Mosquito Exposure Risks in Equine Facilities: An Environmental–Managerial Assessment in Western Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Design
2.2. Environmental Assessment Protocol
2.3. Risk Factor Criteria
2.4. Composite Environmental Risk Score (CERS)
2.5. Statistical Analysis
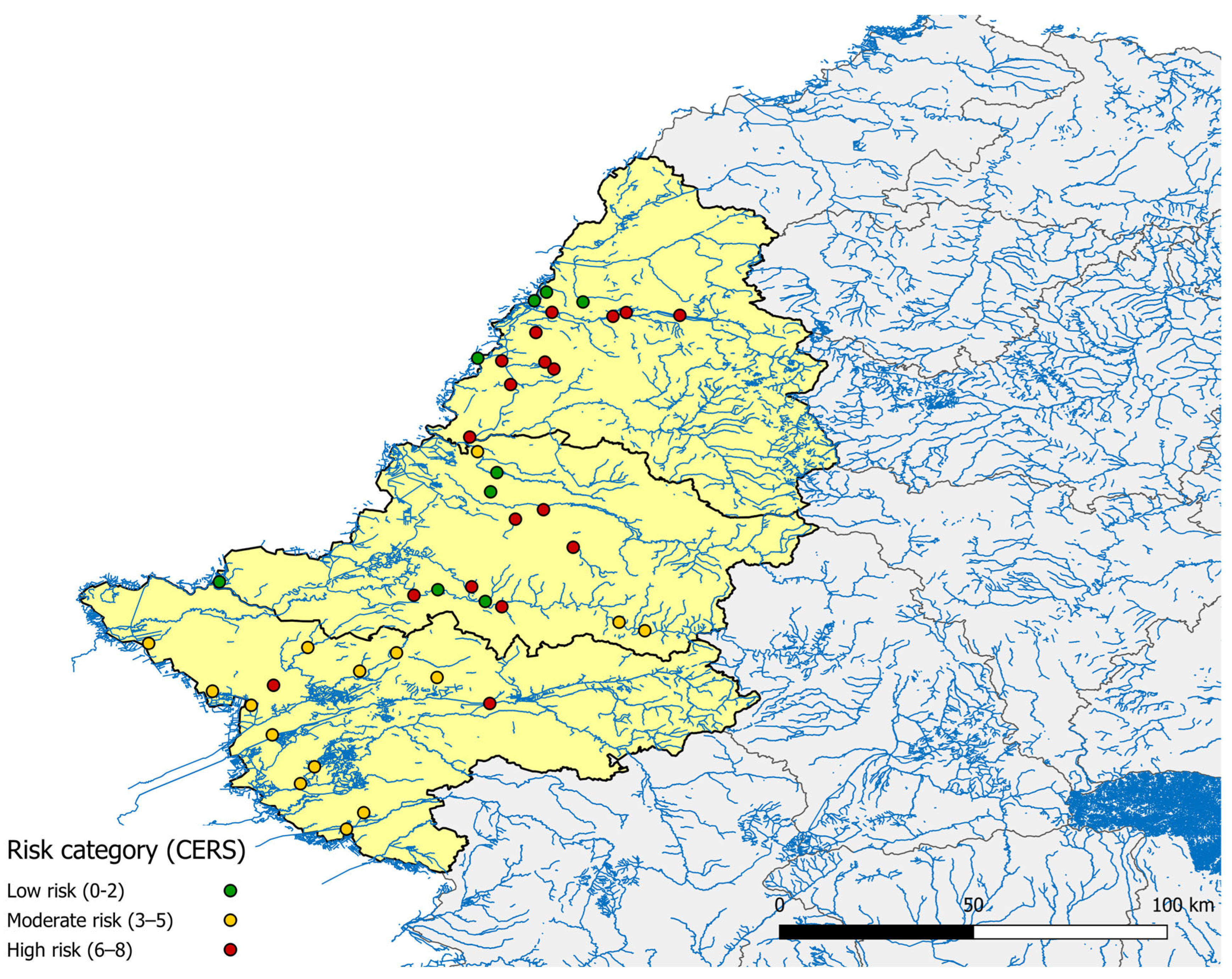
3. Results
3.1. Environmental and Managerial Mosquito-Favoring Factors
3.2. Seroprevalence and Spatial Correlation
4. Discussion
4.1. Interpretation of Findings
4.2. Practical Implications
4.3. Comparison with Other Studies
4.4. Study Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WNV | West Nile Virus |
| IgM | Immunoglobulin M |
| CERS | Composite Environmental Risk Score |
References
- Vargas Campos, C.A.; García-Pérez, S.; Figuerola, J.; la Puente, J.M.-D.; Polo, I.; Rodríguez-De-Fonseca, B.; Fernández-Álvarez, S.; Fraile, V.G.; Martín-Rey, M.; Lacasaña, M.; et al. Comprehensive analysis of West Nile Virus transmission: Environmental, ecological, and individual factors. An umbrella review. One Health 2025, 20, 100984. [Google Scholar] [CrossRef] [PubMed]
- Savuła, G.; Luanda, O.; Aniłă, A.; Aniłă, D. West Nile Virus infections in Romania—Past, present and perspective. Lucr. Științifice Med. Vet. Timișoara 2008, XLI, 301–308. [Google Scholar]
- Simonin, Y. Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]
- Wang, H.R.; Liu, T.; Gao, X.; Wang, H.B.; Xiao, J.H. Impact of climate change on the global circulation of West Nile virus and adaptation responses: A scoping review. Infect. Dis. Poverty 2024, 13, 38. [Google Scholar] [CrossRef]
- Gizaw, Z.; Salubi, E.; Pietroniro, A.; Schuster-Wallace, C.J. Impacts of climate change on water-related mosquito-borne diseases in temperate regions: A systematic review of literature and meta-analysis. Acta Trop. 2024, 258, 107324. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Fernandez-Salas, I.; Contreras-Cordero, J.F.; Marlenee, N.L.; Gonzalez-Rojas, J.I.; Komar, N.; Gubler, D.J.; Calisher, C.H.; Beaty, B.J. Serologic evidence of West Nile virus infection in horses, Coahuila State, Mexico. Emerg. Infect. Dis. 2003, 9, 853–856. [Google Scholar] [CrossRef] [PubMed]
