Development of Sequential Fermentation Starters and Comparison of Quality Characteristics for Black Barley Vinegar Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Barley Makgeolli Preparation
2.2. Seed Vinegar Preparation
2.3. Production of Black Barley Vinegar Using Sequential Starters and Selection of Fermentation Conditions
2.4. Physicochemical Properties
2.5. Organic Acids
2.6. Free Amino Acids
2.7. Taste Fingerprint
2.8. Volatile Compounds
2.9. Statistical Analysis
3. Results and Discussion
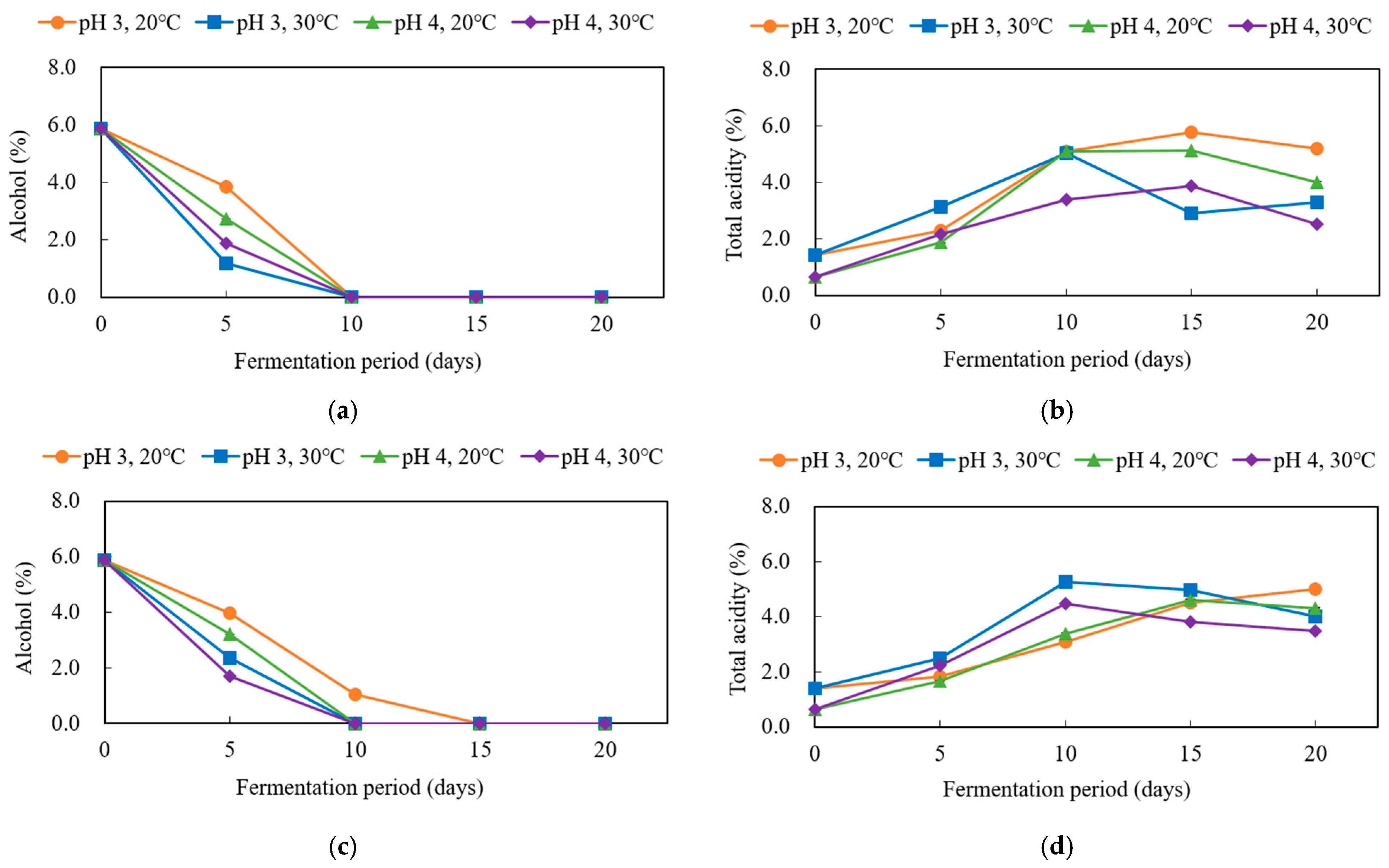
3.1. Production of Black Barley Vinegar Using Sequential Starters and Selection of Optimal Fermentation Conditions
3.2. Organic Acids
3.3. Free Amino Acids
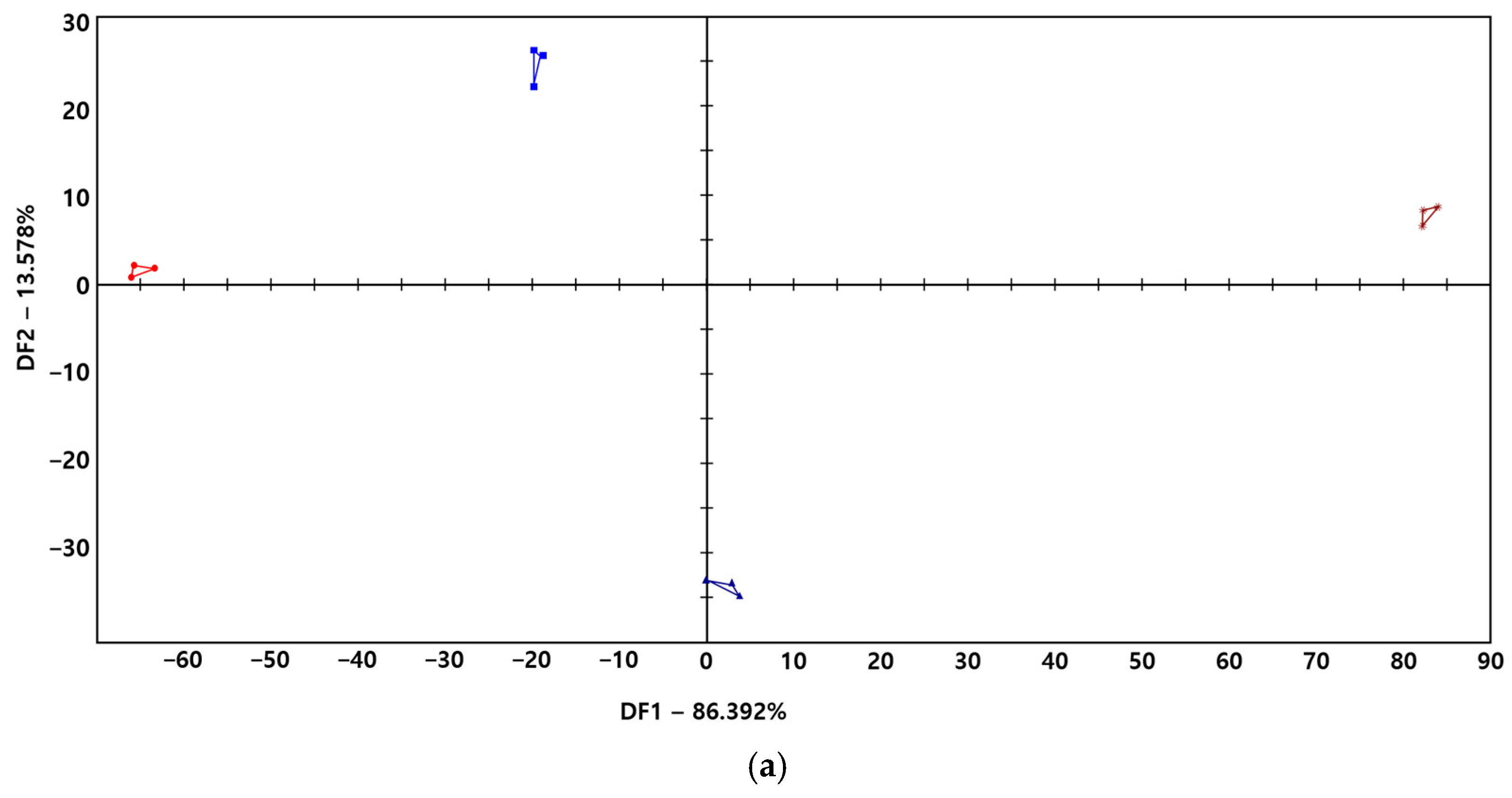
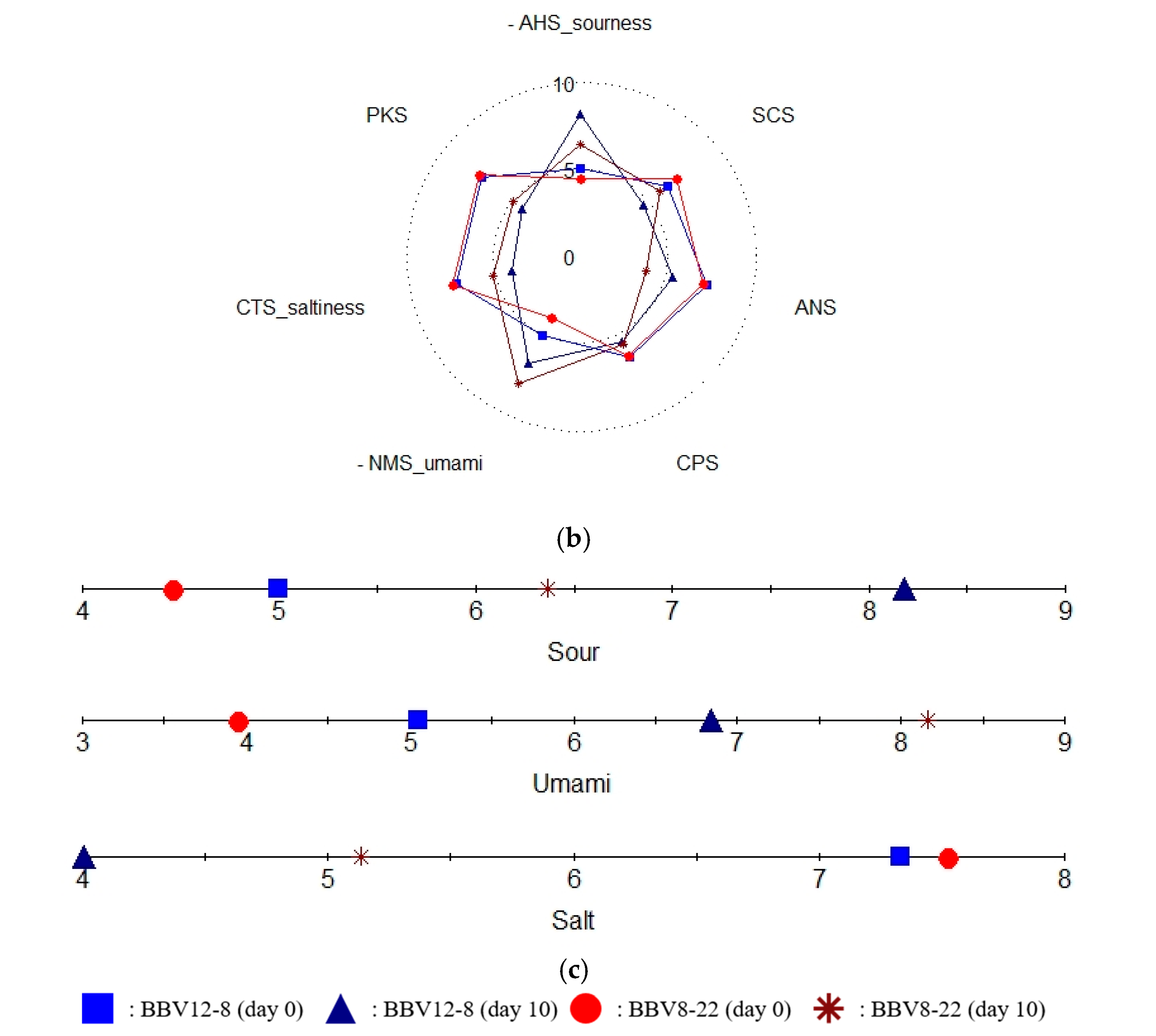
3.4. Taste Fingerprint
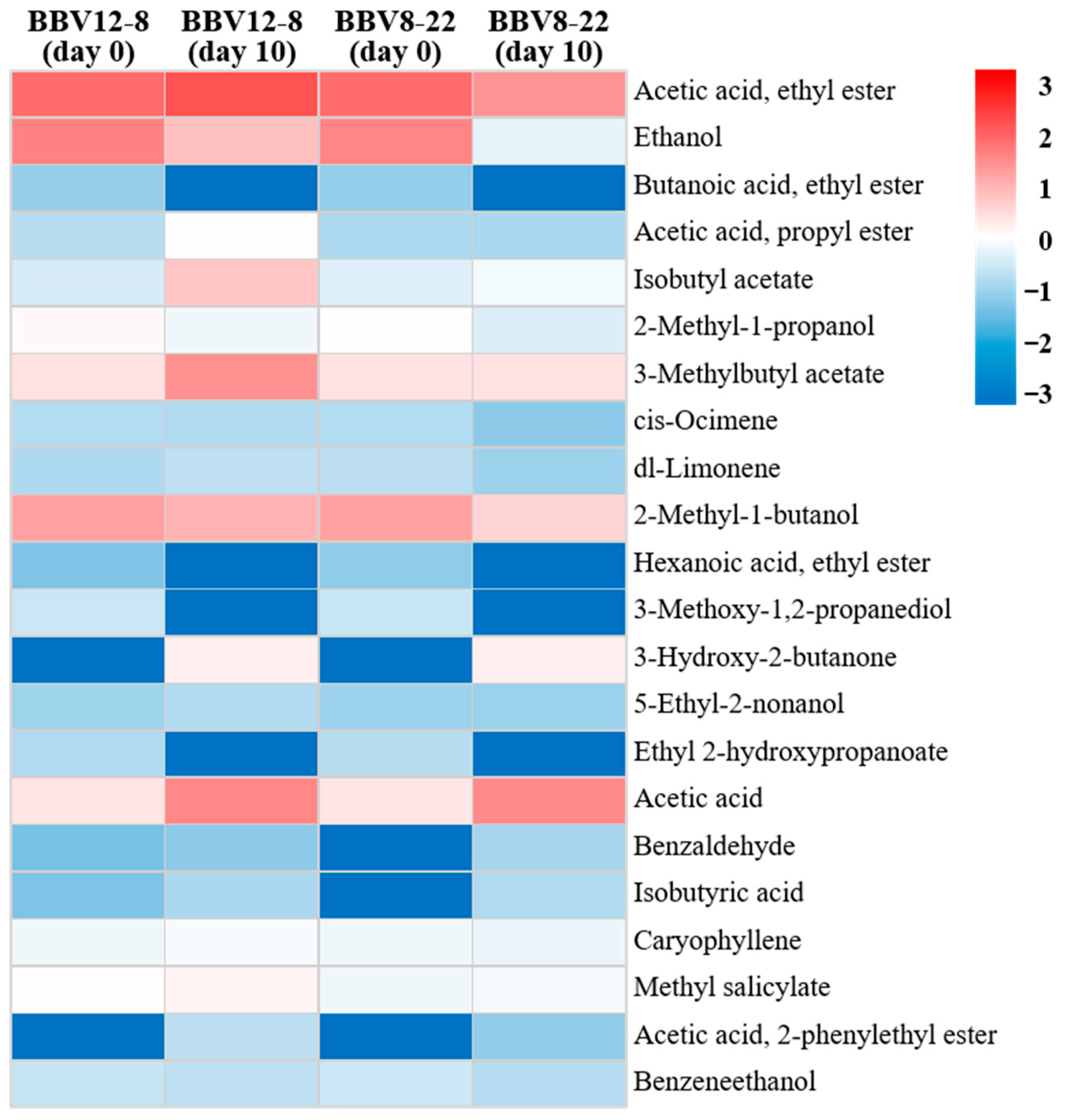
3.5. Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joo, K.-H.; Cho, M.-H.; Park, K.-J.; Jeong, S.-W.; Lim, J.-H. Effects of Fermentation Method and Brown Rice Content on Quality Characteristics of Brown Rice Vinegar. Korean J. Food Preserv. 2009, 16, 33–39. [Google Scholar]
- Chou, C.-H.; Liu, C.-W.; Yang, D.-J.; Wu, Y.-H.S.; Chen, Y.-C. Amino Acid, Mineral, and Polyphenolic Profiles of Black Vinegar, and Its Lipid Lowering and Antioxidant Effects in Vivo. Food Chem. 2015, 168, 63–69. [Google Scholar] [CrossRef]
