Inactivation of Respiratory Syncytial Virus by Ozone Generated via Dielectric Barrier Discharge Technology with Decrease in Intact Viral Surface Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. RSV
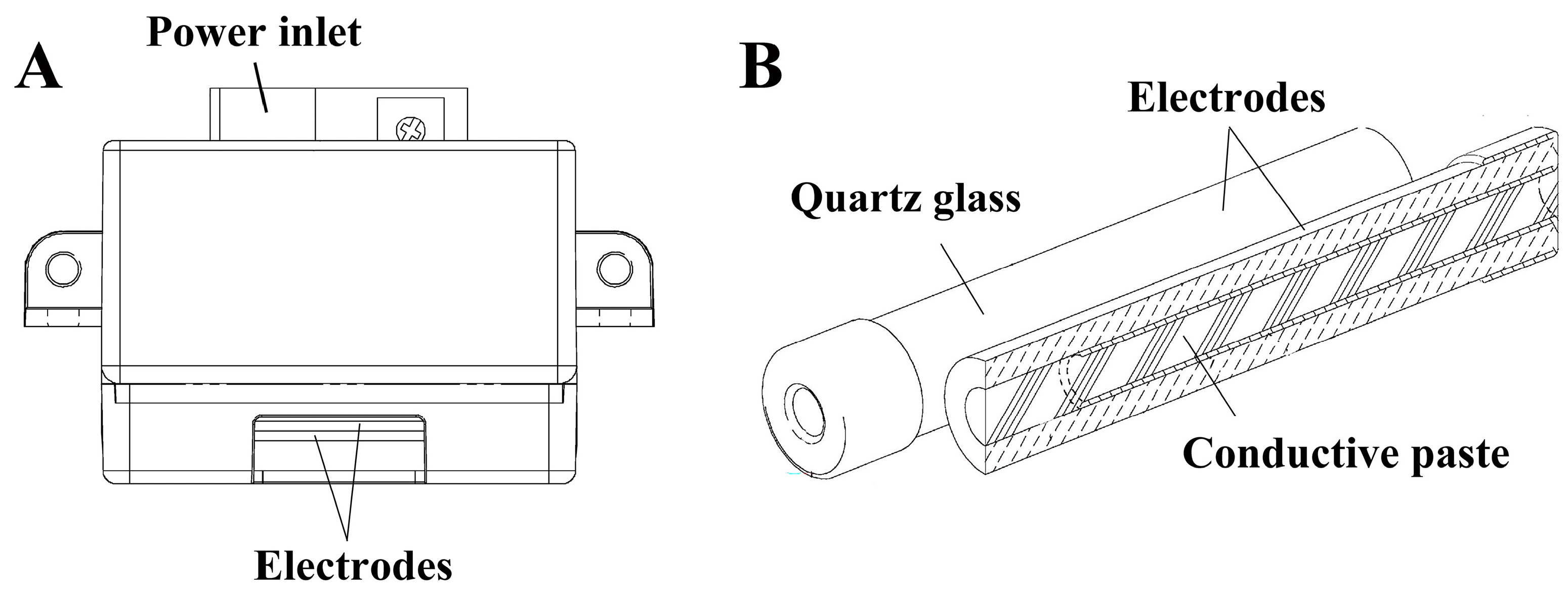
2.2. Configuration of the Ozone Generator
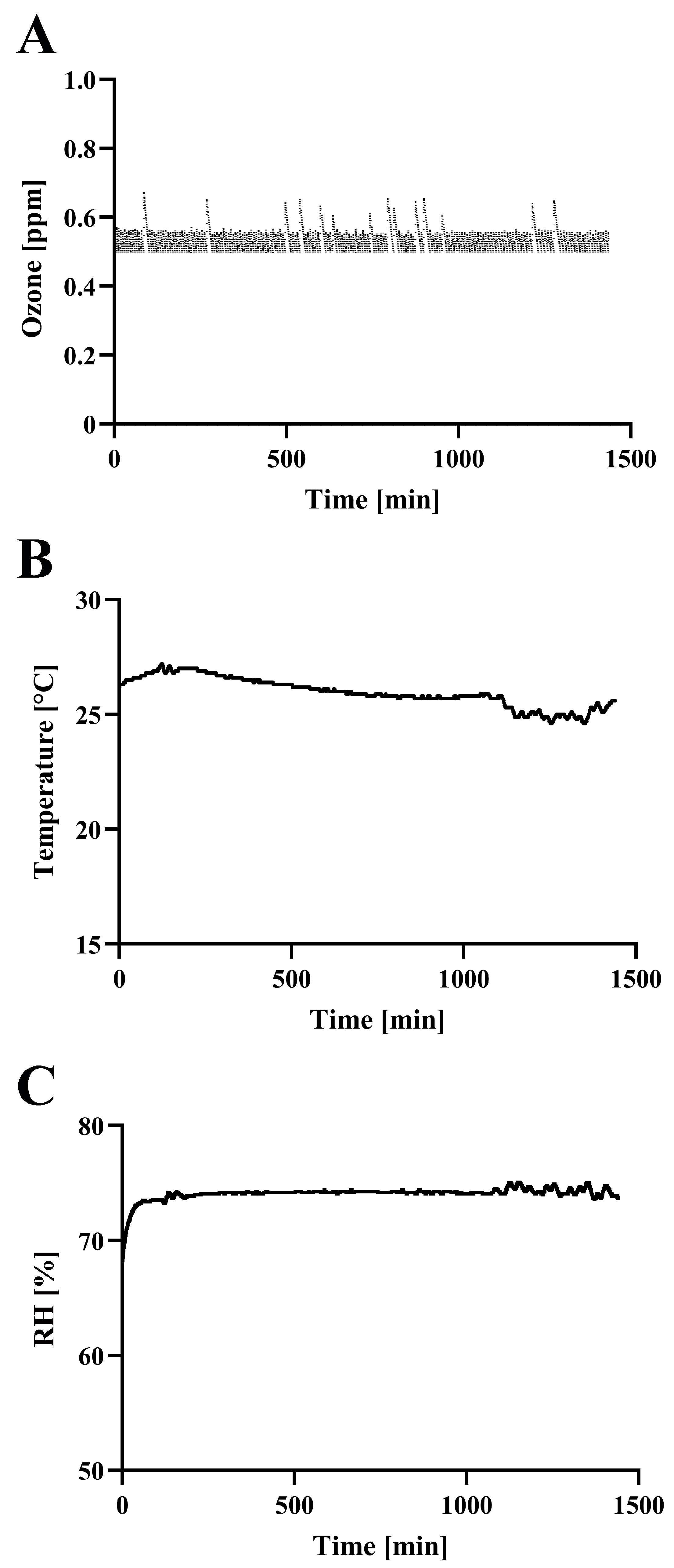
2.3. Ozone Monitoring and Treatment
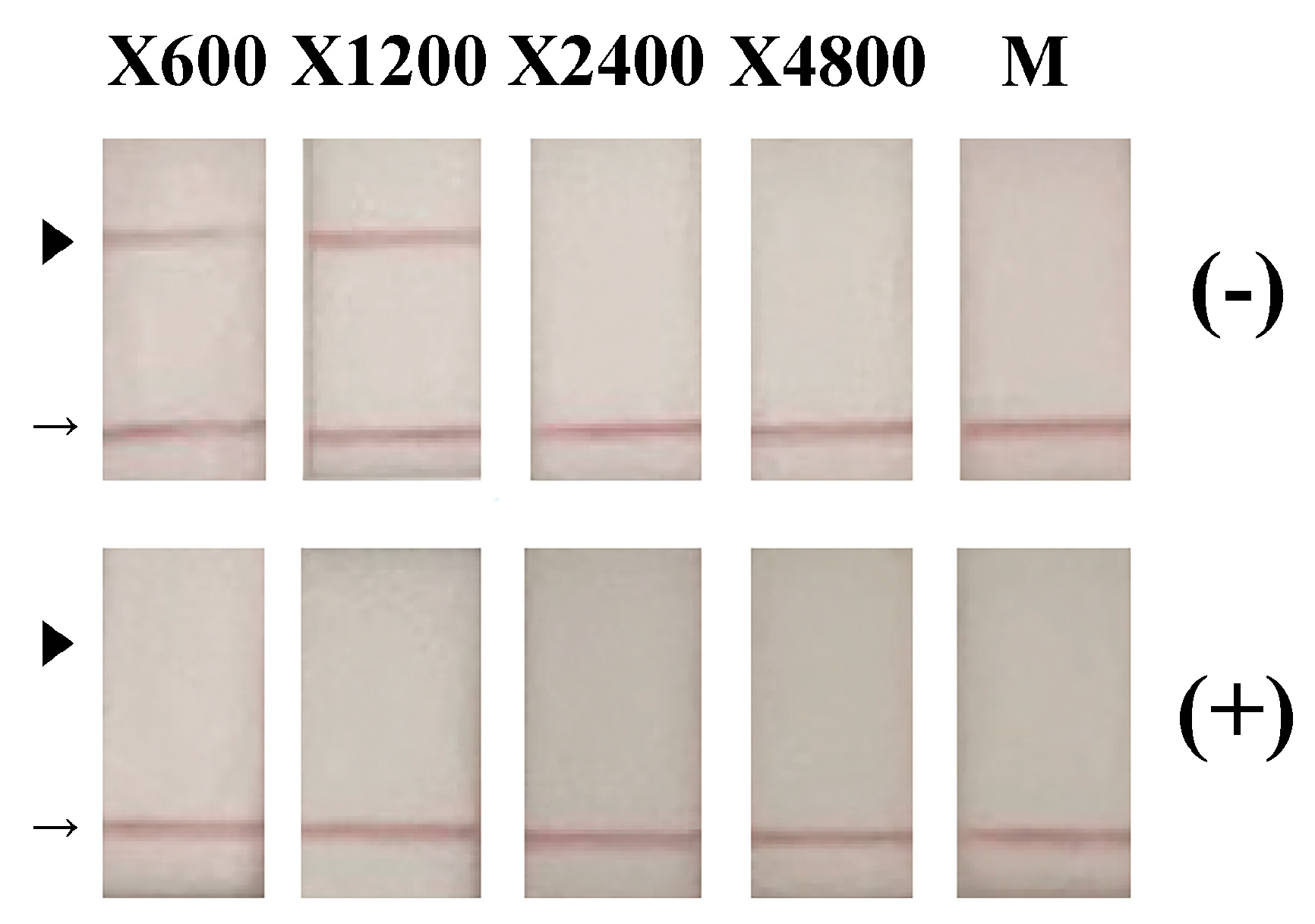
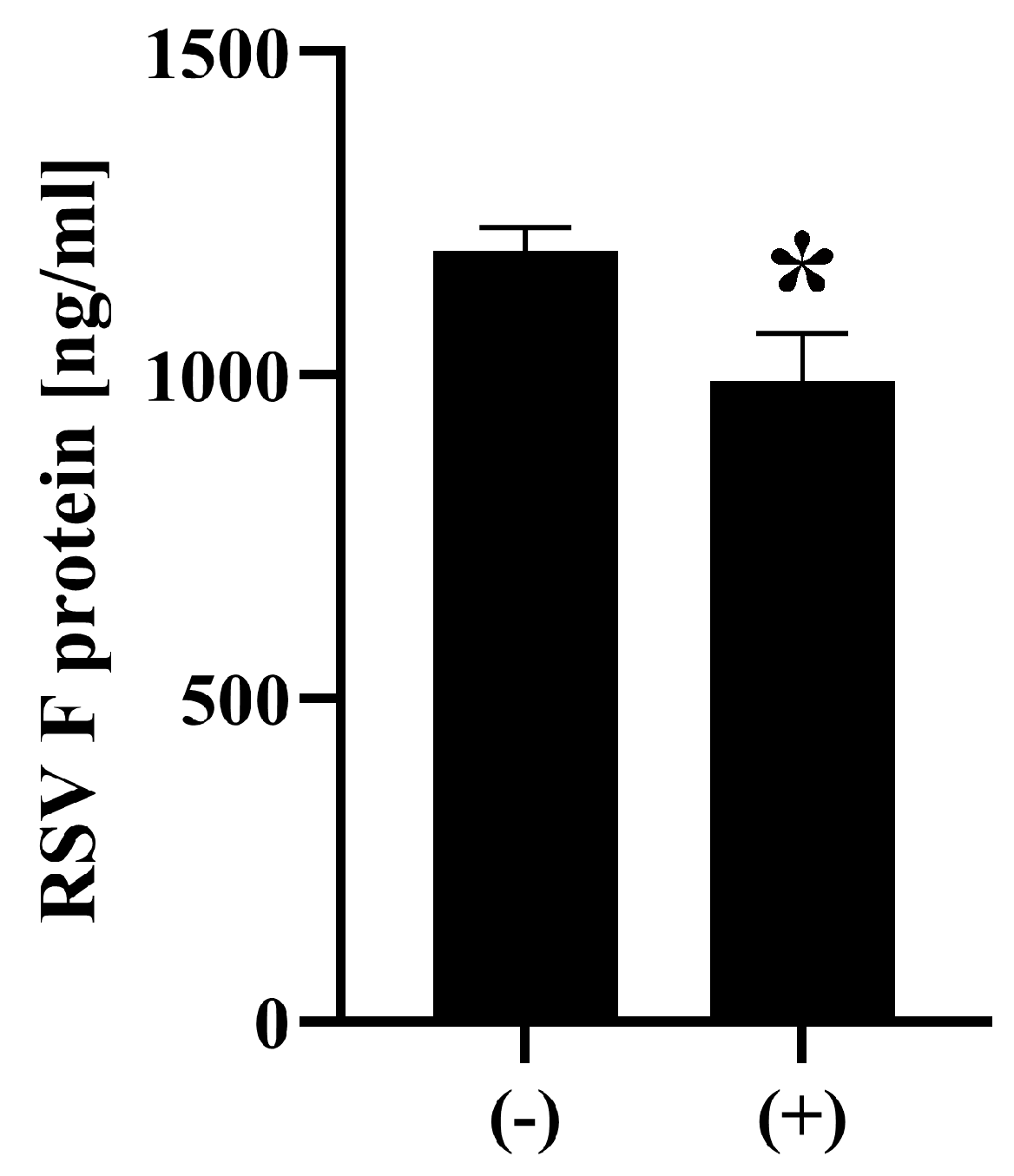
2.4. Detection of RSV Antigens
2.5. Virus Recovery
2.6. Viral Titration Assay
2.7. Viral RNA Extraction and Real-Time Polymerase Chain Reaction (PCR)
2.8. Statistical Analyses
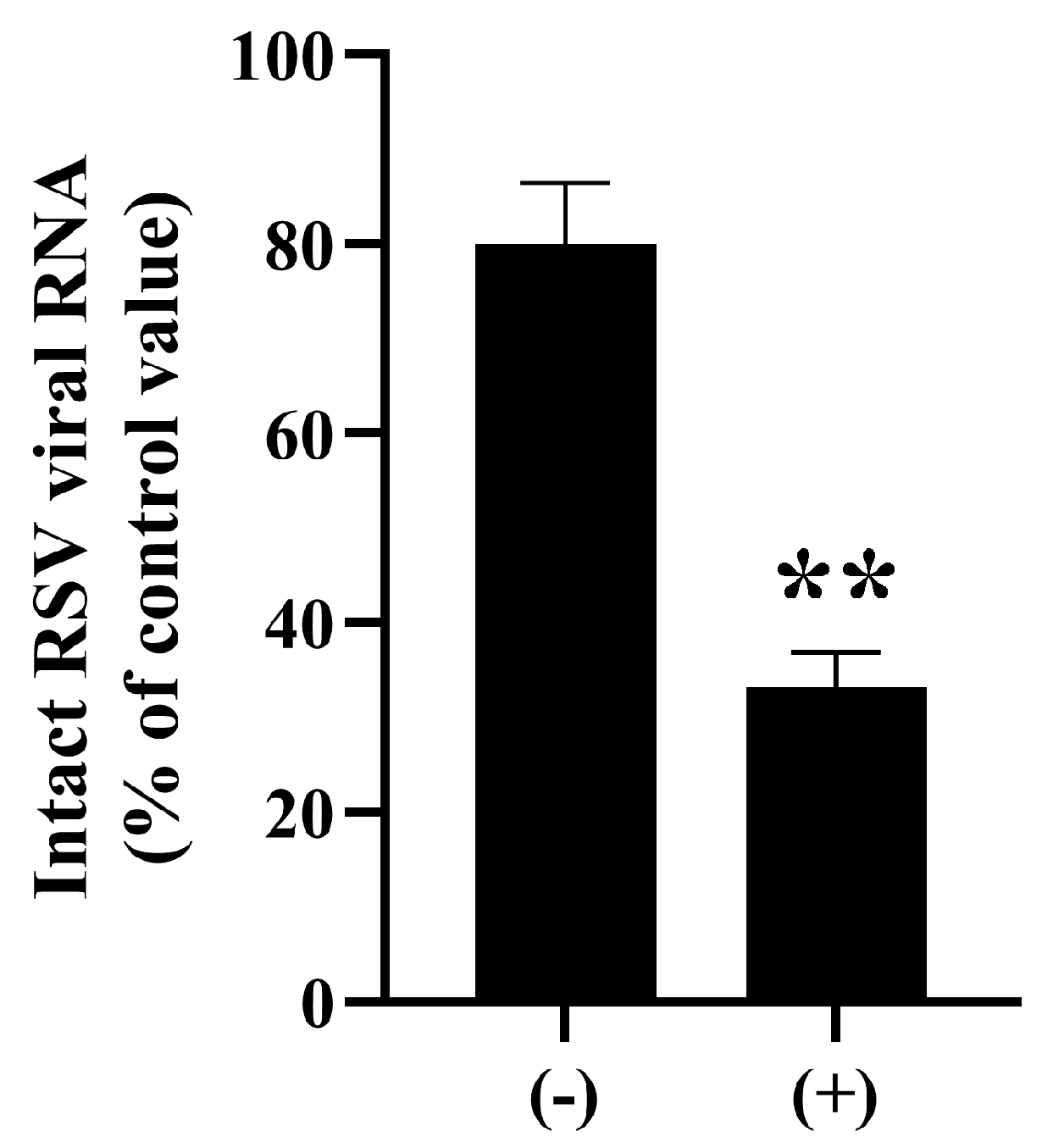
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muthukutty, P.; MacDonald, J.; Yoo, S.Y. Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies. Vaccines 2024, 12, 1220. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Limongelli, L.; Nardone, M.; Carinci, F. Environmental Disinfection Strategies to Prevent Indirect Transmission of SARS-CoV2 in Healthcare Settings. Appl. Sci. 2020, 10, 6291. [Google Scholar] [CrossRef]
- Choi, H.; Chatterjee, P.; Lichtfouse, E.; Martel, J.A.; Hwang, M.; Jinadatha, C.; Sharma, V.K. Classical and alternative disinfection strategies to control the COVID-19 virus in healthcare facilities: A review. Environ. Chem. Lett. 2021, 19, 1945–1951. [Google Scholar] [CrossRef]