- García-Carrasco, J.M.; Muñoz, A.R.; Olivero, J.; Segura, M.; García-Bocanegra, I.; Real, R. West Nile virus in the Iberian Peninsula: Using equine cases to identify high-risk areas for humans. Euro. Surveill. 2023, 28, 2200844. [Google Scholar] [CrossRef]
- Gothe, L.M.R.; Ganzenberg, S.; Ziegler, U.; Obiegala, A.; Lohmann, K.L.; Sieg, M.; Vahlenkamp, T.W.; Groschup, M.H.; Hörügel, U.; Pfeffer, M. Horses as sentinels for the circulation of flaviviruses in Eastern-Central Germany. Viruses 2023, 15, 1108. [Google Scholar] [CrossRef]
- Dinu, S.; Stancu, I.G.; Cotar, A.I.; Ceianu, C.S.; Pintilie, G.V.; Karpathakis, I.; Fălcuță, E.; Csutak, O.; Prioteasa, F.L. Continuous and dynamic circulation of West Nile Virus in mosquito populations in Bucharest area, Romania, 2017–2023. Microorganisms 2024, 12, 2080. [Google Scholar] [CrossRef]
- Horváth, C.; Cazan, C.D.; Mihalca, A.D. Emergence of the invasive Asian bush mosquito, Aedes (Finlaya) japonicus japonicus, in an urban area, Romania. Parasites Vectors 2021, 14, 192. [Google Scholar] [CrossRef]
- Crivei, L.A.; Moutailler, S.; Gonzalez, G.; Lowenski, S.; Crivei, I.C.; Porea, D.; Anita, D.C.; Ratoi, I.A.; Zientara, S.; Oslobanu, L.E.; et al. Detection of West Nile Virus Lineage 2 in Eastern Romania and first identification of Sindbis Virus RNA in mosquitoes analyzed using high-throughput microfluidic real-time PCR. Viruses 2023, 15, 186. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. West Nile virus outbreak in Romania, July to October 1996; ECDC: Stockholm, Sweden, 2011; Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/1104_MIR_West_Nile_outbreak_Romania.pdf (accessed on 23 August 2025).
