1. Introduction
Emerging and reemerging viruses represent a constant epidemic threat not only for human and animal health, but also due to the economic, social, and political effects they may produce globally. Climate change, massive international travel, fast urbanization, increased population density, and proximity to wild animals contribute to the increased number of viral emergencies [
1]. The recent SARS-CoV-2 outbreak dramatically highlighted how unprepared we are to respond to such events and how limited our therapeutic arsenal is against them.
Echoviruses are small (~300 Å in diameter—27 nm), non-enveloped icosahedral viruses with a single-stranded RNA genome that belong to the
Enterovirus B species of the family
Picornaviridae, with at least 28 serotypes [
2]. The primary modes of transmission for enteroviruses (EV) are through the fecal-oral route and respiratory pathways, although instances of vertical and postnatal transmission, particularly in hospital environments, have also been documented [
3]. Infections caused by non-polio EV can lead to several health issues, ranging from mild, nonspecific inflammatory responses to conditions like skin rashes, fevers, diarrhea, herpangina, and hand, foot, and mouth disease (HFMD), or severe respiratory infection and polio-like paralytic illnesses [
4]. Specific echovirus serotypes can cause severe illness in neonates or other vulnerable populations. Echovirus 11 (E11) has been recently associated with more severe clinical features, including inflammatory diseases, acute flaccid paralysis (AFP), severe acute hepatitis, aseptic meningitis, and coagulation disorders [
5,
6,
7]. The European Center for Disease Prevention and Control (ECDC), in conjunction with the World Health Organization (WHO), has observed a growing incidence of severe infections in newborns during 2022–2023 linked to the new E11 lineage 1, which emerged from recombination events that occurred in 2022 [
8]. The new E11 variant has been detected in France, Croatia, Italy, Spain, Sweden, and in the United Kingdom of Great Britain and Northern Ireland [
8]. According to the ECDC, E11 infection should be factored into the differential diagnosis for conditions like hepatitis, sepsis, myocarditis or pericarditis, and critical neurological and respiratory illnesses in infants, to accurately assess its public health implications (Item ID 2023-EIP-00026) [
9].
Currently, there are no targeted pharmaceutical therapies approved for infections caused by non-polio EV, and creating a vaccine that covers all EV serotypes is not achievable due to the high antigenic variability [
5]. The availability of several anti-EV compounds targeting viral or cellular factors has been demonstrated by in vitro studies, including enviroxime, which prevents the synthesis of positive-strand RNA by blocking the assembly of the replication complex [
10]. Despite the latest progress, most of the research is focused on discovering compounds active against
Enterovirus 71 (EV-71) and
Enterovirus 30 (EV-30), which are often associated with meningitis and coagulation disorders [
11]. Therefore, the increasing need for new effective compounds with anti-EV activity is imperative [
12]. This study seeks to uncover new compounds that may exhibit antiviral properties while also enhancing our comprehension of the mechanisms that drive their effectiveness against EV viruses.
An attractive target for the discovery of novel antiviral drugs is represented by the RNA-dependent RNA polymerase (RdRp) enzyme, which plays a crucial role in the replication cycle of most RNA viruses [
13]. This enzyme is highly conserved among evolutionary distant RNA viruses and does not have a homolog in mammalian cells. These characteristics, together with the extensive knowledge of RdRp structure and functions, and the easy availability of biochemical assays for the rapid screening of large libraries of compounds, make this enzyme an optimum target for new antiviral molecules.
In a previous study, Leusciatti et al. [
14] reported the anti-SARS-CoV-2 RdRp activity of newly synthesized isoxazoline-carbocyclic monophosphate nucleotides, determined through computational analysis together with RdRp inhibition and cytotoxicity biological assays. The results obtained showed that the examined compounds could bind the SARS-CoV-2 RdRp nucleotide binding pocket, significantly reducing RNA synthesis [
14]. This study aimed to investigate the potential anti-E11 activity of two compounds,
4a and
4b (isoxazoline-carbocyclic monophosphate nucleotides), previously shown to exhibit moderate anti-SARS-CoV-2 RdRp activity [
14], using in vitro biological assays.
The synthetic approach was based on pericyclic reactions, including hetero Diels–Alder cycloaddition of nitrosocarbonyl intermediates and 1,3-dipolar cycloaddition of nitrile oxides. These strategies enabled the derivatization of aromatic substituents, construction of specific heterobases (e.g., 6-chloropurine), and the selection of the most suitable phosphorylation method to obtain the final nucleotide analogs
4a and
4b [
15].
2. Materials and Methods
2.1. Tested Compounds
Two isoxazoline-carbocyclic monophosphate nucleotides (4a and 4b) were designed and synthesized through the HDA cycloaddition of suitably prepared nitrosocarbonyl intermediates and cyclopentadiene, followed by the 1,3-dipolar cycloaddition reaction of the obtained dipolarophile with the stable anthracenenitrile oxide. The derivatization of the antracene ring with bromine conducted on the regioisomeric cycloadducts and the linear construction of the 6-chloropurine rings will afford the desired nucleosides. These are the precursors of the nucleotides of type 4a, b that can be obtained through phosphorylation according to properly chosen protocols. Compounds 4a and 4b were solubilized in dimethyl sulfoxide (DMSO), stored at −20 °C, and the final dilutions for the experiments were carried out in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA).