- Chu, H.Y.; Kuypers, J.; Renaud, C.; Wald, A.; Martin, E.; Fairchok, M.; Magaret, A.; Sarancino, M.; Englund, J.A. Molecular epidemiology of respiratory syncytial virus transmission in childcare. J. Clin. Virol. 2013, 57, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [Google Scholar] [CrossRef] [PubMed]
- Sze-To, G.N.; Yang, Y.; Kwan, J.K.; Yu, S.C.; Chao, C.Y. Effects of surface material, ventilation, and human behavior on indirect contact transmission risk of respiratory infection. Risk Anal. 2014, 34, 818–830. [Google Scholar] [CrossRef]
- Rechsteiner, J. Thermal inactivation of respiratory syncytial virus in water and hypertonic solutions. J. Gen. Virol. 1969, 5, 397–403. [Google Scholar] [CrossRef]
- Kitai, Y.; Watanabe, O.; Ohmiya, S.; Kisu, T.; Ota, R.; Kawakami, K.; Katoh, H.; Fukuzawa, K.; Takeda, M.; Nishimura, H. Detailed analysis of low temperature inactivation of respiratory syncytial virus. Sci. Rep. 2024, 14, 11823. [Google Scholar] [CrossRef]
- Terrier, O.; Essere, B.; Yver, M.; Barthelemy, M.; Bouscambert-Duchamp, M.; Kurtz, P.; VanMechelen, D.; Morfin, F.; Billaud, G.; Ferraris, O.; et al. Cold oxygen plasma technology efficiency against different airborne respiratory viruses. J. Clin. Virol. 2009, 45, 119–124. [Google Scholar] [CrossRef]