- Oslobanu, L.E.; Pâslaru, A.; Savuța, G. West Nile Virus seroprevalence in horses from Romania: First step in the infection risk assessment. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2015, 72, 5–9. [Google Scholar] [CrossRef]
- Nistor, P.; Stanga, L.; Chirila, A.; Iorgoni, V.; Gligor, A.; Ciresan, A.; Popa, I.; Florea, B.; Imre, M.; Cocioba, V.; et al. Seroprevalence and passive clinical surveillance of West Nile Virus in horses from ecological high-risk areas in Western Romania: Exploratory findings from a cross-sectional study. Microorganisms 2025, 13, 1910. [Google Scholar] [CrossRef]
- Kirov, P.; Iancu, I.; Panayotova, E.; Petrov, R.; Imre, M.; Herman, V.; Hristov, H.; Abudalleh, A.; Alexandrova, R.; Gligor, A. First trans-border serological evidence of West Nile Virus infection in horses in Romania and Bulgaria. Int. J. Vet. Sci. 2025, 14, 777–781. [Google Scholar] [CrossRef]
- Oslobanu, L.E.; Mihu-Pintilie, A.; Aniță, D.; Aniță, A.; Lecollinet, S.; Savuța, G. West Nile virus reemergence in Romania: A serologic survey in host species. Vector Borne Zoonotic Dis. 2014, 14, 330–337. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Franco, J.; León, C.; Barbero-Moyano, J.; García-Miña, M.; Fernández-Molera, V.; Gómez, M.; Cano-Terriza, D.; Gonzálvez, M. High exposure of West Nile Virus in equid and wild bird populations in Spain following the epidemic outbreak in 2020. Transbound. Emerg. Dis. 2022, 69, 14733. [Google Scholar] [CrossRef] [PubMed]
- De Heus, P.; Kolodziejek, J.; Hubálek, Z.; Dimmel, K.; Racher, V.; Nowotny, N.; Cavalleri, J. West Nile Virus and Tick-Borne Encephalitis Virus are endemic in equids in Eastern Austria. Viruses 2021, 13, 1873. [Google Scholar] [CrossRef]
- Calzolari, M.; Angelini, P.; Bolzoni, L.; Bonilauri, P.; Cagarelli, R.; Canziani, S.; Cereda, D.; Cerioli, M.P.; Chiari, M.; Galletti, G.; et al. Enhanced West Nile Virus circulation in the Emilia-Romagna and Lombardy Regions (Northern Italy) in 2018 detected by entomological surveillance. Front. Vet. Sci. 2020, 7, 243. [Google Scholar] [CrossRef]
- Boukraa, S.; De La Grandière, M.; Bawin, T.; Raharimalala, F.; Zimmer, J.; Haubruge, E.; Thiry, E.; Francis, F. Diversity and ecology survey of mosquitoes potential vectors in Belgian equestrian farms: A threat prevention of mosquito-borne equine arboviruses. Prev. Vet. Med. 2016, 124, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.; Archer, D.; Torr, S.; Solomon, T.; Baylis, M. Potential vectors of equine arboviruses in the UK. Vet. Rec. 2016, 180, 103825. [Google Scholar] [CrossRef]
- Zannoli, S.; Sambri, V. West Nile Virus and Usutu Virus co-circulation in Europe: Epidemiology and implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). Wilson method for calculating confidence intervals for proportions. In Engineering Statistics Handbook; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Orawo, L.A. Confidence intervals for the binomial proportion: A comparison of four methods. Open J. Stat. 2021, 11, 806–816. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics; IBM: Armonk, NY, USA, 2025; Available online: https://www.ibm.com/products/spss-statistics (accessed on 15 April 2025).
