The Effects of Feeding ybfQ-Deficient Gut Bacteria on Radio-Tolerance in Symbiotic Caenorhabditis elegans: The Key Role of Isoscoparin
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Culture and Synchronization
2.2. Worm Radiation Exposure
2.3. Worm Chemotaxis and Dietary Assays
2.4. Analysis of Worm Radio-Tolerance
2.5. Assay of Oxidative Stress in Worms
2.6. Worm Transcriptomic Analysis
2.7. Bacterial Growth and Manipulation
2.8. Establishment of Transgenic Bacterial Strains
2.9. Bacterial Metabolomics and Transcriptomic and Analysis
2.10. Quantification and Statistical Analysis
3. Results
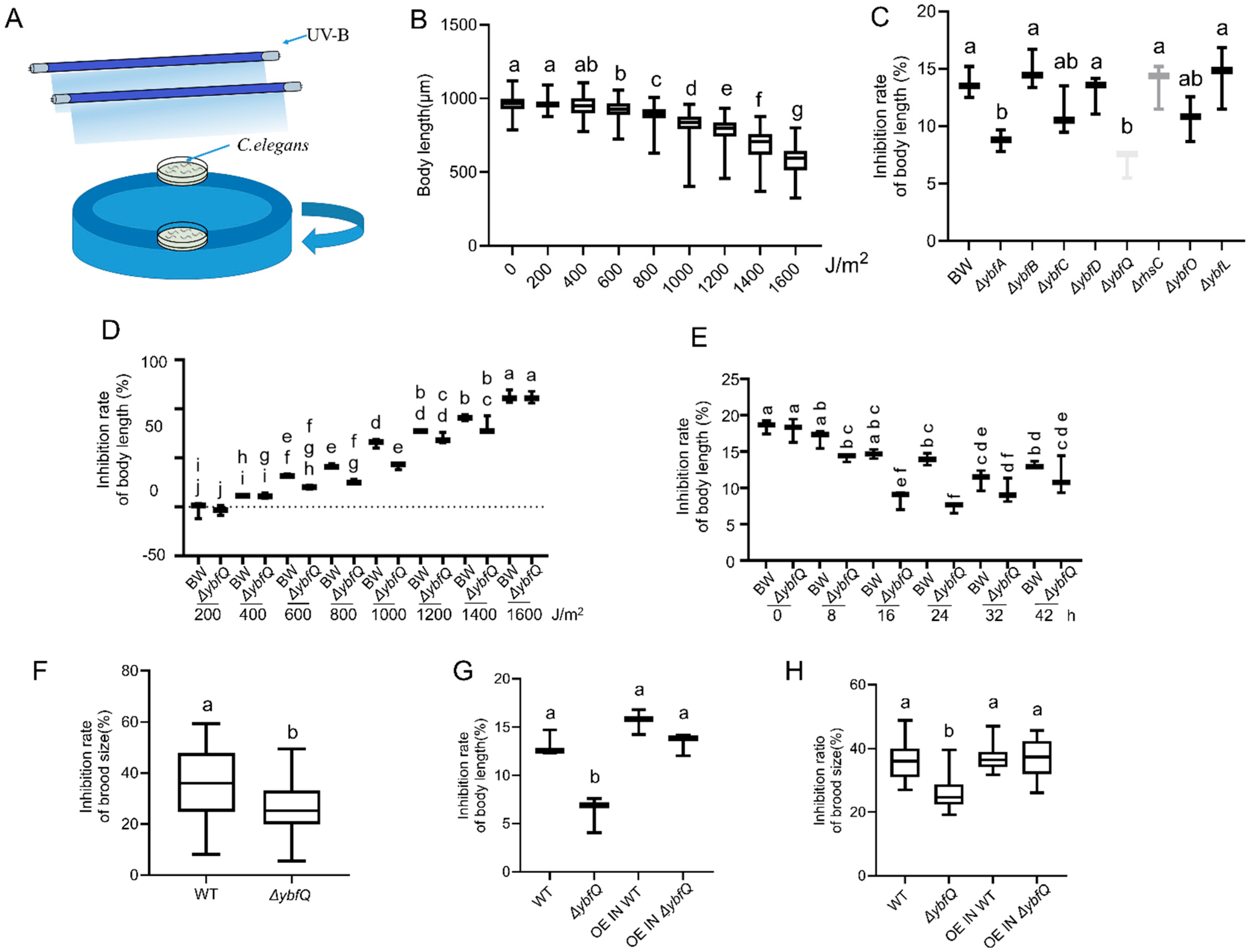
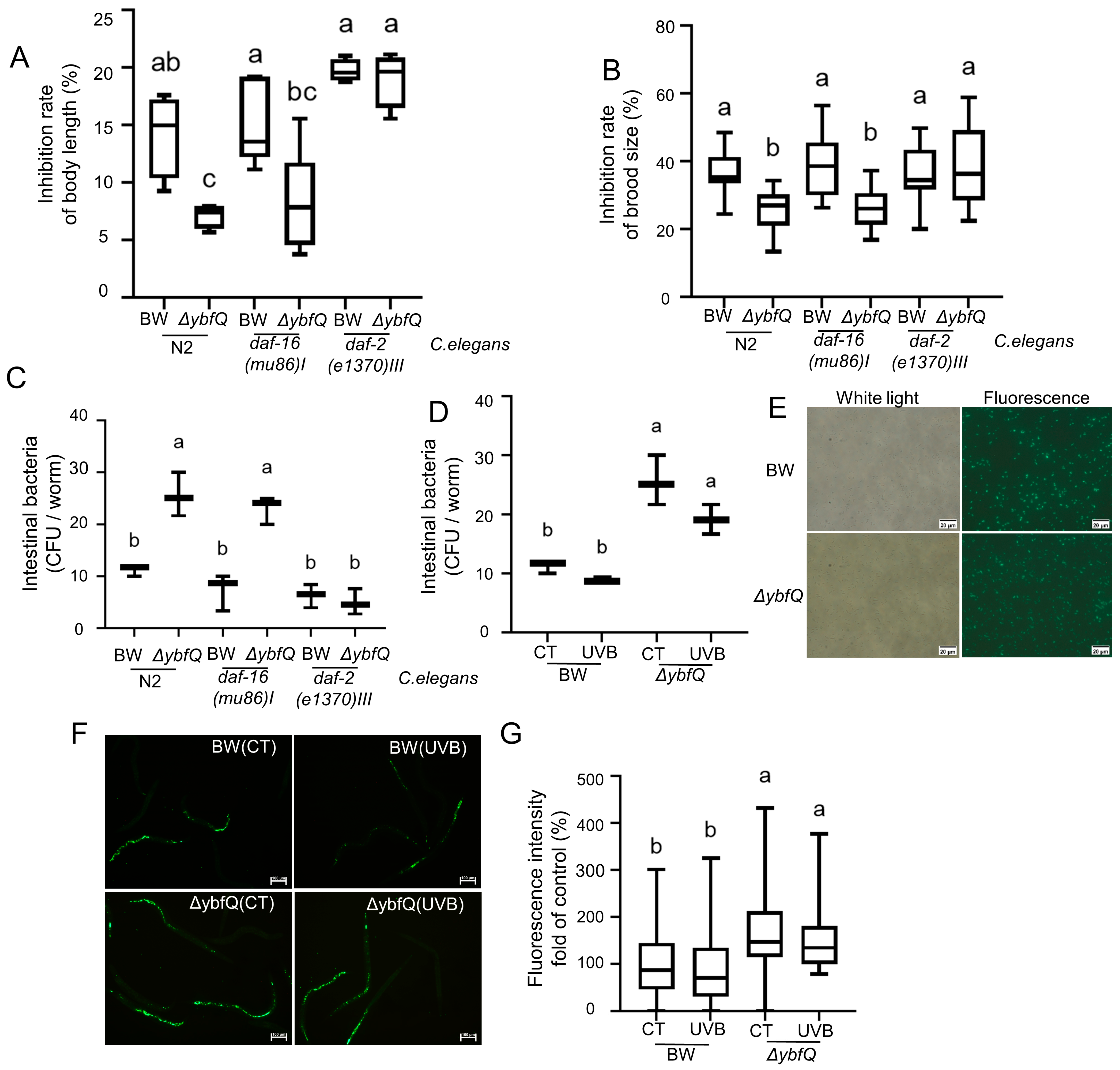
3.1. Radio-Tolerance of Worms Fed with ΔybfQ Bacteria
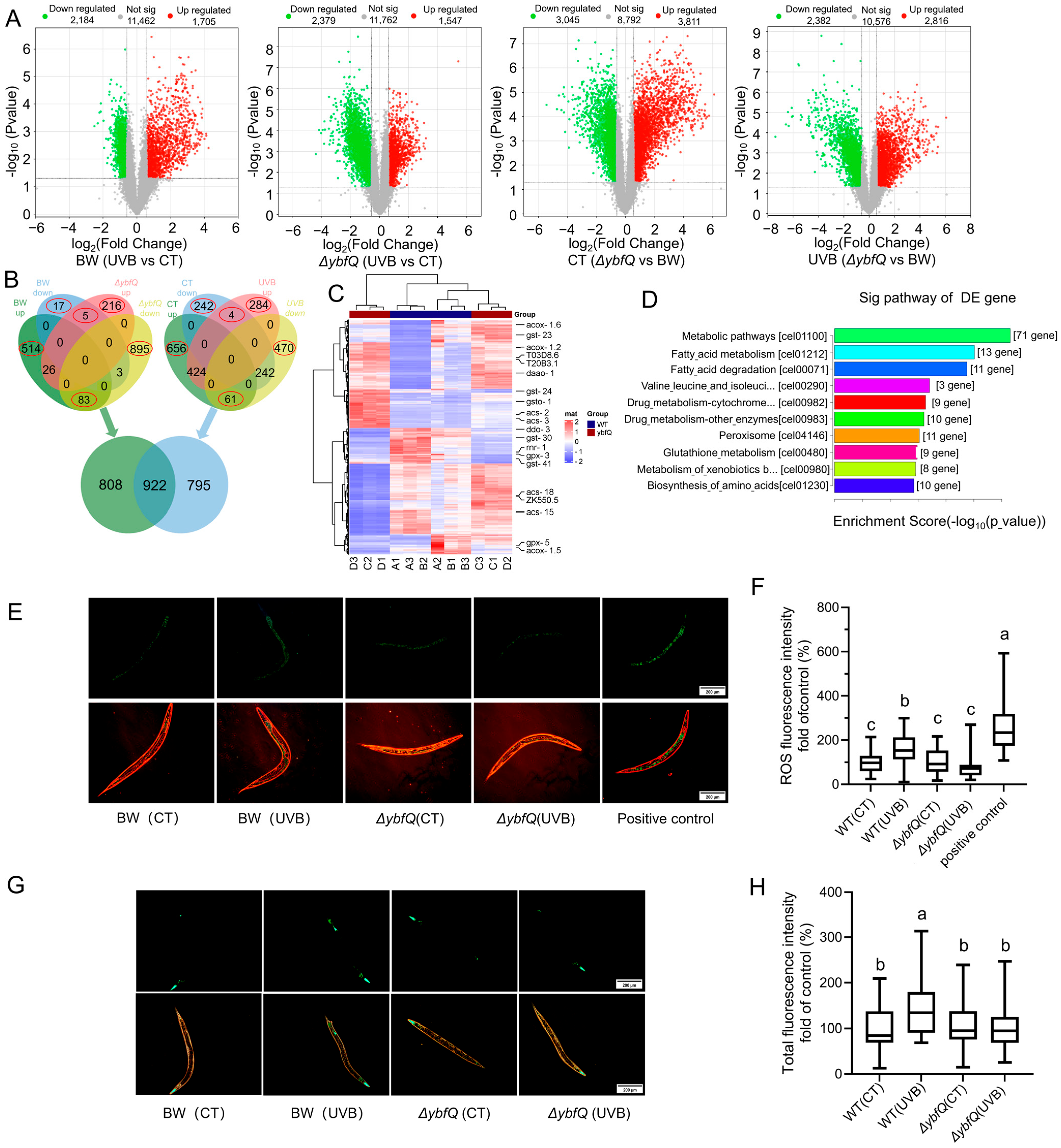
3.2. Pathways Involved in ΔybfQ-Mediated Worm Radio-Tolerance
3.3. Impact of ΔybfQ Feeding on Oxidative Stress in Worms
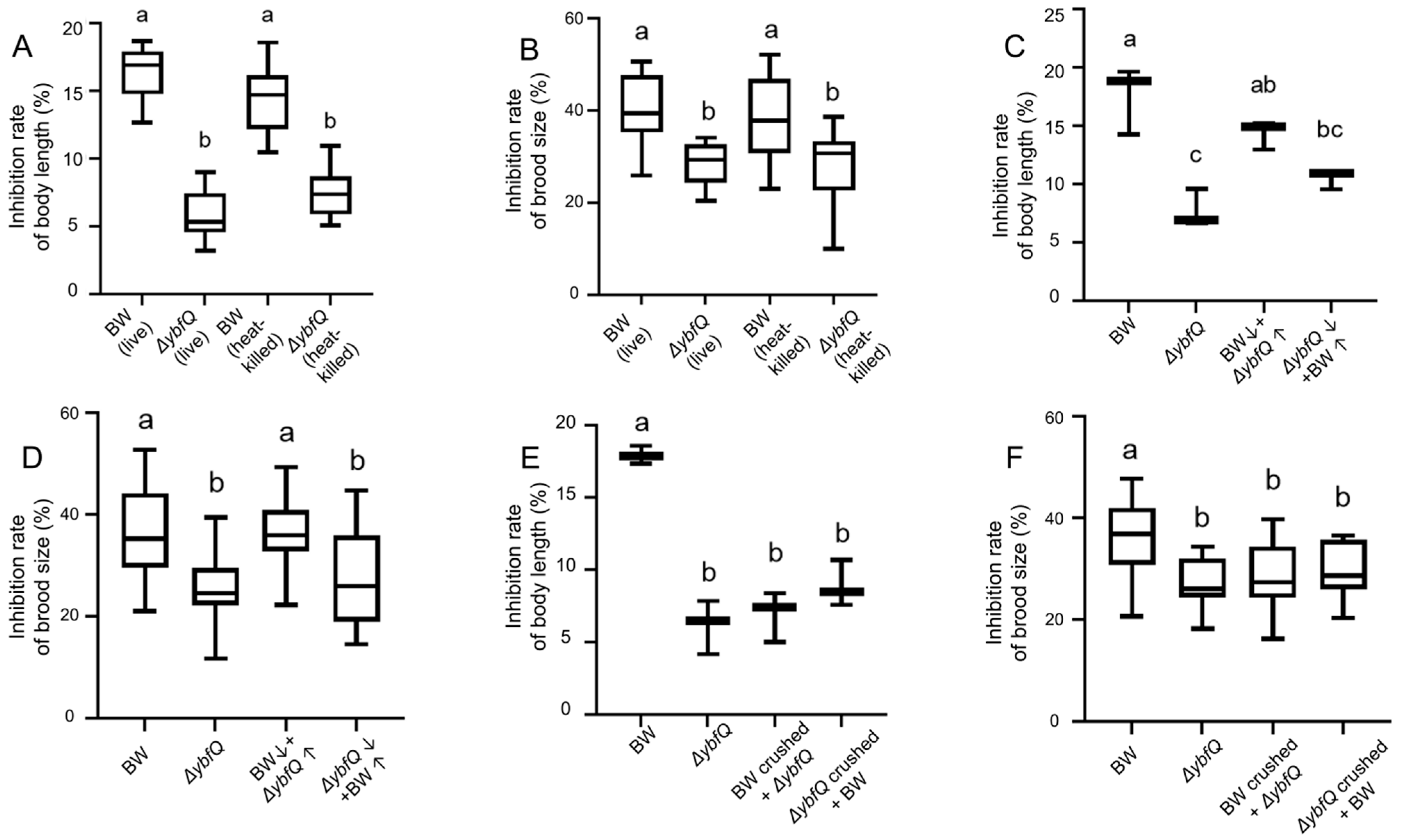
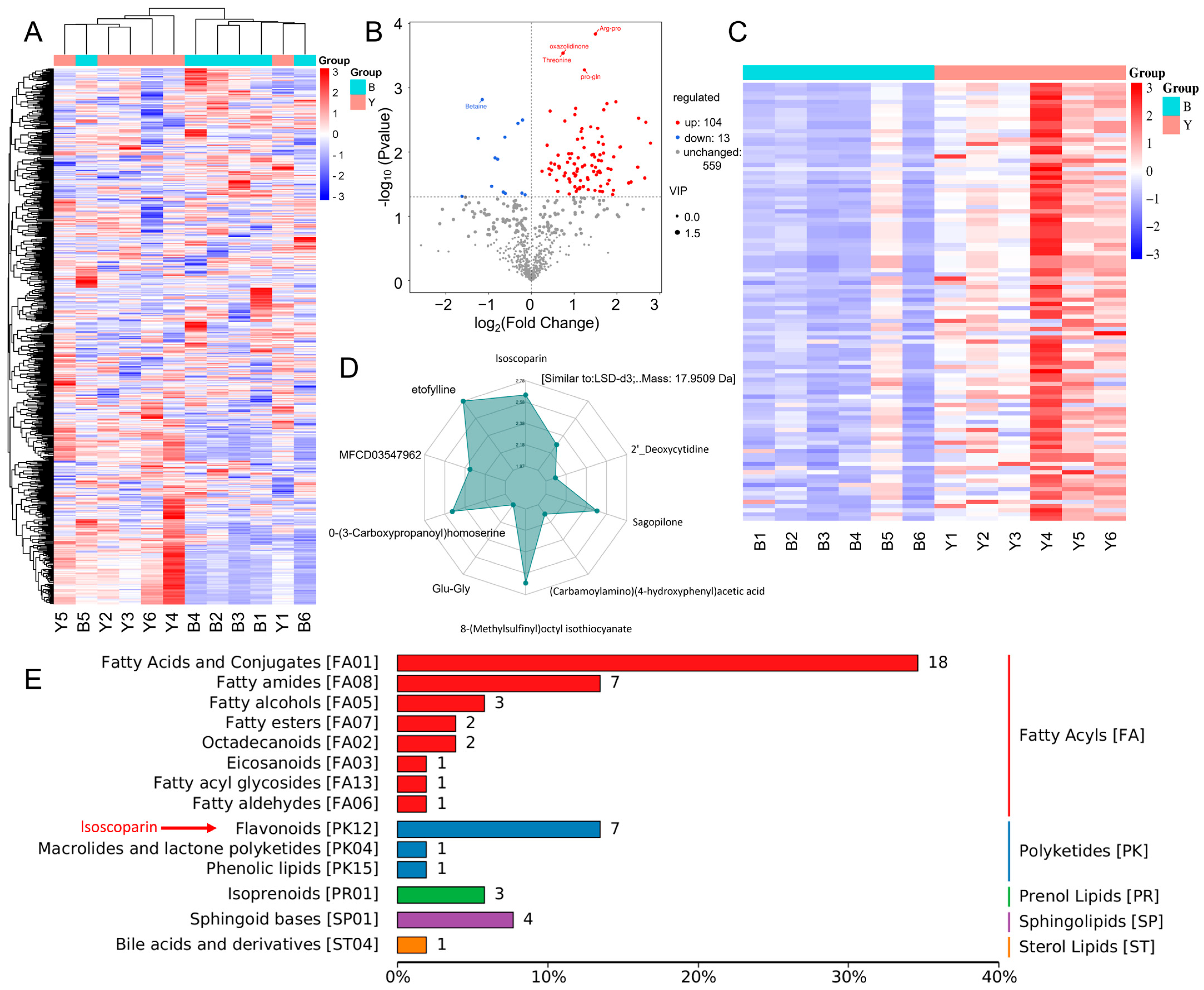
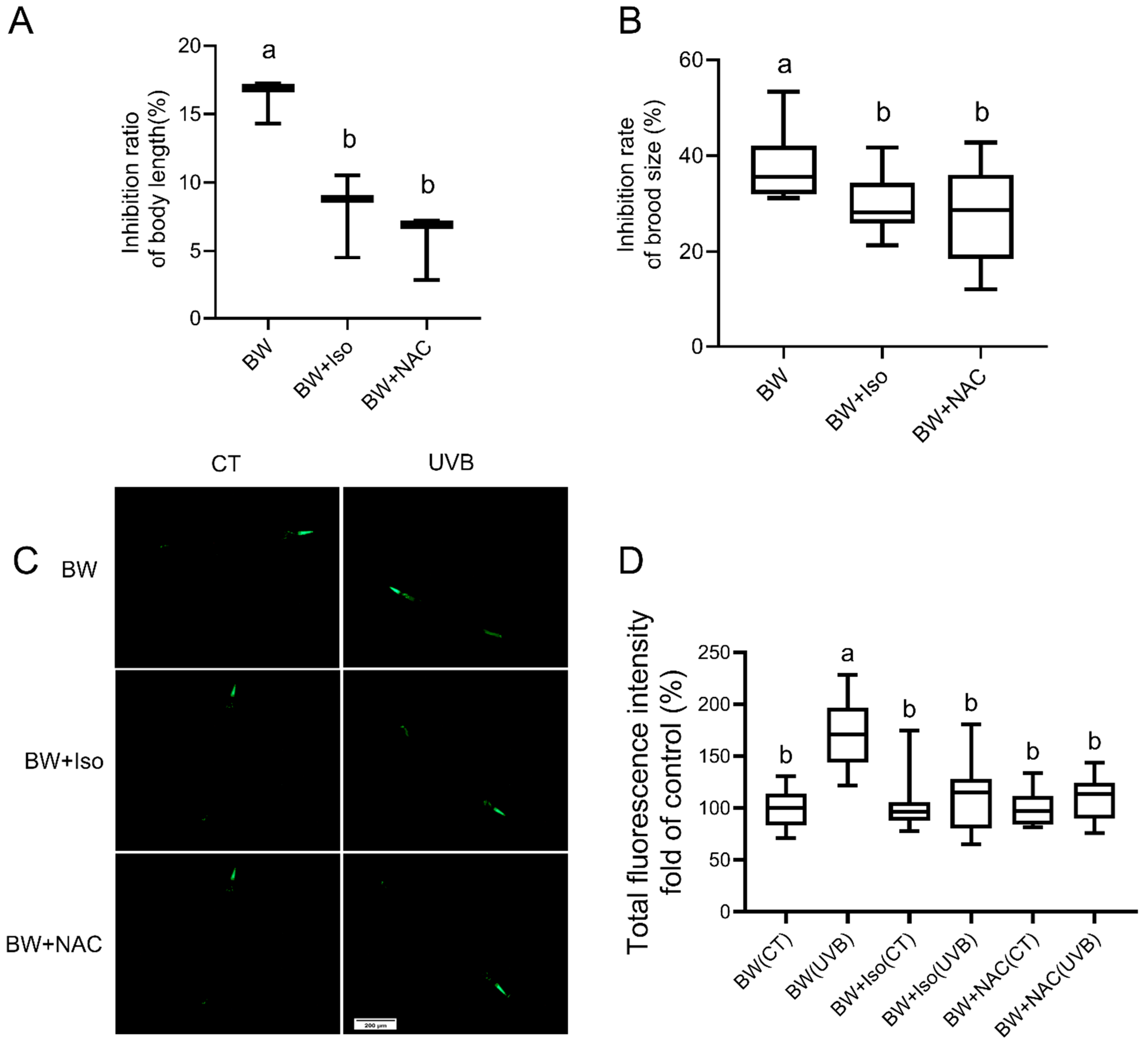
3.4. Involvement of ΔybfQ Metabolites in Modulating Worm Radio-Tolerance
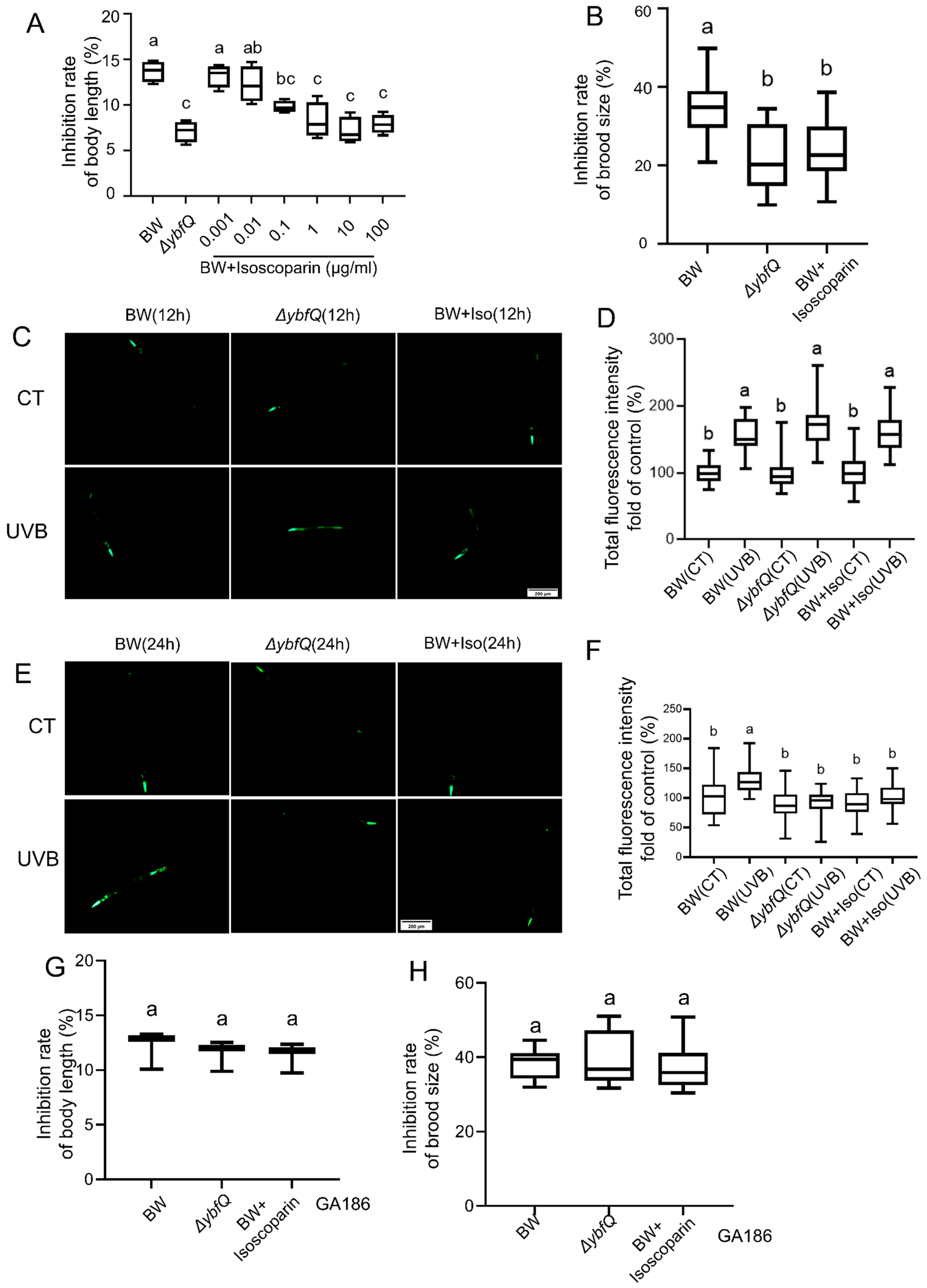
3.5. Contribution of Overproduced Isoscoparin in ΔybfQ to Worm Radio-Tolerance
3.6. Interaction of Isoscoparin and ΔybfQ with Worms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Arsenović, P.; Rozanov, E.; Usoskin, I.; Turney, C.; Sukhodolov, T.; McCracken, K.; Friedel, M.; Anet, J.; Simić, S.; Maliniemi, V.; et al. Global impacts of an extreme solar particle event under different geomagnetic field strengths. Proc. Natl. Acad. Sci. USA 2024, 121, e2321770121. [Google Scholar] [CrossRef]