- Sakanaka, S.; Ishihara, Y. Comparison of Antioxidant Properties of Persimmon Vinegar and Some Other Commercial Vinegars in Radical-Scavenging Assays and on Lipid Oxidation in Tuna Homogenates. Food Chem. 2008, 107, 739–744. [Google Scholar] [CrossRef]
- Park, E.-H.; Choi, C.-Y.; Kwon, H.-J.; Kim, M.-D. Literature Review on Type and Manufacturing Methods of Korean Traditional Vinegar. Food Sci. Ind. 2016, 49, 94–99. [Google Scholar]
- Cejudo-Bastante, C.; Durán-Guerrero, E.; García-Barroso, C.; Castro-Mejías, R. Volatile Compounds, Polyphenols and Sensory Quality in the Production of Tomato Vinegar. J. Food Nutr. Res. 2017, 5, 391–398. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.-A.; Park, G.-G.; Jang, J.-K.; Park, Y.-S. Semi-Continuous Fermentation of Onion Vinegar and Its Functional Properties. Molecules 2017, 22, 1313. [Google Scholar] [CrossRef]
- Chun, J.-E.; Baik, M.-Y.; Kim, B.-Y. Manufacture and Quality Evaluation of Purple Sweet Potato Makgeolli Vinegar Using a 2-Stage Fermentation. Food Sci. Biotechnol. 2014, 23, 1145–1149. [Google Scholar] [CrossRef]
- Karadag, A.; Bozkurt, F.; Bekiroglu, H.; Sagdic, O. Use of Principal Component Analysis and Cluster Analysis for Differentiation of Traditionally-Manufactured Vinegars Based on Phenolic and Volatile Profiles, and Antioxidant Activity. Pol. J. Food Nutr. Sci. 2020, 70, 347–360. [Google Scholar] [CrossRef]