- Söderroos, D.; Ignell, R.; Andersen, H.; Bergvall, K.; Riihimäki, M. The effect of insect bite hypersensitivity on movement activity and behaviour of the horse. Animals 2023, 13, 1283. [Google Scholar] [CrossRef]
- Honnen, A.; Kypke, J.; Hölker, F.; Monaghan, M. Artificial light at night influences clock-gene expression, activity, and fecundity in the mosquito Culex pipiens f. molestus. Sustainability 2019, 11, 6220. [Google Scholar] [CrossRef]
- Bergmann, F.; Trachsel, D.S.; Stoeckle, S.D.; Sierra, J.B.; Lübke, S.; Groschup, M.H.; Gehlen, H.; Ziegler, U. Seroepidemiological survey of West Nile Virus infections in horses from Berlin/Brandenburg and North Rhine-Westphalia, Germany. Viruses 2022, 14, 243. [Google Scholar] [CrossRef]
- Long, M.T.; Gibbs, E.P.; Mellencamp, M.W.; Bowen, R.A.; Seino, K.K.; Zhang, S.; Beachboard, S.E.; Humphrey, P.P. Efficacy, duration, and onset of immunogenicity of a West Nile Virus vaccine, live flavivirus chimera, in horses with a clinical disease challenge model. Equine Vet. J. 2007, 39, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.C. Animal and human vaccines against West Nile Virus. Pathogens 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, F.; Bolici, F.; Fafangel, M.; Jovanovic, V.; Socan, M.; Klepac, P.; Plavsa, D.; Vasic, M.; Bella, A.; Diana, G.; et al. West Nile Virus in Europe: After action reviews of preparedness and response to the 2018 transmission season in Italy, Slovenia, Serbia and Greece. Glob. Health 2020, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Magallanes, S.; Llorente, F.; Ruiz-López, M.J.; la Puente, J.M.-D.; Soriguer, R.; Calderon, J.; Jímenez-Clavero, M.Á.; Aguilera-Sepúlveda, P.; Figuerola, J. Long-term serological surveillance for West Nile and Usutu Virus in horses in South-West Spain. One Health 2023, 17, 100578. [Google Scholar] [CrossRef]
- Leblond, A.; Lecollinet, S. Clinical screening of horses and early warning for West Nile Virus. Equine Vet. Educ. 2017, 29, 325–327. [Google Scholar] [CrossRef]
- Kolodziejek, J.; Jungbauer, C.; Aberle, S.W.; Allerberger, F.; Bagó, Z.; Camp, J.V.; Dimmel, K.; de Heus, P.; Kolodziejek, M.; Schiefer, P.; et al. Integrated analysis of human-animal-vector surveillance: West Nile Virus infections in Austria, 2015–2016. Emerg. Microbes Infect. 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Petrović, T.; Šekler, M.; Petrić, D.; Lazić, S.; Debeljak, Z.; Vidanović, D.; Ćupina, A.I.; Lazić, G.; Lupulović, D.; Kolarević, M.; et al. Methodology and results of integrated WNV surveillance programmes in Serbia. PLoS ONE 2018, 13, e0195439. [Google Scholar] [CrossRef]