2.2. Cells and Viruses
Epithelial monkey kidney VERO 76 cells [ATCC CRL 1587,
Cercopithecus aethiops] were maintained in DMEM supplemented with 1% penicillin-streptomycin, and 2 mM L-glutamine (Sigma-Aldrich), and 10% fetal bovine serum (FBS; Sigma-Aldrich), at 37 °C and 5% CO
2. Echovirus 11 lineage 1 [
16] was maintained in our laboratory and propagated in VERO 76 cells. The virus was stored in small aliquots at −80 °C until use. All experimental work involving viruses was performed in an appropriate biosafety level containment laboratory.
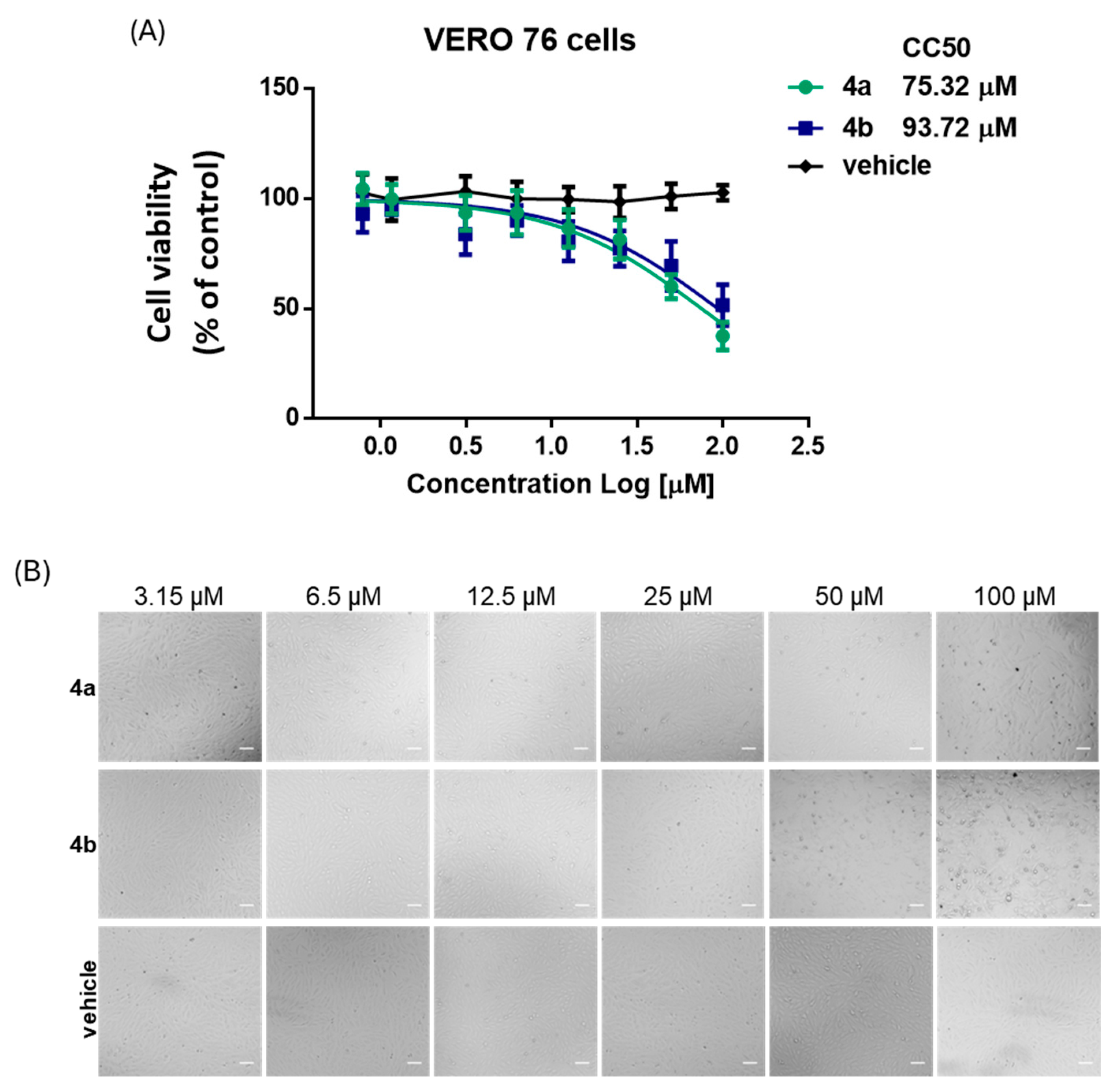
2.3. Cytotoxicity Assays
According to the manufacturer’s instructions, cell viability was measured using the MTS ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) tetrazolium assay (CellTiter 96
® AQueous One Solution, Promega, Madison, WI, USA). Briefly, VERO 76 cells were seeded in 96-well plates at an initial density of 2 × 10
4 cells/mL in complete medium. After overnight incubation at 37 °C/5% CO
2, cells were treated with base-2 serial dilutions (0.781–100 µM) of compounds
4a and
4b, prepared from 10 mM stock solutions in DMSO. For each concentration, a vehicle-only control containing the same final DMSO percentage (up to 1%
v/v at 100 µM) was included. After 48 h of incubation, 20 µL of MTS reagent were added directly to each well and the plates were incubated according to the manufacturer’s protocol. The conversion of MTS into a soluble formazan product by metabolically active cells was quantified by measuring the absorbance at 490 nm. For data analysis, the optical density (OD) of each well was first corrected by subtracting the background OD (medium + MTS without cells). The percentage of cell viability was then calculated by comparing the corrected OD of treated samples with the mean corrected OD of untreated control cells (0 µM), which was set as 100% viability:
Two independent experiments were performed, each in triplicate (n = 6 per condition). Dose–response curves were generated and CC50 values were determined by nonlinear regression using GraphPad Prism software v. 8.0 (San Diego, CA, USA).