- Krilov, L.R.; Harkness, S.H. Inactivation of respiratory syncytial virus by detergents and disinfectants. Pediatr. Infect. Dis. J. 1993, 12, 582–584. [Google Scholar] [CrossRef]
- Platt, J.; Bucknall, R.A. The disinfection of respiratory syncytial virus by isopropanol and a chlorhexidine-detergent handwash. J. Hosp. Infect. 1985, 6, 89–94. [Google Scholar] [CrossRef]
- Hilmarsson, H.; Traustason, B.S.; Kristmundsdottir, T.; Thormar, H. Virucidal activities of medium- and long-chain fatty alcohols and lipids against respiratory syncytial virus and parainfluenza virus type 2: Comparison at different pH levels. Arch. Virol. 2007, 152, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Friesland, M.; Frericks, N.; Wetzke, M.; Haid, S.; Steinmann, J.; Todt, D.; Pietschmann, T.; Steinmann, E. Virucidal activity of oral, hand, and surface disinfectants against respiratory syncytial virus. J. Hosp. Infect. 2023, 141, 25–32. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- McDonnell, G.; Hansen, J. Block’s Disinfection, Sterilization, and Preservation, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021. [Google Scholar]
- Dubuis, M.E.; Racine, E.; Vyskocil, J.M.; Turgeon, N.; Tremblay, C.; Mukawera, E.; Boivin, G.; Grandvaux, N.; Duchaine, C. Ozone inactivation of airborne influenza and lack of resistance of respiratory syncytial virus to aerosolization and sampling processes. PLoS ONE 2021, 16, e0253022, Erratum in PLoS ONE 2021, 20, e0334842. [Google Scholar] [CrossRef]