- Louw, N.L.; Lele, K.; Ye, R.; Edwards, C.B.; Wolfe, B.E. Microbiome Assembly in Fermented Foods. Annu. Rev. Microbiol. 2023, 77, 381–402. [Google Scholar] [CrossRef]
- Han, D.; Yang, Y.; Guo, Z.; Dai, S.; Jiang, M.; Zhu, Y.; Wang, Y.; Yu, Z.; Wang, K.; Rong, C.; et al. A Review on the Interaction of Acetic Acid Bacteria and Microbes in Food Fermentation: A Microbial Ecology Perspective. Foods 2024, 13, 2534. [Google Scholar] [CrossRef]
- Gullo, M.; Caggia, C.; De Vero, L.; Giudici, P. Characterization of Acetic Acid Bacteria in “Traditional Balsamic Vinegar”. Int. J. Food Microbiol. 2006, 106, 209–212. [Google Scholar] [CrossRef]
- Baek, S.Y.; Park, H.Y.; Lee, C.H.; Yeo, S.-H. Comparison of the fermented property and isolation of acetic-acid bacteria from traditional Korean vinegar. Korean J. Food Preserv. 2014, 21, 903–907. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars. Fermentation 2023, 9, 447. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.-Y.; Jeong, W.S.; Gwon, H.-M.; Kim, S.Y.; Yeo, S.-H. Culture and function-related characteristics of six acetic acid bacterial strains isolated from farm-made fermented vinegars. Korean J. Food Preserv. 2022, 29, 142–156. [Google Scholar] [CrossRef]