- Rios, L.M.; Sheu, J.J.; Day, J.F.; Maruniak, J.E.; Seino, K.; Zaretsky, H.; Long, M.T. Environmental risk factors associated with West Nile Virus clinical disease in Florida horses. Med. Vet. Entomol. 2009, 23, 357–366. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Arenas-Montes, A.; Napp, S.; Jaén-Téllez, J.A.; Fernández-Morente, M.; Fernández-Molera, V.; Arenas, A. Seroprevalence and risk factors associated with West Nile Virus in horses from Andalusia, Southern Spain. Vet. Microbiol. 2012, 160, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Sandoz, A.; Lefebvre, G.; Zeller, H.; Bicout, D.J. Remote sensing-based identification of environmental risk factors associated with West Nile disease in horses in Camargue, France. Prev. Vet. Med. 2007, 79, 20–31. [Google Scholar] [CrossRef]
- Ganzenberg, S.; Sieg, M.; Ziegler, U.; Pfeffer, M.; Vahlenkamp, T.W.; Hörügel, U.; Groschup, M.H.; Lohmann, K.L. Seroprevalence and risk factors for equine West Nile Virus infections in Eastern Germany, 2020. Viruses 2022, 14, 1191. [Google Scholar] [CrossRef]
- Selim, A.; Megahed, A.; Kandeel, S.; Alouffi, A.; Alshazi, M. West Nile Virus seroprevalence and associated risk factors among horses in Egypt. Sci. Rep. 2021, 11, 4496. [Google Scholar] [CrossRef]
- Cendejas, P.; Goodman, A. Vaccination and Control Methods of West Nile Virus Infection in Equids and Humans. Vaccines 2024, 12, 485. [Google Scholar] [CrossRef]
- Olagunju, E.; Ayewumi, I.; Adeleye, B. Effects of Livestock-Keeping on the Transmission of Mosquito-Borne Diseases. Zoonoses 2024, 4, 966. [Google Scholar] [CrossRef]
- Naveed, A.; Eertink, L.; Wang, D.; Li, F. Lessons Learned from West Nile Virus Infection: Vaccinations in Equines and Their Implications for One Health Approaches. Viruses 2024, 16, 781. [Google Scholar] [CrossRef]
- Dapari, R.; Fadzil, M.; Hanzir, M.; Jais, J.; Safarudin, N.; Albar, A. Factors Associated with Mosquito Control among Construction Workers: A Systematic Review. PLoS ONE 2024, 19, e0303330. [Google Scholar] [CrossRef]
- Tai, Z.; Connelly, C.; Lange, S.; Foley, N.; De, J.; Rivera, L.; Lozano, S.; Nett, R. A Scoping Review to Determine if Adverse Human Health Effects Are Associated with Use of Organophosphates for Mosquito Control. J. Med. Entomol. 2024, 62, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.; Rudd, S.; Mena, O.; Hudspeth, P.; Barboza-Corona, J.; Park, H.; Bideshi, D. The Perpetual Vector Mosquito Threat and Its Eco-Friendly Nemeses. Biology 2024, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Franco, A.; Godinho, F.; Huff, R.; Candido, D.; Da Cruz Cardoso, J.; Hua, X.; Claro, I.; Morais, P.; Franceschina, C.; et al. Molecular Epidemiology of Western Equine Encephalitis Virus, South America, 2023–2024. Emerg. Infect. Dis. 2024, 30, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, O.; Tolsá-García, M.; Arana-Guardía, R.; Rodríguez-Valencia, V.; Talaga, S.; Pontifes, P.; Machain-Williams, C.; Suzán, G.; Roiz, D. Seasonal Mosquito (Diptera: Culicidae) Dynamics and the Influence of Environmental Variables in a Land Use Gradient from Yucatán, Mexico. Acta Trop. 2024, 257, 107275. [Google Scholar] [CrossRef] [PubMed]
