.; Lu, W.; Zhao, X.; Wan, B.; Liao, Y.; Zhao, Q.; Netherton, C.L.; et al. Identification and Characterization of a Novel Epitope of ASFV-Encoded dUTPase by Monoclonal Antibodies. Viruses 2021, 13, 2175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Liu, J.; Niu, B.; Zhu, Y.M.; Zhao, D.M.; Chen, W.Y.; Liu, R.Q.; Bu, Z.G.; Hua, R.H. Comprehensive mapping of antigenic linear B-cell epitopes on K205R protein of African swine fever virus with monoclonal antibodies. Virus Res. 2023, 328, 199085. [Google Scholar] [CrossRef] [PubMed]
- Potocnakova, L.; Bhide, M.; Pulzova, L.B. An Introduction to B-Cell Epitope Mapping and In Silico Epitope Prediction. J. Immunol. Res. 2016, 2016, 6760830. [Google Scholar] [CrossRef] [PubMed]
- Koltsov, A.; Sukher, M.; Krutko, S.; Belov, S.; Korotin, A.; Rudakova, S.; Morgunov, S.; Koltsova, G. Towards Safe African Swine Fever Vaccines: The Gene as a Tool to Reduce Virulence and a Promising Serological DIVA Marker Candidate. Animals 2024, 14, 2469. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Zhang, C.A.; Lin, J.; Wang, Y.; Wu, S.H.; Sun, Y.; Zhang, B.W.; Lv, H.; Ji, X.M.; Lu, Y.; et al. Selection of specific nanobodies against peanut allergen through unbiased immunization strategy and the developed immuno-assay. Food Sci. Hum. Well 2023, 12, 745–754. [Google Scholar] [CrossRef]
- Tian, Y.; Liang, C.; Zhou, J.; Sun, F.; Liu, Y.; Chen, Y.; Zhu, X.; Liu, H.; Ding, P.; Liu, E.; et al. Identification of a novel B-cell epitope of the African swine fever virus p34 protein and development of an indirect ELISA for the detection of serum antibodies. Front. Microbiol. 2023, 14, 1308753. [Google Scholar] [CrossRef]
| Primer | Sequence of Nucleotide (5′→3′) |
|---|---|
| p22-F | CGCGGATCCAAGAAGCAGCAGCCTCCTAAGAAG |
| p22-R | CCGCTCGAGTTAGGCGTGCTTGTGGTTTCTAGG |
| S1-F | TTGGTCTCTAGGTAAGAAGCAGCAGCCTCCTAAGAAG |
| S1-R | CCGCTCGAGTTATCTGTAGAAGTTAGGGGTGAACTCAC |
| S2-F | TTGGTCTCTAGGTATCAAGATCGATTCTAAGATCTCTTCTTG |
| S2-R | CCGCTCGAGTTAGCCCCAAGAACAGTACTTCTCACAC |
| S3-F | TTGGTCTCTAGGTCCTTCTGAGTCTCACTCTCCTCAG |
| S3-R | CCGCTCGAGTTAGGCGTGCTTGTGGTTTCTAGG |
| Temperature for Expression (°C) | Concentration of IPTG (mM) | Incubation Time (h) | |
|---|---|---|---|
| Group 1 | 16 | 0.2 | 8 |
| Group 2 | 25 | 0.2 | 12 |
| Group 3 | 37 | 0.2 | 16 |
| Group 4 | 16 | 0.5 | 12 |
| Group 5 | 25 | 0.5 | 16 |
| Group 6 | 37 | 0.5 | 8 |
| Group 7 | 16 | 0.8 | 16 |
| Group 8 | 25 | 0.8 | 8 |
| Group 9 | 37 | 0.8 | 12 |
| p22 Protein in Supernatant | p22 Protein in Precipitate | Ratio of Supernatant to Precipitate | |||||
|---|---|---|---|---|---|---|---|
| IntDen | Lane IntDen | Percentage (%) | IntDen | Lane IntDen | Percentage (%) | ||
| Group 1 | 4.829 | 26.036 | 18.547 | 1.523 | 12.275 | 12.407 | 3.171 |
| Group 2 | 5.198 | 30.261 | 17.177 | 1.265 | 11.234 | 11.260 | 4.109 |
| Group 3 | 2.916 | 44.269 | 6.587 | 2.664 | 20.662 | 12.893 | 1.095 |
| Group 4 | 8.315 | 44.837 | 18.545 | 3.528 | 20.007 | 17.634 | 2.357 |
| Group 5 | 5.457 | 33.381 | 16.348 | 3.551 | 23.088 | 15.380 | 1.537 |
| Group 6 | 4.634 | 54.733 | 8.467 | 6.721 | 38.989 | 17.238 | 0.689 |
| Group 7 | 11.392 | 57.270 | 19.892 | 1.769 | 26.171 | 6.759 | 6.440 |
| Group 8 | 7.397 | 44.687 | 16.553 | 2.599 | 28.571 | 9.097 | 2.846 |
| Group 9 | 3.757 | 52.452 | 7.163 | 3.311 | 34.899 | 9.487 | 1.135 |
| Name | Sequence of Amino Acid | Position |
|---|---|---|
| P1 | CKKQQPPKK | 30–37 |
| P2 | CVRGSCSSLS | 52–60 |
| P3 | CKMDKRNIKIDSKIS | 66–79 |
| P4 | CADEQQEFGKTRH | 95–106 |
| P5 | CKITPSPSESHSP | 109–120 |
| P6 | CWGTDDCTG | 130–137 |
| P7 | CVGDEKEGT | 141–148 |
| P8 | CYVYNNPHH | 150–157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhou, J.; Chen, Y.; Liu, H.; Qi, Y.; Liang, C.; Zhu, X.; Liu, E.; Wu, S.; Ding, P.; et al. Identification and Characterization of Three Novel B-Cell Epitopes in African Swine Fever Virus p22 Protein. Microorganisms 2025, 13, 2666. https://doi.org/10.3390/microorganisms13122666
Li Z, Zhou J, Chen Y, Liu H, Qi Y, Liang C, Zhu X, Liu E, Wu S, Ding P, et al. Identification and Characterization of Three Novel B-Cell Epitopes in African Swine Fever Virus p22 Protein. Microorganisms. 2025; 13(12):2666. https://doi.org/10.3390/microorganisms13122666
Chicago/Turabian StyleLi, Zehui, Jingming Zhou, Yumei Chen, Hongliang Liu, Yanhua Qi, Chao Liang, Xifang Zhu, Enping Liu, Sixuan Wu, Peiyang Ding, and et al. 2025. "Identification and Characterization of Three Novel B-Cell Epitopes in African Swine Fever Virus p22 Protein" Microorganisms 13, no. 12: 2666. https://doi.org/10.3390/microorganisms13122666
APA StyleLi, Z., Zhou, J., Chen, Y., Liu, H., Qi, Y., Liang, C., Zhu, X., Liu, E., Wu, S., Ding, P., & Wang, A. (2025). Identification and Characterization of Three Novel B-Cell Epitopes in African Swine Fever Virus p22 Protein. Microorganisms, 13(12), 2666. https://doi.org/10.3390/microorganisms13122666