- Blanchard, E.L.; Lawrence, J.D.; Noble, J.A.; Xu, M.; Joo, T.; Ng, N.L.; Schmidt, B.E.; Santangelo, P.J.; Finn, M.G. Enveloped Virus Inactivation on Personal Protective Equipment by Exposure to Ozone. medRxiv 2020. [Google Scholar] [CrossRef]
- Tizaoui, C.; Stanton, R.; Statkute, E.; Rubina, A.; Lester-Card, E.; Lewis, A.; Holliman, P.; Worsley, D. Ozone for SARS-CoV-2 inactivation on surfaces and in liquid cell culture media. J. Hazard. Mater. 2022, 428, 128251. [Google Scholar] [CrossRef] [PubMed]
- Vyskocil, J.M.; Turgeon, N.; Turgeon, J.G.; Duchaine, C. Ozone treatment in a wind tunnel for the reduction of airborne viruses in swine buildings. Aerosol Sci. Technol. 2020, 54, 1471–1478. [Google Scholar] [CrossRef]
- Nishimura, H.; Sakata, S.; Dapat, I.; Segawa, M.; Mizutani, Y.; Imaizumi, J.; Shirato, K.; Ohmiya, S.; Katsumi, M.; Yokoyama, T. Synergistic Inactivation of Airborne Viruses by Low-Concentration Ozone with High Humidity and Temperature. Microbiol. Immunol. 2025, 69, 280–288. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a Practical Method for Using Ozone Gas as a Virus Decontaminating Agent. Ozone: Sci. Eng. 2009, 31, 216–223. [Google Scholar] [CrossRef]