- Umar, S.A.; Tasduq, S.A. Ozone Layer Depletion and Emerging Public Health Concerns—An Update on Epidemiological Perspective of the Ambivalent Effects of Ultraviolet Radiation Exposure. Front. Oncol. 2022, 12, 866733. [Google Scholar] [CrossRef]
- Guerrero-Navarro, L.; Jansen-Dürr, P.; Cavinato, M. Synergistic interplay of UV radiation and urban particulate matter induces impairment of autophagy and alters cellular fate in senescence-prone human dermal fibroblasts. Aging Cell 2024, 23, e14086. [Google Scholar] [CrossRef]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M.; Stone, E.M.; Stolte, H.; Cashman, K.D.; Macdonald, H.; Lanham-New, S.; Hiom, S.; Webb, A.; Fraser, D. UK Food Standards Agency Workshop Report: An investigation of the relative contributions of diet and sunlight to vitamin D status. Br. J. Nutr. 2010, 104, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Liu, Y.X.; Liu, Y.D.; Ho, C.K.; Tsai, Y.T.; Wen, D.S.; Huang, L.; Zheng, D.N.; Gao, Y.; Zhang, Y.F.; et al. Salvianolic acid B protects against UVB-induced skin aging via activation of NRF2. Phytomed. Int. J. Phytother. Phytopharm. 2024, 130, 155676. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Park, B.; Seo, S.A.; Cho, J.G.; Zhang, M.; Yi, T.H. Dietary Foeniculum vulgare Mill extract attenuated UVB irradiation-induced skin photoaging by activating of Nrf2 and inhibiting MAPK pathways. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 1273–1284. [Google Scholar] [CrossRef]