- Hidalgo, C.; Vegas, C.; Mateo, E.; Tesfaye, W.; Cerezo, A.B.; Callejón, R.M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Torija, M.J. Effect of Barrel Design and the Inoculation of Acetobacter pasteurianus in Wine Vinegar Production. Int. J. Food Microbiol. 2010, 141, 56–62. [Google Scholar] [CrossRef]
- Kong, H.; Kim, S.H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Microbiome and Volatile Metabolic Profile of Acetic Acid Fermentation Using Multiple Starters for Traditional Grain Vinegar. Fermentation 2023, 9, 423. [Google Scholar] [CrossRef]
- Cho, C.-W.; Han, C.; Rhee, Y.; Lee, Y.-C.; Shin, K.-S.; Hong, H.-D. Immunostimulatory Effects of Polysaccharides Isolated from Makgeolli (Traditional Korean Rice Wine). Molecules 2014, 19, 5266–5277. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Song, J.-Y.; Park, H.-J.; Kim, J.-M.; No, J. The Chemical Composition and Fermentation Properties of Colored Barley. Korean J. Food Cook. Sci. 2022, 38, 356–366. [Google Scholar]
- Park, S.-M.; Choi, Y.-M.; Kim, Y.-H.; Ham, H.-M.; Jeong, H.-S.; Lee, J.-S. Antioxidant Content and Activity in Methanolic Extracts from Colored Barley. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1043–1047. [Google Scholar] [CrossRef]