In addition to the MTS assay, cytotoxicity of the compounds under study has also been assayed by optical microscopy analysis using the same method described below for the CPE inhibition assay.
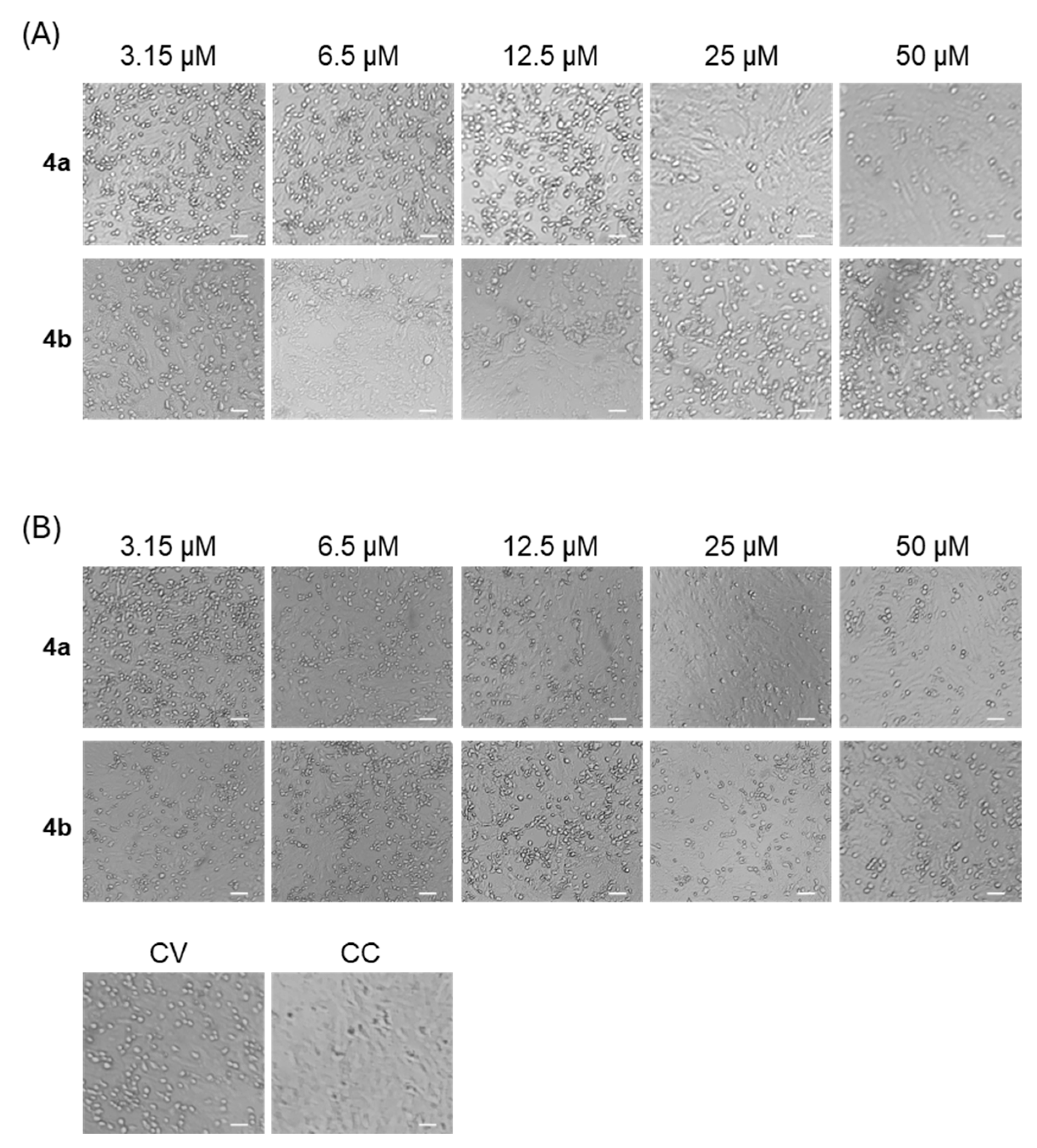
2.4. Cytopathic Effect Inhibition Assay
Protection of VERO 76 cells from E11-induced cytopathic effect (CPE) was evaluated using a CPE inhibition assay at a multiplicity of infection (MOI) of 0.025. In this assay, increased crystal violet staining indicates preservation of cell viability, reflecting protection against virus-induced cytopathic damage mediated by the tested compounds. The MOI of 0.025 was selected to allow multi-cycle infection, thereby maximizing the detection of compounds that limit viral propagation. Under these conditions, complete CPE was consistently observed in VERO 76 cells within 48 hours.
- (A)
A co-treatment assay was performed as a screening test to determine the antiviral activity of 4a and 4b as anti-E11 agents. Each compound was added to the cell monolayer (3.2–100 μM) at the same time as viral infection for 48 h at 37 °C.
- (B)
A post-infection assay was performed to assess the possible ability of 4a and 4b to interfere with the viral replication due to the RdPR binding. VERO 76 cells in 96-well plates were infected with E11 for 1 h in DMEM at 37 °C to allow viral adsorption and penetration. After removal of the virus inoculum, the cells were washed once with culture medium and treated for 48 h with different concentrations of 4a and 4b (3–100 μM).