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomed. Int. J. Phytother. Phytopharm. 2018, 42, 100–111. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Daniel, N.; Lécuyer, E.; Chassaing, B. Host/microbiota interactions in health and diseases-Time for mucosal microbiology! Mucosal Immunol. 2021, 14, 1006–1016. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- Jansma, J.; El Aidy, S. Understanding the host-microbe interactions using metabolic modeling. Microbiome 2021, 9, 16. [Google Scholar] [CrossRef]
- Curtis, M.M.; Sperandio, V. A complex relationship: The interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol. 2011, 4, 133–138. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Huang, Z.Q.; Liu, K.; Li, D.Z.; Mo, T.L.; Liu, Q. The role of intestinal microbiota and metabolites in intestinal inflammation. Microbiol. Res. 2024, 288, 127838. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Le, H.H.; Lee, M.T.; Besler, K.R.; Johnson, E.L. Host hepatic metabolism is modulated by gut microbiota-derived sphingolipids. Cell Host Microbe 2022, 30, 798–808.e797. [Google Scholar] [CrossRef]
- Teng, Y.; Mu, J.; Xu, F.; Zhang, X.; Sriwastva, M.K.; Liu, Q.M.; Li, X.; Lei, C.; Sundaram, K.; Hu, X.; et al. Gut bacterial isoamylamine promotes age-related cognitive dysfunction by promoting microglial cell death. Cell Host Microbe 2022, 30, 944–960.e948. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: Impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Xin, J.Y.; Wang, J.; Ding, Q.Q.; Chen, W.; Xu, X.K.; Wei, X.T.; Lv, Y.H.; Wei, Y.P.; Feng, Y.; Zu, X.P. Potential role of gut microbiota and its metabolites in radiation-induced intestinal damage. Ecotoxicol. Environ. Saf. 2022, 248, 114341. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.W.; Cui, M.; Li, Y.; Dong, J.L.; Zhang, S.Q.; Zhu, C.C.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.C.; et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.W.; Lu, H.Y.; Tang, L.F.; Tang, F.L.; Zhu, R.Q.; Wang, D.F.; Cai, S.; Tian, Y.; Li, M. Probiotic Consortia Protect the Intestine Against Radiation Injury by Improving Intestinal Epithelial Homeostasis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, J.; Xiao, H.; Zhang, S.; Wang, B.; Cui, M.; Fan, S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes 2020, 11, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions. Front. Cell. Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, A.; Soares, R.; Barata, P. The Effects of Ionizing Radiation on Gut Microbiota: What Can Animal Models Tell Us?-A Systematic Review. Curr. Issues Mol. Biol. 2023, 45, 3877–3910. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Qin, T.; Lai, P.; Jing, Y.; Zhang, Z.; Zhou, G.; Gao, P.; Ding, G. Effects of X-ray cranial irradiation on metabolomics and intestinal flora in mice. Ecotoxicol. Environ. Saf. 2024, 270, 115898. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kanca, O.; Wangler, M.F.; Bellen, H.J. Integrating non-mammalian model organisms in the diagnosis of rare genetic diseases in humans. Nat. Rev. Genet. 2024, 25, 46–60. [Google Scholar] [CrossRef]