- Morrison, C.; Atkinson, A.; Zamyadi, A.; Kibuye, F.; McKie, M.; Hogard, S.; Mollica, P.; Jasim, S.; Wert, E.C. Critical Review and Research Needs of Ozone Applications Related to Virus Inactivation: Potential Implications for SARS-CoV-2. Ozone Sci. Eng. 2020, 43, 2–20. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Y.; Yadav, A.K.; Ji, B.; Kang, P.; Wei, T. Ozone based inactivation and disinfection in the pandemic time and beyond: Taking forward what has been learned and best practice. Sci. Total Environ. 2023, 862, 160711. [Google Scholar] [CrossRef]
- Bayarri, B.; Cruz-Alcalde, A.; Lopez-Vinent, N.; Mico, M.M.; Sans, C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021, 415, 125658. [Google Scholar] [CrossRef]
- Cordoba-Lanus, E.; Garcia-Perez, O.; Rodriguez-Esparragon, F.; Bethencourt-Estrella, C.J.; Torres-Mata, L.B.; Blanco, A.; Villar, J.; Sanz, O.; Diaz, J.J.; Martin-Barrasa, J.L.; et al. Ozone treatment effectively eliminates SARS-CoV-2 from infected face masks. PLoS ONE 2022, 17, e0271826. [Google Scholar] [CrossRef]
- Yano, H.; Nakano, R.; Suzuki, Y.; Nakano, A.; Kasahara, K.; Hosoi, H. Inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by gaseous ozone treatment. J. Hosp. Infect. 2020, 106, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of COVID-19. Gases 2021, 1, 19–32. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics, 51st ed.; The Chemical Rubber Co.: Cleveland, OH, USA, 1970; pp. 67, 68, 112. [Google Scholar]