Each treatment was performed in triplicate. For all assays, VERO 76 cells were seeded in 96-well plates at a density of 2 × 104 cells/well and allowed to form confluent monolayers by overnight incubation at 37 °C. After 48 h, CPE inhibition was evaluated by optical microscopy observation. Cells were fixed with 4% formaldehyde for 15 min at room temperature and stained with 0.1% (w/v) crystal violet for 30 min. The absorbance was measured at 595 nm.
Dose–response curves derived from these absorbance values were used to determine the half-maximal effective concentration (EC50) for each compound. Although no antiviral drugs are approved for enterovirus infections, Remdesivir (Cat. No. HY-104077, MedChemExpress, Shanghai, China) was included for comparison based on its documented in vitro activity against other enteroviruses and its mechanism of action targeting the viral RNA-dependent RNA polymerase. All experiments were carried out in triplicate.
2.5. Plaque Assay
E11 infectious titers were determined by plaque assay. Briefly, 100 µL of culture supernatant from infected cells were serially diluted (10-1–10-4) in DMEM containing 1% FBS. VERO 76 cells were seeded in 24-well plates 24 h before infection to obtain 90% confluency. Cells were inoculated with 50 µl of each dilution and incubated for 1 h at 37 °C with gentle rocking. After adsorption, the inoculum was removed, and cells were overlaid with DMEM containing 1% FBS and carboxymethyl cellulose (CMC). Following 72 h incubation, monolayers were fixed, stained with crystal violet, and plaques were counted. Viral titers were expressed as plaque-forming units per milliliter (PFU/mL) according to:
2.6. E11 Genome Quantification in Infected VERO 76 Cells and Supernatant
Quantification of E11 genomic RNA was used as a hallmark of RNA virus replication within VERO 76-infected cells. The viral RNA was extracted from both cell culture supernatants and cell-associated fractions and quantified by one-step qRT-PCR using the automated Elite InGenius one-step RNA Enterovirus ELITe MGB
® Kit, according to the manufacturer’s protocols. The experiment was performed in triplicate and RNA yield was reported as the mean value of a triplicate assay. To improve the accuracy of extracellular RNA quantification, particularly at 48 h post-infection (p.i.), a secondary analysis was performed using a two-step SYBR Green RT-qPCR. In particular, specific primers targeting the gene encoding the RNA-dependent RNA polymerase (RdRp) of E11 were designed using the Primer3 software (version 4.1.0). The sequences were as follows: Forward primer: 5′-TGCAAGGAAAAGGGGTGGTT-3′ and Reverse primer: 5′-AGAGACAAAGGTGGTGAGCG. To account for variability in RNA recovery and cDNA synthesis, an in-house transcribed synthetic RNA was added to each sample prior to RNA extraction [
17]. Reverse transcription was carried out with a high-capacity cDNA synthesis kit, followed by quantitative PCR using SYBR Green Master Mix on a real-time PCR instrument. Data were analyzed using the ΔΔCt method, with normalization to the synthetic RNA spike-in.
2.7. Molecular Docking Studies
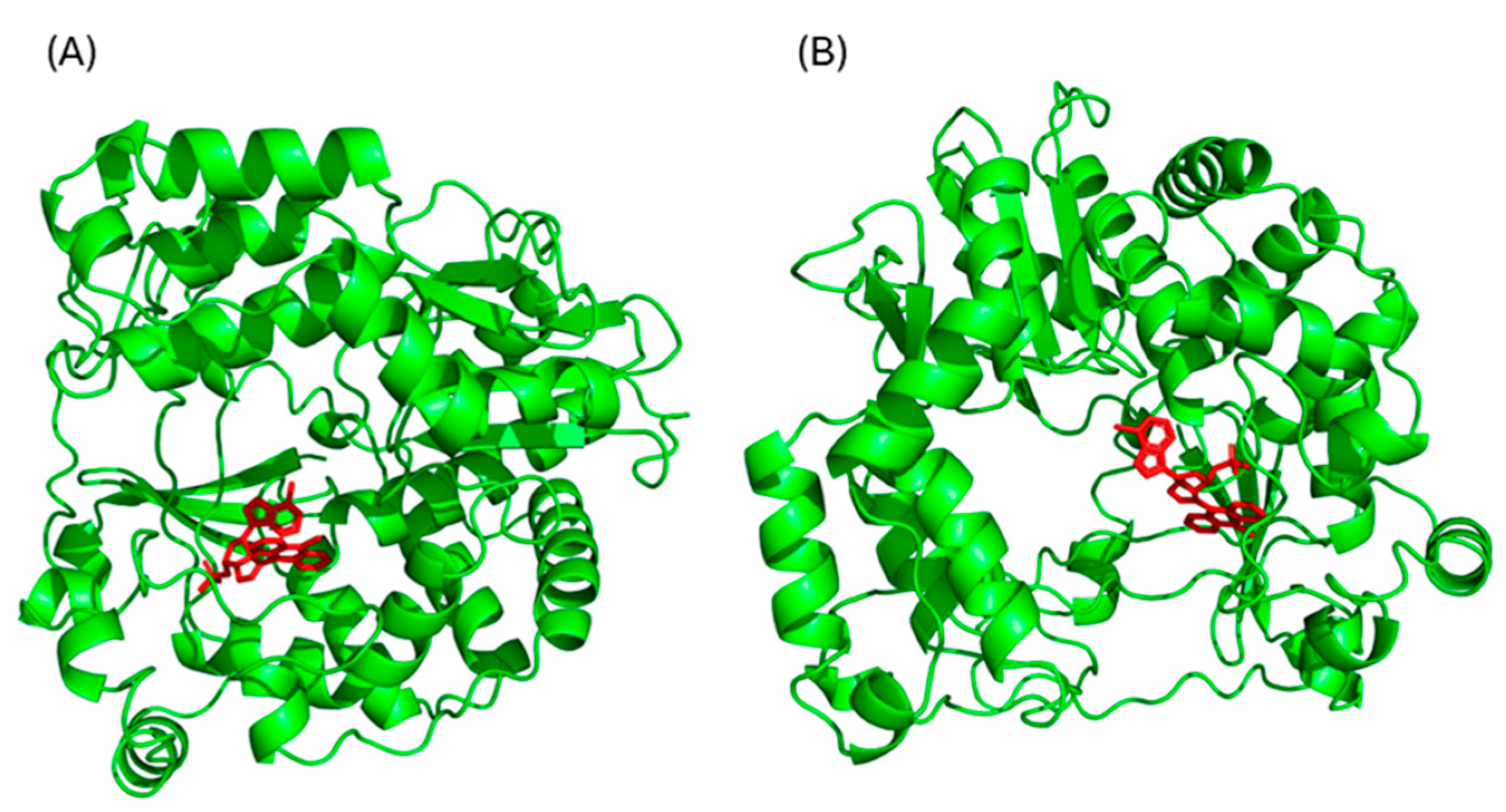
The chemical structures of compounds 4a and 4b were drawn using ChemDraw (freeware version 12.01, PerkinElmer Waltham, MA, USA) and converted into PDB format. These structures were refined, and all water molecules and non-essential ligands were removed using AutoDockTools (Scripps Research Institute, MGLTools, version 1.5.7; La Jolla, CA, USA). Since no crystal structure of E11 RdRp was available, a homology model was built using the Swiss-Model server, an automated platform for comparative 3D protein modeling. The model was based on the RNA polymerase of Coxsackievirus B3 (PDB ID: 4Y2A), which showed the highest sequence identity and resolution among the available templates.