- Sharma, K.; Pooranachithra, M.; Balamurugan, K.; Goel, G. Multivariate Analysis of Increase in Life Span of Caenorhabditis elegans Through Intestinal Colonization by Indigenous Probiotic Strains. Probiot. Antimicrob. Proteins 2019, 11, 865–873. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Olmedo, M.; Holdorf, A.D.; Shang, Y.; Artal-Sanz, M.; Yilmaz, L.S.; Walhout, A.J.M. A Delicate Balance between Bacterial Iron and Reactive Oxygen Species Supports Optimal C. elegans Development. Cell Host Microbe 2019, 26, 400–411.e403. [Google Scholar] [CrossRef]
- Kodoyianni, V.; Maine, E.M.; Kimble, J. Molecular basis of loss-of-function mutations in the glp-1 gene of Caenorhabditis elegans. Mol. Biol. Cell 1992, 3, 1199–1213. [Google Scholar] [CrossRef]
- Weidhaas, J.B.; Eisenmann, D.M.; Holub, J.M.; Nallur, S.V. A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 9946–9951. [Google Scholar] [CrossRef]
- Kumar, S.; Egan, B.M.; Kocsisova, Z.; Schneider, D.L.; Murphy, J.T.; Diwan, A.; Kornfeld, K. Lifespan Extension in C. elegans Caused by Bacterial Colonization of the Intestine and Subsequent Activation of an Innate Immune Response. Dev. Cell 2019, 49, 100–117.e106. [Google Scholar] [CrossRef]
- Singh, J.; Aballay, A. Microbial Colonization Activates an Immune Fight-and-Flight Response via Neuroendocrine Signaling. Dev. Cell 2019, 49, 89–99.e84. [Google Scholar] [CrossRef]
