Microbial Diversity Analysis of Soil in the Rhizosphere of Securidaca longipedunculata (African Violet Tree)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Isolation of Rhizospheric Microorganisms
2.3. Colony Selection and Biochemical Characterization
2.3.1. Colony Selection
2.3.2. Catalase Activity
2.3.3. Urease Test
2.3.4. Methyl Red Test
2.3.5. Oxidase Activity
2.4. Determination of Plant Growth-Promoting Properties: In Vitro
2.4.1. Growth in Nitrogen Free Environment
2.4.2. Phosphate Solubilization
2.4.3. Indole Acetic Acid (IAA) Production
2.4.4. Ammonia Production
2.5. DNA Extraction and Polymerase Chain Reaction (PCR) Amplification
2.6. Antifungal Assays
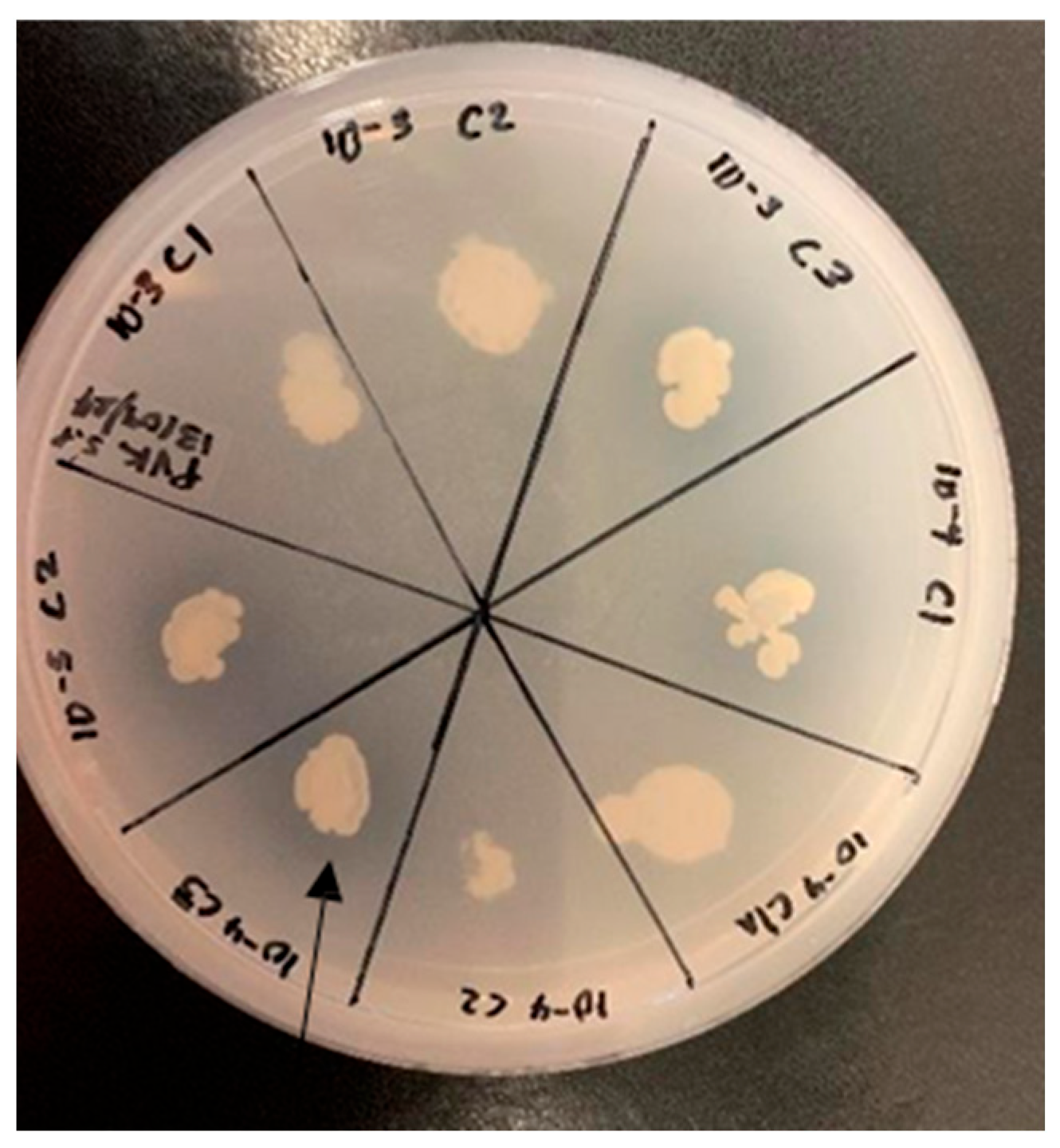
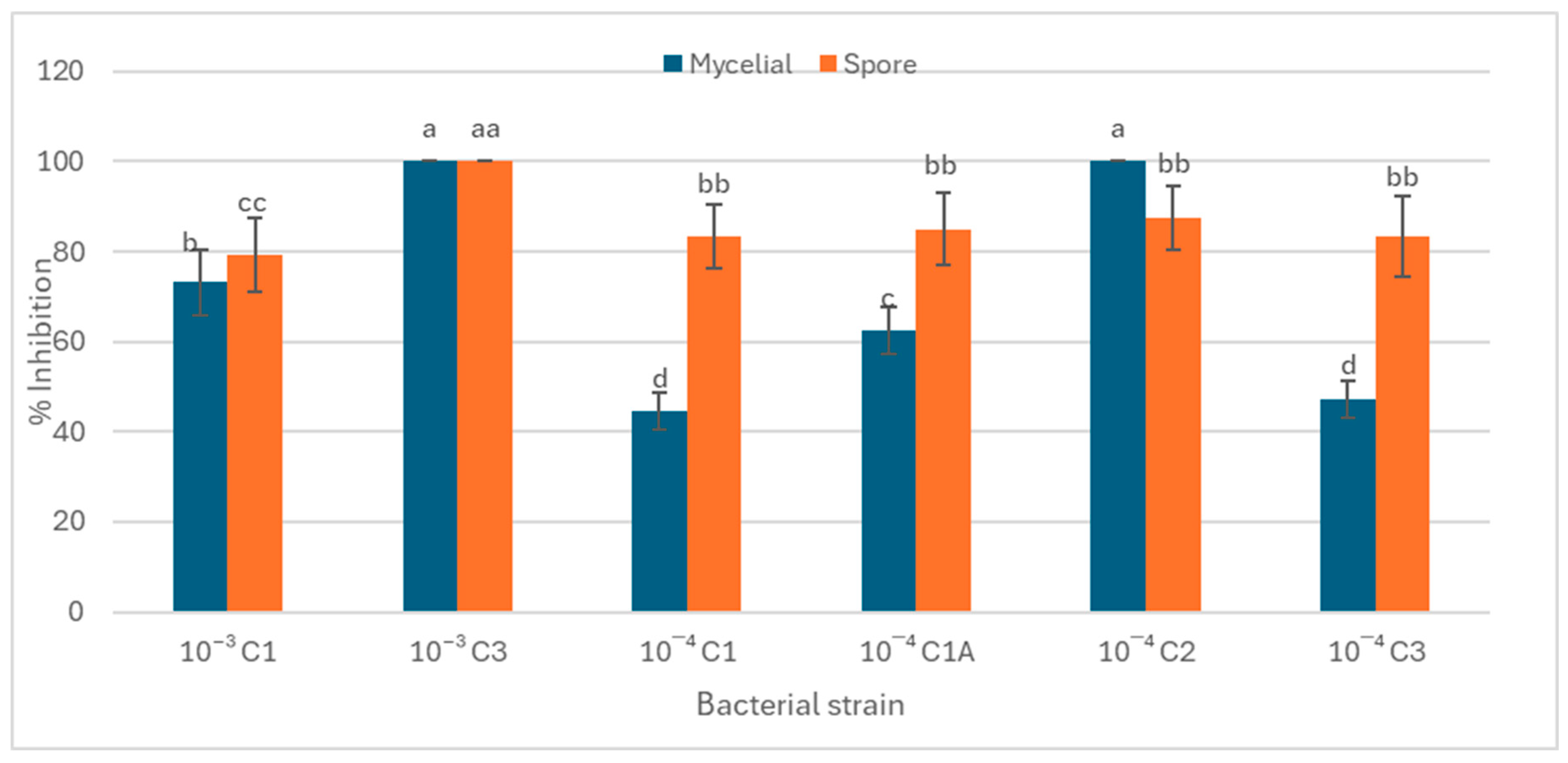
2.6.1. Mycelial Growth Inhibition Assay
2.6.2. Spore Germination Inhibition Assay
2.7. In Vivo Plant Growth Promotion Assays
2.8. Statistical Analysis
3. Results
3.1. Isolation, Morphological and Biochemical Characterization of Bacterial Endophytes
3.2. Plant Growth Promotion: In Vitro
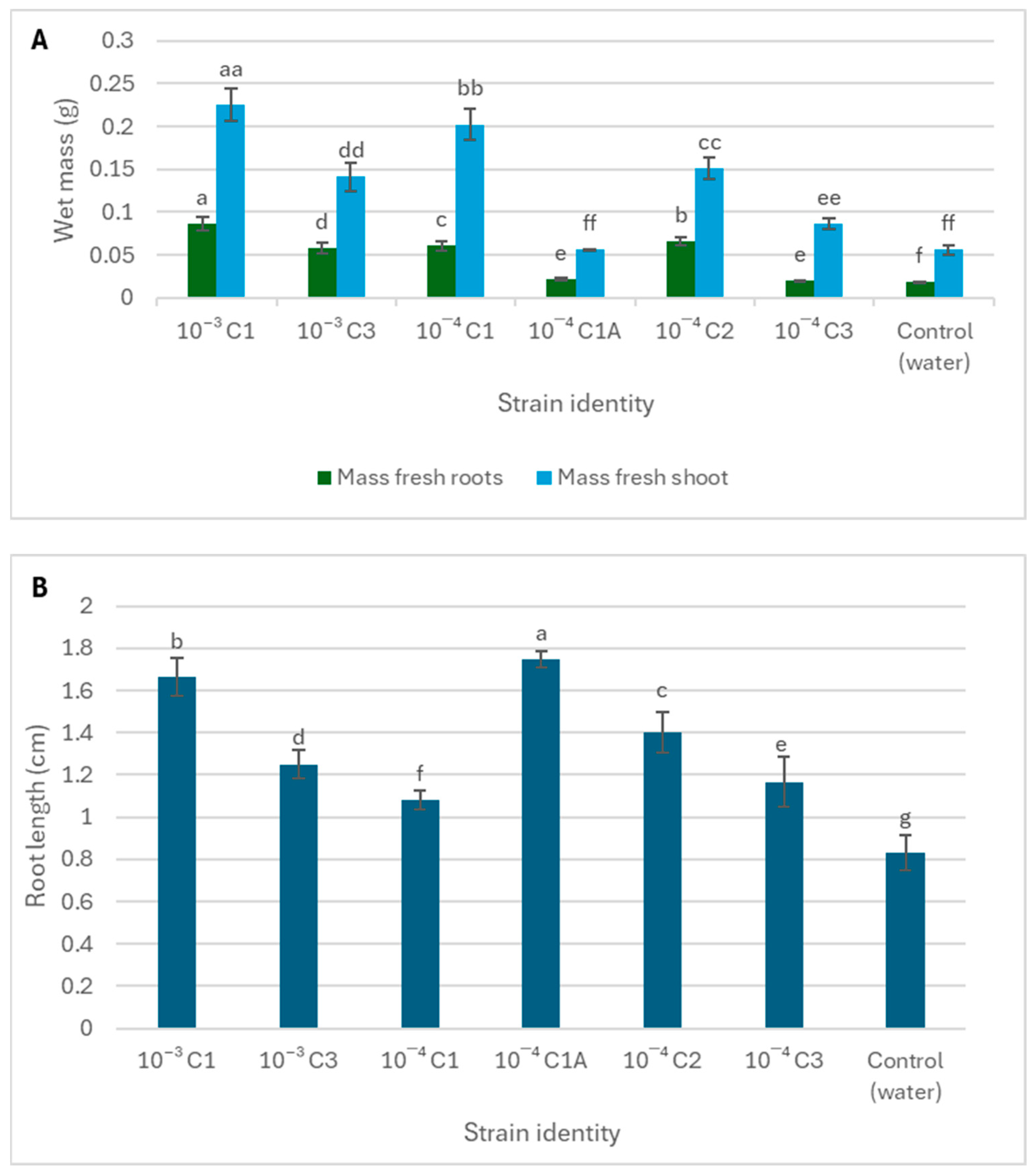
3.3. The Role of Rhizospheric Bacteria in Antifungal Activity and In Vivo Plant Growth Promotion
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IAA | Indole acetic acid |
| HGT | Horizontal gene transfer |
| PDA | Potato dextrose agar |
| PDB | Potato dextrose broth |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| PVK | Pikovskaya |
| PGP | Plant growth promoting |
| PCR | Polymerase chain reaction |
| dNTPs | Deoxynucleotide triphosphates |
| RGI | Relative germination inhibition |
References
- Lijalem, T.; Feyissa, T. In vitro propagation of Securidaca longipedunculata (Fresen) from shoot tip: An endangered medicinal plant. J. Genet. Eng. Biotechnol. 2020, 18, 3. [Google Scholar] [CrossRef]
- Shai, K.; Lebelo, S.L.; Manyeula, F.; Mabelebele, M.; Sebola, A.N. Assessment of Securidaca longipedunculata (violet tree) effect on Semen Quality and Blood Sex Hormone Levels in Indigenous Goats. Vet. Anim. Sci. 2025, 30, 100531. [Google Scholar] [CrossRef]