- Velde, F.; Overgaard, H.; Bastien, S. An Integrated Human Behavioral Model for Mosquito-Borne Disease Control: A Scoping Review of Behavior Change Theories Used to Identify Key Behavioral Determinants. Heliyon 2024, 10, e26488. [Google Scholar] [CrossRef]
- Mader, E.; Clements, N.; Lehane, Á.; Gangloff-Kaufmann, J.; Crans, S.; Horton, C.; Safi, A. A Qualitative Analysis of Perceived Risks and Benefits of Mosquito Abatement and Bite Prevention Strategies in Northeastern U.S. Communities. J. Med. Entomol. 2024, 62, 435–448. [Google Scholar] [CrossRef]
- Al-Eitan, L.; Alnimri, M.; Ali, H.; Alkhawaldeh, M.; Mihyar, A. Mosquito-Borne Diseases: Assessing Risk and Strategies to Control Their Spread in the Middle East. J. Biosaf. Biosecurity 2024, 6, 1–12. [Google Scholar] [CrossRef]
- Vissani, M.; Alamos, F.; Tordoya, M.; Minatel, L.; Schammas, J.; Santos, M.; Trono, K.; Barrandeguy, M.; Balasuriya, U.; Carossino, M. Outbreak of Western Equine Encephalitis Virus Infection Associated with Neurological Disease in Horses Following a Nearly 40-Year Intermission Period in Argentina. Viruses 2024, 16, 1594. [Google Scholar] [CrossRef]
- Nejati, J.; Azari-Hamidian, S.; Oshaghi, M.; Vatandoost, H.; White, V.; Moosa-Kazemi, S.; Bueno-Marí, R.; Hanafi-Bojd, A.; Endersby-Harshman, N.; Axford, J.; et al. The Monsoon-Associated Equine South African Pointy Mosquito Aedes caballus: The First Comprehensive Record from Southeastern Iran with a Description of Ecological, Morphological, and Molecular Aspects. PLoS ONE 2024, 19, e0298412. [Google Scholar] [CrossRef]
- Wouters, R.; Beukema, W.; Schrama, M.; Biesmeijer, K.; Braks, M.; Helleman, P.; Schaffner, F.; Van Slobbe, J.; Stroo, A.; Van Der Beek, J. Local Environmental Factors Drive Distributions of Ecologically-Contrasting Mosquito Species (Diptera: Culicidae). Sci. Rep. 2024, 14, 19315. [Google Scholar] [CrossRef]
- Ripoll, L.; Iserte, J.; Cerrudo, C.; Presti, D.; Serrat, J.; Poma, R.; Mangione, F.; Micheloud, G.; Gioria, V.; Berrón, C.; et al. Insect-Specific RNA Viruses Detection in Field-Caught Aedes aegypti Mosquitoes from Argentina Using NGS Technology. PLoS Neglected Trop. Dis. 2025, 19, e0012792. [Google Scholar] [CrossRef]
- Chuchuy, A.; Rodriguero, M.; Alonso, A.; Stein, M.; Micieli, M. Wolbachia Infection in Natural Mosquito Populations from Argentina. Parasitol. Res. 2024, 123, 343. [Google Scholar] [CrossRef]
- Abbasi, M.; Yousefi, S. Assessing Insecticide Susceptibility of Culex pipiens Linnaeus (Diptera: Culicidae) in the Aras River Basin: Implications for Disease Control. BMC Infect. Dis. 2025, 25, 247. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hoffmann, A.; Champer, J. Gene Drive and Symbiont Technologies for Control of Mosquito-Borne Diseases. Annu. Rev. Entomol. 2024, 70, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Huang, M.; Zhao, J. Innovative Strategies and Challenges in Mosquito-Borne Disease Control Amidst Climate Change. Front. Microbiol. 2024, 15, 1488106. [Google Scholar] [CrossRef] [PubMed]