- Song, E.S.; Park, S.J.; Won, M.H.; Choi, J.S.; Kim, J.G.; Kang, M.H. Antioxidant Capacity of Colored Barley Extracts by Varieties. J. Korean Soc. Food Sci. Nutr. 2005, 34, 1491–1497. [Google Scholar] [CrossRef]
- Gullo, M.; Giudici, P. Acetic Acid Bacteria in Traditional Balsamic Vinegar: Phenotypic Traits Relevant for Starter Cultures Selection. Int. J. Food Microbiol. 2008, 125, 46–53. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jang, H.W. Quality characteristics of black barley makgeolli using nuruk produced with useful molds. Food Sci. Preserv. 2025, 32, 124–135. [Google Scholar] [CrossRef]
- Song, S.H. Analysis of Microflora Profile in Korean Traditional Nuruk. J. Microbiol. Biotechnol. 2013, 23, 40–46. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Sung, K.-W.; Bae, H.-W.; Yi, Y.-H. pH, Acidity, Color, Reducing Sugar, Total Sugar, Alcohol and Organoleptic Characteristics of Puffed Rice Powder Added Takju during Fermentation. Korean J. Food Sci. Technol. 2007, 39, 266–271. [Google Scholar]
- Gil, N.-Y.; Gwon, H.-M.; Yeo, S.-H.; Kim, S.-Y. Optimization of ‘Nuruk’, alcohol, and acetic acid fermentations for producing vinegar from Acorus gramineus roots. Korean J. Food Preserv. 2020, 27, 936–945. [Google Scholar] [CrossRef]
- Jo, Y.; Chung, N.; Park, S.W.; Noh, B.S.; Jeong, Y.-J.; Kwon, J.-H. Application of E-Tongue, E-Nose, and MS-E-Nose for Discriminating Aged Vinegars Based on Taste and Aroma Profiles. Food Sci. Biotechnol. 2016, 25, 1313–1318. [Google Scholar] [CrossRef]