- Zhang, K.; Liu, J.; Lv, H.; Zeng, X.; Ling, Z.; Ding, L.; Jin, C. Advances in Ozone Technology for Environmental, Energy, Food and Medical Applications. Processes 2025, 13, 1126. [Google Scholar] [CrossRef]
- Mohammed, S.M.; Sukkar, K.A.; Al-Ezzi, A.A.R.; Qarahgouli, A.R. A critical review of optimizing pollutant removal from refinery wastewater using ozone and nanocatalyst-assisted bubble column reactors. Desalin. Water Treat. 2025, 323, 101343. [Google Scholar] [CrossRef]
- OHNIT Co., Ltd. Compact Ozonizer Capable of Stable Low-Concentration Ozone Generation. Available online: https://www.ohnit.co.jp/product/sfg1210-f/ (accessed on 2 November 2025).
- OHNIT Co., Ltd. Small Ozone Generator [For DC12V] SFG1210 Series. Available online: https://mono.ipros.com/en/product/detail/2000034499/ (accessed on 2 November 2025).
- Nakanishi, M.; Nieda, M. Low Temperature Plasma Generator. Patent JP5405296B2, 3 March 2008. Available online: https://patents.google.com/patent/JP5405296B2/en?oq=JP+5405296+B2 (accessed on 2 November 2025).
- Mazur-Panasiuk, N.; Botwina, P.; Kutaj, A.; Woszczyna, D.; Pyrc, K. Ozone Treatment Is Insufficient to Inactivate SARS-CoV-2 Surrogate under Field Conditions. Antioxidants 2021, 10, 1480. [Google Scholar] [CrossRef]