Molecular docking studies were performed using the AutoDockTools 1.5.7 software (Scripps Research Institute, MGLTools, version 1.5.7; La Jolla, CA, USA), with which initially all small molecules, such as water, salts, and ligands, were removed. The docking protocol using AutoDockTools (Scripps Research Institute, MGLTools, version 1.5.7; La Jolla, CA, USA) was constructed using a box with dimensions of 15 × 15 × 15 Å, centered on coordinates [60.8, 44.7, 58.8] with a grid spacing of 1 Å, using the default settings of the Lamarckian genetic algorithm. To best center the grid box, the coordinates of the complex ligand were extrapolated from the crystallographic structure of 4Y2A and were used for our analysis. The protein–ligand interactions were analyzed and visualized using the Pymol (version 2.6, Schrödinger, Portland, OR) and BIOVIA Discovery Studio 2024 ( version 24.1.0.23298, Dassault Systèmes, San Diego, CA, USA) software.
2.8. Statistics
All statistical analyses were performed using GraphPad Prism V.8.0 for Windows (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard deviation (SD) from at least three independent experiments. Dose–response curves were generated to determine the CC50 values by nonlinear regression. Comparisons between groups were carried out using one-way ANOVA, followed by Bonferroni’s post hoc test for multiple comparisons. Differences were considered statistically significant at p < 0.05.
4. Discussion
Enteroviruses continue to significantly impact global health, particularly related to the emergence of novel E11 variants [
5,
7]. This alarming development has spurred increased efforts toward repurposing existing drugs and developing novel antiviral therapies targeting enteroviruses [
20,
21,
22]. Although numerous compounds have demonstrated promising anti-enteroviral activity in vitro, only a limited number have shown efficacy in vivo [
12,
13,
14,
23,
24]. Among those, only few viral capsid inhibitors (disoxaril, pleconaril, pirodavir, vapendavir and pocapavir) have progressed to clinical evaluation [
25,
26,
27,
28]. However, their therapeutic use is frequently limited by adverse effects such as crystalluria, menstrual irregularities, and the emergence of drug-resistant viral variants [
25,
28]. Thus, we are still far from having identified compounds capable of providing an optimal therapeutic treatment for enterovirus infections, including those caused by echovirus variants. Non-structural proteins represent innovative targets for the development of broad-spectrum antivirals. Notably, recent studies have shown that both coronaviruses and enteroviruses share a conserved mechanism for initiating viral RNA synthesis, which involves the covalent modification of specific non-structural proteins (nsps) [
29]. In particular, in echoviruses, the viral protein genome-linked (VPg) protein can initiate the RNA synthesis after its cleavage by the viral protease 3 CD and covalent attachment of uridine monophosphate (UMPylation) by 3D
pol [
30]. Similarly, the non-structural protein 12 of coronaviruses (CoV RdRp nsp12) uridylates the non-structural protein 8 (nsp8), which then primes RNA synthesis from a poly(A) template.
A class of promising antiviral drugs under investigation is represented by molecules inhibiting RdRp complex formation through binding with specific nsps 28.
In general, the interaction between antiviral compounds and viral non-structural proteins results in the inhibition of key enzymatic functions essential for the viral replication cycle. Within this class of antivirals are the two isoxazoline-carbocyclic monophosphate nucleotides,
4a and
4b, which were originally designed and synthesized as inhibitors of non-structural protein 12 (nsp12) of SARS-CoV-2 [
11]. These compounds were shown to bind putatively to allosteric sites on nsp12 distinct from those targeted by Remdesivir, leading to a measurable reduction in RdRp activity. These findings support the potential of
4a and
4b as novel antiviral agents, warranting investigation of their efficacy against other RNA viruses, such as enteroviruses.