- Fomnya, H.J.; Ngulde, S.I.; Saka, S.; Gana, S.M.; Umaru, B.; Auwal, M.S. Anticonvulsant and anxiolytic activities of methanol extract of Securidaca longipedunculata Fres. root bark in mice. J. Appl. Pharm. Sci. 2023, 13, 176–183. [Google Scholar] [CrossRef]
- Daboué, E.M.; Béné, A.; Dimobe, K.; Dayamba, D.S.; Zouré, A.B.; Ouattara, B.; Sabo, P.; Tuina, S.; Neya, O.; Vinceti, B.; et al. Variability in fruit morphology and germination capacity of the tropical medicinal species Securidaca longipedunculata Fres. Seeds 2024, 3, 639–655. [Google Scholar] [CrossRef]
- Mongalo, N.I.; McGaw, L.J.; Finnie, J.F.; Van Staden, J. Securidaca longipedunculata Fresen (Polygalaceae): A review of its ethnomedicinal uses, phytochemistry, pharmacological properties and toxicology. J. Ethnopharmacol. 2015, 165, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Mubin, S.; Hassan, S.S.U.; Tagde, P.; Ullah, O.; Rahman, M.H.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical profiling and bio-potentiality of genus scutellaria: Biomedical approach. Biomolecules 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Neelu Joshi, N.J.; Alok Shukla, A.S.; Nailwal, T.K. Taxonomic and phytomedicinal properties of Oroxylum indicum (L.) Vent: A wonderful gift of nature. J. Med. Plants Res. 2014, 8, 1148–1155. [Google Scholar] [CrossRef]
- Abubakar, U.S.; Khalifa, B.I.; Abdu, F.; Sanusi, M.; Gawuna, T.A.; Adamu, J.G.; Rogo, S.S. Threatened medicinal plants of Kano flora and the need for urgent conservation. Int. J. Conserv. Sci. 2018, 9, 173. [Google Scholar]
- Obode, O.C.; Adebayo, A.H.; Omonhinmin, C.A.; Yakubu, O.F. A systematic review of medicinal plants used in Nigeria for hypertension management. Int. J. Pharm. Res. 2020, 12, 2231. [Google Scholar]
- Saranraj, P.; Sivasakthi, S.; Deepa, M.S. Phytochemistry of pharmacologically important medicinal plants—A review. Int. J. Curr. Res. Chem. Pharm. Sci. 2016, 3, 56–66. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W. The role of botanical gardens in scientific research, conservation, and citizen science. Plant Divers. 2018, 40, 181–188. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of beneficial microorganisms in soil remediation and quality improvement of medicinal plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.; Pieterse, C.M. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Giron, D.; Dedeine, F.; Dubreuil, G.; Huguet, E.; Mouton, L.; Outreman, Y.; Vavre, F.; Simon, J.C. Influence of microbial symbionts on plant–insect interactions. In Advances in Botanical Research; Academic Press: Cambridge, UK, 2017; Volume 81, pp. 225–257. [Google Scholar]
- Lyu, D.; Msimbira, L.A.; Nazari, M.; Antar, M.; Pagé, A.; Shah, A.; Monjezi, N.; Zajonc, J.; Tanney, C.A.; Backer, R.; et al. The coevolution of plants and microbes underpins sustainable agriculture. Microorganisms 2021, 9, 1036. [Google Scholar] [CrossRef]
- Boufares, K.; Kouadria, M.; Karima, M.; Merdjet, Y.N. Investigating the effects of plant growth-promoting rhizobacteria isolates on germination and physiology status of durum wheat under salt stress. Acta Agric. Slov. 2023, 119, 1–9. [Google Scholar] [CrossRef]
- Lehman, D.C. Gram-Negative Bacteria. In Textbook of Diagnostic Microbiology-E-Book: Textbook of Diagnostic Microbiology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; p. 181. [Google Scholar]
- Bangun, A. Taxonomic Study of Pasteurella Anatipestifer; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 1981. [Google Scholar]
- Oakey, H.J.; Ellis, J.T.; Gibson, L.F. A biochemical protocol for the differentiation of current genomospecies of Aeromonas. Zentralblatt Bakteriol. 1996, 284, 32–46. [Google Scholar] [CrossRef]
- Jensen, H. The azotobacteriaceae. Bacteriol. Rev. 1954, 18, 195–214. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of Phosphorus in Soil in Connection with Vital Activity of Some Microbial Species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Sarwar, M.; Kremer, R.J. Determination of bacterially derived auxins using a microplate method. Lett. Appl. Microbiol. 1995, 20, 282–285. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef]
- Lanyi, B. 1 Classical and rapid identification methods for medically important bacteria. In Methods in Microbiology; Academic Press: Cambridge, UK, 1988; Volume 19, pp. 1–67. [Google Scholar]