- Sakudo, A.; Baba, K.; Tsukamoto, M.; Ikuta, K. Use of anionic polymer, poly(methyl vinyl ether-maleic anhydride)-coated beads for capture of respiratory syncytial virus. Bioorg Med. Chem. Lett. 2009, 19, 4488–4491, Erratum in Bioorg Med. Chem. Lett. 2011, 21, 879. [Google Scholar] [CrossRef]
- Miernyk, K.; Bulkow, L.; DeByle, C.; Chikoyak, L.; Hummel, K.B.; Hennessy, T.; Singleton, R. Performance of a rapid antigen test (Binax NOW® RSV) for diagnosis of respiratory syncytial virus compared with real-time polymerase chain reaction in a pediatric population. J. Clin. Virol. 2011, 50, 240–243. [Google Scholar] [CrossRef]
- Sakudo, A.; Toyokawa, Y.; Imanishi, Y.; Murakami, T. Crucial roles of reactive chemical species in modification of respiratory syncytial virus by nitrogen gas plasma. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 131–136. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Monti, A.; Doti, N.; Sanna, G.; Manzin, A.; De Filippis, A.; Galdiero, M. Hylin-a1: A Pan-Inhibitor against Emerging and Re-Emerging Respiratory Viruses. Int. J. Mol. Sci. 2023, 24, 13888. [Google Scholar] [CrossRef]
- Sino Biological, Inc. Human Respiratory Syncytial Virus (RSV) (A2) Fusion Glycoprotein/RSV-F ELISA Kit. Cat: KIT11049.. Available online: https://www.sinobiological.com/elisa-kits/rsv-fusion-kit11049 (accessed on 2 November 2025).