Given the clinical relevance of the new E11 variant and the conserved RNA synthesis mechanism, in this study, the potential anti-E11 activity of nucleotides 4a and 4b was tested by in vitro infection. In line with the results previously observed on Hep-2 and HepG2 cell lines, exposure of cells to high concentrations of 4a and 4b is associated with a certain cytotoxicity.
According to a previous study investigating the inhibition of Echovirus 71 RdRp by the adenosine analog NITD008 [
31], the ability of nucleotides
4a and
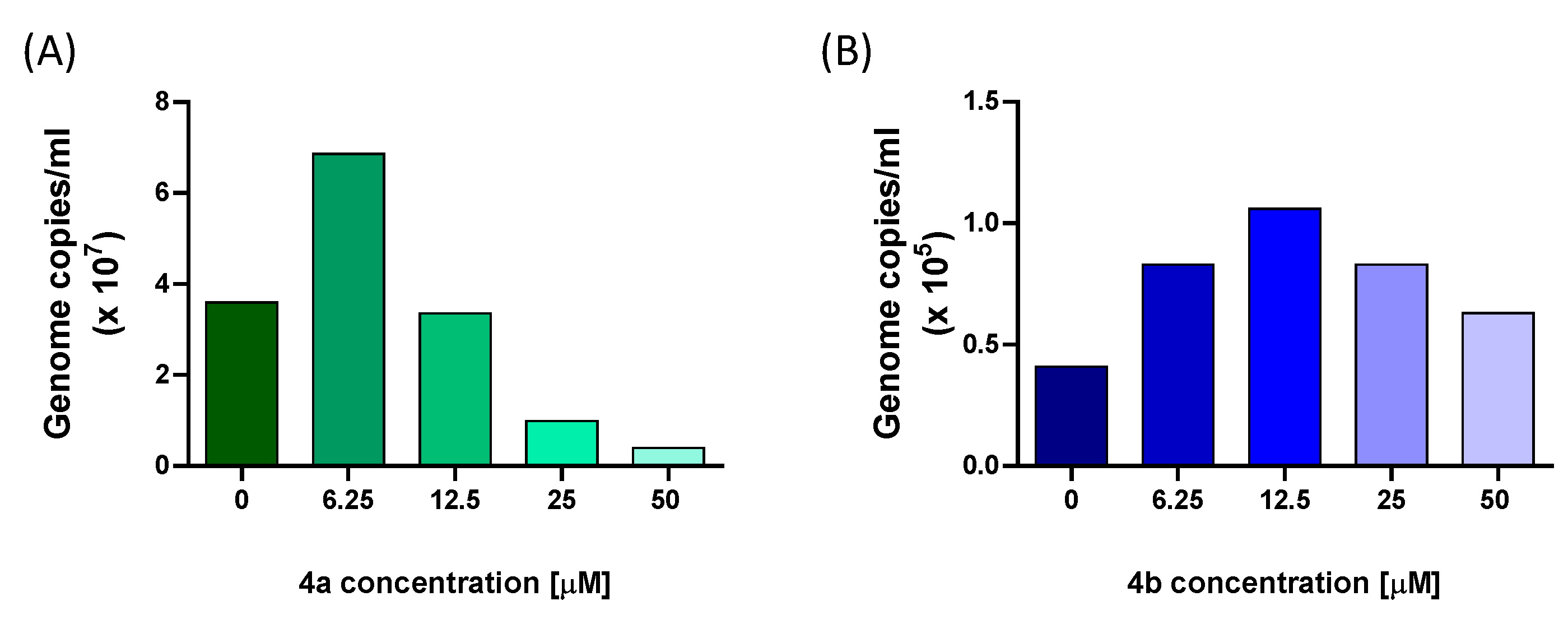
4b to interfere with E11 viral replication was further assessed by measuring the levels of viral RNA in the supernatant by qRT-PCR. The results showed that incubation with
4a caused a reduction in viral load by 72% at 25 μM and 89% at 50 μM. Differently, treatment with nucleotide
4b resulted in a weaker inhibition of E11 infection. These findings confirmed, at the molecular level, the observations regarding the reduction in CPE and affirmed the more potent inhibitory properties of nucleotide
4a against E11. In line with these observations, quantitative analysis of dose–response curves showed that
4a displayed a markedly lower EC
50 and a more favorable Selectivity Index compared to
4b, further supporting the superior antiviral profile of
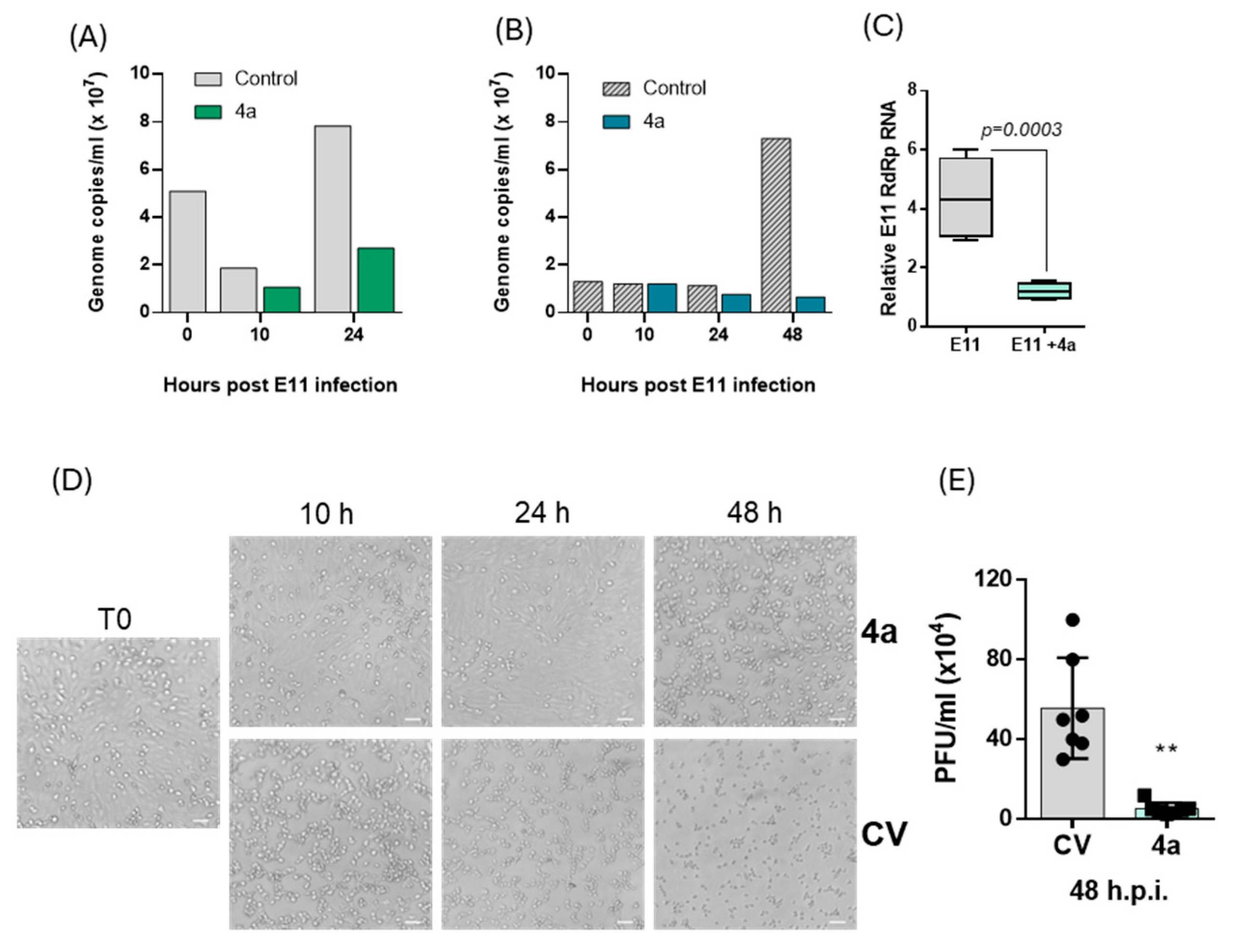
4a. Due to the limited information on the E11 life cycle, a quantification of the viral RNA was conducted at different time points from T0 to 48 h p.i. The E11 RNA load was analyzed in the VERO 76 cells supernatant, which mainly contained released infectious particles. In line with Echovirus 71 and Echovirus 30 in vitro infection studies [
3], and in accordance with the CPE assay, the results of this experiment revealed an initial decrease in viral load in the supernatant from T0 to 10 h p.i., followed by an increase in E11 RNA from 10 h to 48 h p.i. Since we hypothesized that compound
4a could act by inhibiting the RNA-dependent RNA polymerase (RdRp), which is translated and becomes active in enteroviruses no earlier than 3–4 h post-infection, in this study we focused our kinetic analysis on timepoints from 10 h onwards. Earlier phases, such as viral adsorption, uncoating, and initial translation of the viral polyprotein, occur within the first 1–2 h p.i. and are not directly targeted by RdRp inhibitors. Nevertheless, future experiments including intermediate timepoints such as 6 h p.i. may help to define the onset of antiviral activity with greater temporal resolution.