- Jabborova, D.; Mamarasulov, B.; Davranov, K.; Enakiev, Y.; Bisht, N.; Singh, S.; Stoyanov, S.; Garg, A.P. Diversity and plant growth properties of rhizospheric bacteria associated with medicinal plants. Indian J. Microbiol. 2024, 64, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sharma, R.K.; Sharma, V.; Singh, T.; Kumar, R.; Kumari, D. Isolation, morphological identification and in vitro antibacterial activity of endophytic bacteria isolated from Azadirachta indica (neem) leaves. Vet. World 2017, 10, 510. [Google Scholar] [CrossRef]
- Verma, P.; Hiremani, N.S.; Gawande, S.P.; Sain, S.K.; Nagrale, D.T.; Narkhedkar, N.G.; Prasad, Y.G. Modulation of plant growth and antioxidative defense system through endophyte biopriming in cotton (Gossypium spp.) and non-host crops. Heliyon 2022, 8, 9487. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.L.; Kour, D.; Kaur, T.; Sheikh, I.; Yadav, A.N.; Kumar, V.; Suman, A.; Dhaliwal, H.S. Endophytic microbes from diverse wheat genotypes and their potential biotechnological applications in plant growth promotion and nutrient uptake. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 969–979. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Ivashechkin, A.A.; Alekhin, A.I.; Sergeeva, Y.E. Fungal spores: Dormancy, germination, chemical composition, and role in biotechnology. Appl. Biochem. Microbiol. 2012, 48, 1–11. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Jan, M.; Muhammad, S.; Jin, W.; Zhong, W.; Zhang, S.; Lin, Y.; Zhou, Y.; Liu, J.; Liu, H.; Munir, R. Modulating root system architecture: Cross-talk between auxin and phytohormones. Front. Plant Sci. 2024, 15, 1343928. [Google Scholar] [CrossRef] [PubMed]
- Podgórska-Kryszczuk, I.; Solarska, E.; Kordowska-Wiater, M. Biological control of Fusarium culmorum, Fusarium graminearum and Fusarium poae by antagonistic yeasts. Pathogens 2022, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Duncan, N.; Horta, A.; Conallin, J.; Marsden, T.; Lynch, A.J.; Stuart, I. Reframing Fish Passage Prioritization for Human Nutrition Outcomes. Environ. Manag. 2025, 75, 3401–3417. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Christensen, H.; Olsen, J.E.D. Introduction to Phylogenetic Analysis of Molecular Sequence Data. In Introduction to Bioinformatics in Microbiology; Springer International Publishing: Cham, Switzerland, 2023; pp. 111–130. [Google Scholar]