- Saunders, J.L.; Daniels, I.A.; Edwards, T.L.; Relich, R.F.; Zhao, Y.; Smith, L.A.; Gaston, B.M.; Davis, M.D. Effects of pH alteration on respiratory syncytial virus in human airway epithelial cells. ERJ Open Res. 2023, 9, 00404–02022. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hayasaki-Kajiwara, Y.; Baba, T.; Okaga, S.; Kakui, M.; Shishido, T. Stability of Respiratory Syncytial Virus in Nasal Aspirate from Patients Infected with RSV. Influenza Other Respir. Viruses 2024, 18, e70058. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.B.; Sharma, M.; Petric, M. Inactivation of Norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect. 2007, 66, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Wong, P.K.; Engelbrecht, R.S.; Chian, E.S. Mechanism of enteroviral inactivation by ozone. Appl. Environ. Microbiol. 1981, 41, 718–723. [Google Scholar] [CrossRef]
- Ishizaki, K.; Shinriki, N.; Ikehata, A.; Ueda, T. Degradation of Nucleic Acids with Ozone. I. Degradation of Nucleobases, Ribonucleosides and Ribonucleoside-5’-monophosphates. Chem. Pharm. Bull. 1981, 29, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.K.; Ohmine, S.; Tomer, D.P.; Jensen, K.J.; Johnson, F.B.; Kirsi, J.J.; Robison, R.A.; O’Neill, K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods 2008, 153, 74–77. [Google Scholar] [CrossRef]
- Dubuis, M.E.; Dumont-Leblond, N.; Laliberte, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS ONE 2020, 15, e0231164, Erratum in PLoS ONE 2025, 20, e0334631. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Lu, Y.; Ren, Y.; Chen, Z.; Chen, M. The virus removal in UV irradiation, ozonation and chlorination. Water Cycle 2021, 2, 23–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakudo, A.; Moriyama, R.; Nieda, M. Inactivation of Respiratory Syncytial Virus by Ozone Generated via Dielectric Barrier Discharge Technology with Decrease in Intact Viral Surface Protein. Microorganisms 2025, 13, 2611. https://doi.org/10.3390/microorganisms13112611
Sakudo A, Moriyama R, Nieda M. Inactivation of Respiratory Syncytial Virus by Ozone Generated via Dielectric Barrier Discharge Technology with Decrease in Intact Viral Surface Protein. Microorganisms. 2025; 13(11):2611. https://doi.org/10.3390/microorganisms13112611
Chicago/Turabian StyleSakudo, Akikazu, Ryoya Moriyama, and Masanori Nieda. 2025. "Inactivation of Respiratory Syncytial Virus by Ozone Generated via Dielectric Barrier Discharge Technology with Decrease in Intact Viral Surface Protein" Microorganisms 13, no. 11: 2611. https://doi.org/10.3390/microorganisms13112611
APA StyleSakudo, A., Moriyama, R., & Nieda, M. (2025). Inactivation of Respiratory Syncytial Virus by Ozone Generated via Dielectric Barrier Discharge Technology with Decrease in Intact Viral Surface Protein. Microorganisms, 13(11), 2611. https://doi.org/10.3390/microorganisms13112611