The evaluation of viral progeny generation carried out in the presence of nucleotide 4a at 25 μM showed a remarkable drop of E11 RNA load in supernatant of the treated group compared to the control at 24 h (31%) and 48 h p.i. (91%). According to the RNA load decrease in the supernatant, a reduction in intracellular E11 RNA was observed in the treated group. Altogether, the molecular analysis coupled with the CPE reduction assays results strongly suggest the inhibitory activity of nucleotide 4a on both E11 RNA synthesis and viral particle release. To validate whether the reduction in viral RNA corresponded to a loss of infectious virus, we performed a plaque assay on supernatants collected at 48 h p.i. The marked decrease in PFU/mL in 4a-treated cultures confirmed a profound impairment in the production of infectious E11 progeny, strengthening the antiviral evidence obtained through CPE and qRT-PCR analyses. Finally, the yield of E11 viral RNA in cells subjected to 4a treatment was quantified over time. The results confirmed a continuous decrease in E11 genome levels in the supernatant while the quantification of genome inside the cell monolayer revealed a 60% decrease at 10 h p.i. followed by an E11 genome accumulation at 48 h. The increase in viral RNA observed within cells at 48 h could be explained as the result of the incomplete suppression of RNA synthesis by nucleotide 4a, and the detection of genome fragments which will not become infectious virus particles.
In parallel with the antiviral evaluation, the cytotoxic potential of the compounds was assessed using the MTS assay. The analysis confirmed a dose-dependent reduction in cell viability, with CC50 values of 75.3 ± 10.6 µM for 4a and 93.7 ± 19.2 µM for 4b. Compound 4a maintained a cell viability above 80% up to 25 µM, whereas for compound 4b a modest reduction (~77%) was already observed at the same concentration. Microscopic inspection corroborated these data, showing no appreciable morphological alterations under these conditions. Overall, the pronounced antiviral effects of 4a at sub-cytotoxic concentrations clearly outweighed the modest impact on cell viability, highlighting its favorable selectivity profile. By contrast, the narrower therapeutic window of 4b, combined with its weaker antiviral activity, further supports 4a as a more promising candidate for subsequent investigations.