- Portal-Celhay, C.; Bradley, E.R.; Blaser, M.J. Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 2012, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.R.; de Araújo, L.C.A.; Dos Santos da Rocha, P.; Agarrayua, D.A.; Ávila, D.S.; Carollo, C.A.; Silva, D.B.; Estevinho, L.M.; de Picoli Souza, K.; Dos Santos, E.L. Baru Pulp (Dipteryx alata Vogel): Fruit from the Brazilian Savanna Protects against Oxidative Stress and Increases the Life Expectancy of Caenorhabditis elegans via SOD-3 and DAF-16. Biomolecules 2020, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Mo, A.; Liang, Y.; Cao, X.; Jiang, J.; Liu, Y.; Cao, X.; Qiu, Y.; He, D. Polymer chain extenders induce significant toxicity through DAF-16 and SKN-1 pathways in Caenorhabditis elegans: A comparative analysis. J. Hazard. Mater. 2024, 473, 134730. [Google Scholar] [CrossRef]
- Bohin, N.; McGowan, K.P.; Keeley, T.M.; Carlson, E.A.; Yan, K.S.; Samuelson, L.C. Insulin-like Growth Factor-1 and mTORC1 Signaling Promote the Intestinal Regenerative Response After Irradiation Injury. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.A.; Chen, W.C.; Tan, M.W. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell 2008, 7, 879–893. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Peng, Z.Y.; Peng, M.J.; Yan, W.B.; Ouyang, Y.Z.; Zhu, H.L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Kim, B.; Woo, S.; Kim, M.J.; Kwon, S.W.; Lee, J.; Sung, S.H.; Koh, H.J. Identification and quantification of flavonoids in yellow grain mutant of rice (Oryza sativa L.). Food Chem. 2018, 241, 154–162. [Google Scholar] [CrossRef]