- Farina, D.; Bianco, A.; Manzulli, V.; Castellana, S.; Parisi, A.; Caruso, M.; Fraccalvieri, R.; Serrecchia, L.; Rondinone, V.; Pace, L.; et al. Antimicrobial and Phylogenomic Characterization of Bacillus cereus Group Strains Isolated from Different Food Sources in Italy. Antibiotics 2024, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Goraj, W.; Szafranek-Nakonieczna, A.; Grządziel, J.; Polakowski, C.; Słowakiewicz, M.; Zheng, Y.; Gałązka, A.; Stępniewska, Z.; Pytlak, A. Microbial involvement in carbon transformation via CH4 and CO2 in saline sedimentary pool. Biology 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Bruno, L.B.; Banu, J.R. Alleviation of environmental stress in plants: The role of beneficial Pseudomonas spp. Crit. Rev. Environ. Sci. Technol. 2017, 47, 372–407. [Google Scholar] [CrossRef]
- Leitão, F.; Alves, M.; Henriques, I.; Pinto, G. Endophytic Bacterial Consortia Isolated from Disease-Resistant Pinus pinea L. Increase Germination and Plant Quality in Susceptible Pine Species (Pinus radiata D. Don). Forests 2025, 16, 1161. [Google Scholar] [CrossRef]
- Suman, A.; Govindasamy, V.; Ramakrishnan, B.; Aswini, K.; SaiPrasad, J.; Sharma, P.; Pathak, D.; Annapurna, K. Microbial community and function-based synthetic bioinoculants: A perspective for sustainable agriculture. Front. Microbiol. 2022, 12, 805498. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Kour, D.; Kour, H.; Khan, S.S.; Khan, R.T.; Bhardwaj, M.; Kailoo, S.; Kumari, C.; Rasool, S.; Yadav, A.N.; Sharma, Y.P. Biodiversity and functional attributes of rhizospheric microbiomes: Potential tools for sustainable agriculture. Curr. Microbiol. 2023, 80, 192. [Google Scholar] [CrossRef]

| Selective bacterial strains | ||||||
| Characteristics | ||||||
| 10−3 C1 | 10−3 C3 | 10−4 C1 | 10−4 C1A | 10−4 C2 | 10−4 C3 | |