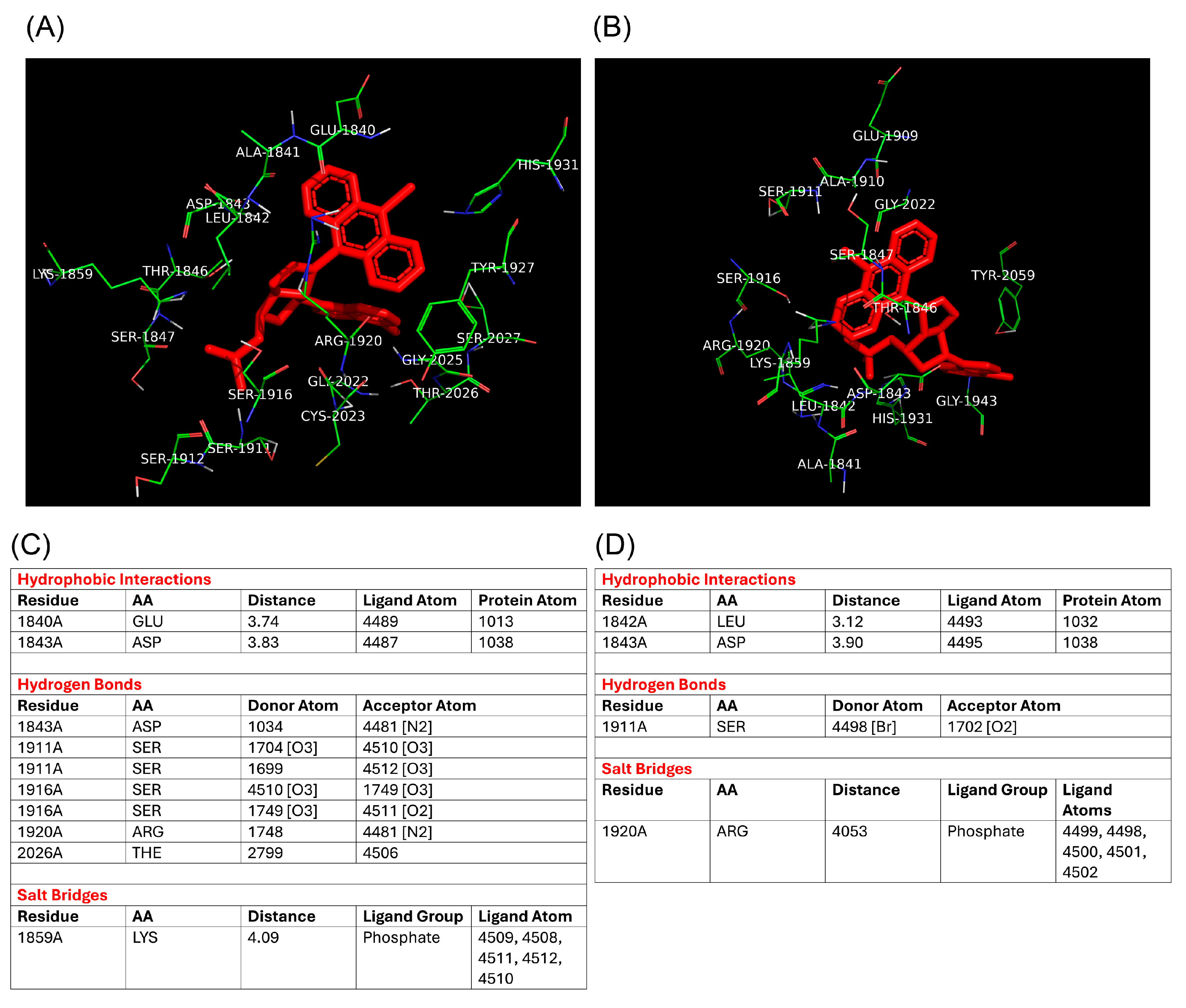
Despite the lack of a crystallographic structure for E11 RdRp, a homology-based model using the closely related Coxsackievirus B3 RdRp provided a reliable structural framework for docking simulations. Compound 4a exhibited a lower binding energy (−8.89 kcal/mol) compared to 4b (−8.11 kcal/mol), and a stronger predicted inhibition constant (302.19 nM vs. 1.14 µM), suggesting a tighter interaction with the viral polymerase. Additionally, molecular docking identified specific interactions of 4a with catalytically relevant residues such as Arg1920 and Ser1911, highlighting potential key contacts responsible for its inhibitory activity. These in silico observations are in line with the biological assays and support the hypothesis that RdRp is the molecular target of compound 4a in E11.
In conclusion, the results of this study provided preliminary insights into the kinetics of infection of the new Echovirus 11 variant in VERO 76 cells. Moreover, the antiviral screening tests conducted in this study support the promising antiviral properties of the two monophosphate nucleotides 4a and 4b previously identified as possible SARS-CoV-2 inhibitors. In particular, the data obtained revealed the effectiveness of 4a against additional RNA viruses, especially against the new Echovirus 11 variant.
Although additional investigations are required to broaden the insights on the mechanism of action of these molecules, our findings strongly indicate that nucleotide 4a is an RdRp inhibitor, demonstrating its capability to cause dramatic reductions in E11 genome replication and viral particle formation in VERO 76 cells.