| No. | Assessment Criterion | Response Options |
|---|---|---|
| 1 | Presence of stagnant water within 100 m of the stable | Yes/No |
| 2 | Presence of open containers collecting rainwater | Yes/No |
| 3 | Vegetation density and maintenance status | Dense/Moderate/Well-maintained |
| 4 | Use of insect screens in stable buildings | Yes/No |
| 5 | Routine use of insect repellents or insecticides | Yes/No (specify type) |
| 6 | Horses kept outdoors overnight | Yes/No (Open pasture/Sheltered pen) |
| 7 | Stable hygiene and structural maintenance | Good/Moderate/Poor |
| 8 | Presence of manure waste management protocols | Yes/No |
| 9 | Personnel knowledge about mosquito-borne WNV transmission | Yes/No |
| 10 | Neurological clinical cases in horses in past 2 years | None/Suspected/Confirmed |
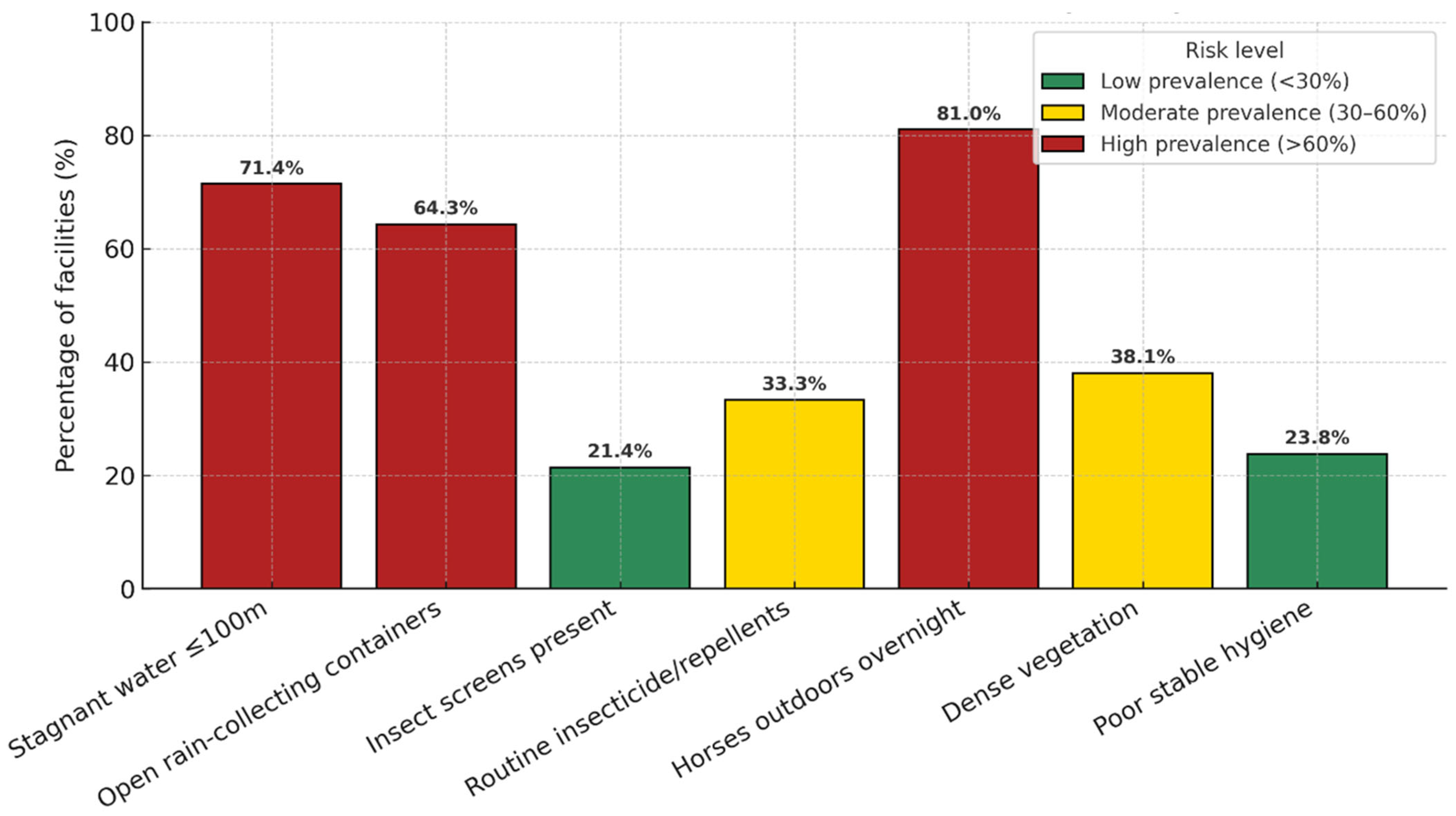
| Indicator | n/N | % | 95% CI |
|---|---|---|---|
| Stagnant water ≤ 100 m | 30/42 | 71.4 | 56.4–82.8 |
| Open rain-collecting containers | 27/42 | 64.3 | 49.2–77.0 |
| Insect screens present | 9/42 | 21.4 | 11.7–35.9 |
| Routine repellents/insecticides | 14/42 | 33.3 | 21.0–48.4 |
| Horses outdoors overnight | 34/42 | 81.0 | 66.7–90.0 |
| Dense/overgrown vegetation | 16/42 | 38.1 | 25.0–53.2 |
| Poor stable hygiene | 10/42 | 23.8 | 13.5–38.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, P.; Stanga, L.; Chirila, A.; Iorgoni, V.; Gligor, A.; Ciresan, A.; Florea, B.; Bota, C.; Cocioba, V.; Popa, I.; et al. Mosquito Exposure Risks in Equine Facilities: An Environmental–Managerial Assessment in Western Romania. Microorganisms 2025, 13, 2637. https://doi.org/10.3390/microorganisms13112637
Nistor P, Stanga L, Chirila A, Iorgoni V, Gligor A, Ciresan A, Florea B, Bota C, Cocioba V, Popa I, et al. Mosquito Exposure Risks in Equine Facilities: An Environmental–Managerial Assessment in Western Romania. Microorganisms. 2025; 13(11):2637. https://doi.org/10.3390/microorganisms13112637
Chicago/Turabian StyleNistor, Paula, Livia Stanga, Andreia Chirila, Vlad Iorgoni, Alexandru Gligor, Alexandru Ciresan, Bogdan Florea, Carina Bota, Vlad Cocioba, Ionela Popa, and et al. 2025. "Mosquito Exposure Risks in Equine Facilities: An Environmental–Managerial Assessment in Western Romania" Microorganisms 13, no. 11: 2637. https://doi.org/10.3390/microorganisms13112637
APA StyleNistor, P., Stanga, L., Chirila, A., Iorgoni, V., Gligor, A., Ciresan, A., Florea, B., Bota, C., Cocioba, V., Popa, I., Orghici, G., Iancu, I., Maris, C. H., Degi, J., & Herman, V. (2025). Mosquito Exposure Risks in Equine Facilities: An Environmental–Managerial Assessment in Western Romania. Microorganisms, 13(11), 2637. https://doi.org/10.3390/microorganisms13112637