| Morphological characterization | ||||||
| Gram Staining | Positive | Negative | Negative | Positive | Positive | Positive |
| Shape | Rod | Rod | Rod | Rod | Rod | Rod |
| Form | Round | Round | Round | Round | Round | Round |
| Surface | Smooth | Shiny | Smooth | Smooth | Smooth | Smooth |
| Color | Creamy white | Creamy white | Creamy white | Creamy white | Creamy white | Creamy white |
| Size | Medium | Small | Small | Medium | Medium | Small |
| Elevation | Entire | Entire | Entire | Entire | Entire | Entire |
| Opacity | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque |
| Biochemical characterization | ||||||
| Catalase | + | + | + | + | + | + |
| Urea | − | + | − | − | − | + |
| Methyl Red | + | + | + | + | + | + |
| Oxidase | + | + | + | + | + | + |
| Strain | Phosphate Solubilization | Growth in Nitrogen Free Environment | Ammonia Solubilization | IAA Production |
|---|---|---|---|---|
| 10−3 C1 | − | + | + | − |
| 10−3 C3 | + | + | + | − |
| 10−4 C1 | + | + | + | + |
| 10−4 C1A | − | − | + | − |
| 10−4 C2 | − | − | + | − |
| 10−4 C3 | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zondi, S.; Serepa-Dlamini, M.H.; Maumela, P. Microbial Diversity Analysis of Soil in the Rhizosphere of Securidaca longipedunculata (African Violet Tree). Microorganisms 2025, 13, 2636. https://doi.org/10.3390/microorganisms13112636
Zondi S, Serepa-Dlamini MH, Maumela P. Microbial Diversity Analysis of Soil in the Rhizosphere of Securidaca longipedunculata (African Violet Tree). Microorganisms. 2025; 13(11):2636. https://doi.org/10.3390/microorganisms13112636
Chicago/Turabian StyleZondi, Sphelele, Mahloro Hope Serepa-Dlamini, and Pfariso Maumela. 2025. "Microbial Diversity Analysis of Soil in the Rhizosphere of Securidaca longipedunculata (African Violet Tree)" Microorganisms 13, no. 11: 2636. https://doi.org/10.3390/microorganisms13112636
APA StyleZondi, S., Serepa-Dlamini, M. H., & Maumela, P. (2025). Microbial Diversity Analysis of Soil in the Rhizosphere of Securidaca longipedunculata (African Violet Tree). Microorganisms, 13(11), 2636. https://doi.org/10.3390/microorganisms13112636