- Lee, H.; Yeong Yang, J.; Eun Ra, J.; Ahn, H.J.; Ja Lee, M.; Young Kim, H.; Song, S.Y.; Hyun Kim, D.; Hwan Lee, J.; Duck Seo, W. Elucidation of phenolic metabolites in wheat seedlings (Triticum aestivum L.) by NMR and HPLC-Q-Orbitrap-MS/MS: Changes in isolated phenolics and antioxidant effects through diverse growth times. Food Chem. X 2023, 17, 100557. [Google Scholar] [CrossRef]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Li, H.; Zhang, Y.; Ren, L.; Chen, C.; Wang, X.; Yu, J.; Li, Z.; Liu, Y. Tris(1,3-dichloro-2-propyl)phosphate Reduces the Lifespan via Activation of an Unconventional Insulin/Insulin-Like Growth Factor-1 Signaling Pathway. Environ. Sci. Technol. 2020, 54, 10783–10796. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.S.; Hong, S.M. Long-term consumption of caffeine improves glucose homeostasis by enhancing insulinotropic action through islet insulin/insulin-like growth factor 1 signaling in diabetic rats. Metab. Clin. Exp. 2007, 56, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Lappano, R.; Bonavita, E.; Maggiolini, M.; Clarke, R.B.; Belfiore, A.; De Francesco, E.M. Insulin/IGF Axis and the Receptor for Advanced Glycation End Products: Role in Meta-inflammation and Potential in Cancer Therapy. Endocr. Rev. 2023, 44, 693–723. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yu, Y.; An, L.; Wang, W.; Fu, X.; Chen, J.; Ma, J. Ellagic Acid Increases Stress Resistance via Insulin/IGF-1 Signaling Pathway in Caenorhabditis elegans. Molecules 2022, 27, 6168. [Google Scholar] [CrossRef]
- Nieuwenhuis, E.E.; Matsumoto, T.; Lindenbergh, D.; Willemsen, R.; Kaser, A.; Simons-Oosterhuis, Y.; Brugman, S.; Yamaguchi, K.; Ishikawa, H.; Aiba, Y.; et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Investig. 2009, 119, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Nair, T.; Weathers, B.A.; Stuhr, N.L.; Nhan, J.D.; Curran, S.P. Serotonin deficiency from constitutive SKN-1 activation drives pathogen apathy. Nat. Commun. 2024, 15, 8129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Zhang, J.; Qiao, S.; Xu, J.; Li, J.; Zhang, W.; Yi, Q.; Wu, Y.; Wang, T.; Bian, P. The Effects of Feeding ybfQ-Deficient Gut Bacteria on Radio-Tolerance in Symbiotic Caenorhabditis elegans: The Key Role of Isoscoparin. Microorganisms 2025, 13, 2626. https://doi.org/10.3390/microorganisms13112626
Ding L, Zhang J, Qiao S, Xu J, Li J, Zhang W, Yi Q, Wu Y, Wang T, Bian P. The Effects of Feeding ybfQ-Deficient Gut Bacteria on Radio-Tolerance in Symbiotic Caenorhabditis elegans: The Key Role of Isoscoparin. Microorganisms. 2025; 13(11):2626. https://doi.org/10.3390/microorganisms13112626
Chicago/Turabian StyleDing, Liu, Jingjing Zhang, Shanpeng Qiao, Jiyu Xu, Jing Li, Wenjing Zhang, Qiyi Yi, Yuejin Wu, Ting Wang, and Po Bian. 2025. "The Effects of Feeding ybfQ-Deficient Gut Bacteria on Radio-Tolerance in Symbiotic Caenorhabditis elegans: The Key Role of Isoscoparin" Microorganisms 13, no. 11: 2626. https://doi.org/10.3390/microorganisms13112626
APA StyleDing, L., Zhang, J., Qiao, S., Xu, J., Li, J., Zhang, W., Yi, Q., Wu, Y., Wang, T., & Bian, P. (2025). The Effects of Feeding ybfQ-Deficient Gut Bacteria on Radio-Tolerance in Symbiotic Caenorhabditis elegans: The Key Role of Isoscoparin. Microorganisms, 13(11), 2626. https://doi.org/10.3390/microorganisms13112626