- Kong, H.; Kim, S.-H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Microbiome Analysis of Traditional Grain Vinegar Produced under Different Fermentation Conditions in Various Regions in Korea. Foods 2022, 11, 3573. [Google Scholar] [CrossRef]
- Adachi, O.; Fujii, Y.; Ano, Y.; Moonmangmee, D.; Toyama, H.; Shinagawa, E.; Theeragool, G.; Lotong, N.; Matsushita, K. Membrane-Bound Sugar Alcohol Dehydrogenase in Acetic Acid Bacteria Catalyzes L-Ribulose Formation and NAD-Dependent Ribitol Dehydrogenase Is Independent of the Oxidative Fermentation. Biosci. Biotechnol. Biochem. 2001, 65, 115–125. [Google Scholar] [CrossRef]
- Baek, C.; Baek, S.; Lee, S.H.; Kang, J.-E.; Choi, H.-S.; Kim, J.-H.; Yeo, S.-H. Characterization of Acetobacter sp. Strain CV1 Isolated from a Fermented Vinegar. Microbiol. Biotechnol. Lett. 2015, 43, 126–133. [Google Scholar] [CrossRef]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Chang, J.; Fang, T. Survival of Escherichia coli O157:H7 and Salmonella Enterica Serovars Typhimurium in Iceberg Lettuce and the Antimicrobial Effect of Rice Vinegar against E. coli O157:H7. Food Microbiol. 2007, 24, 745–751. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Deng, Y.; Beuchat, L.R. Behavior of Acid-Adapted and Unadapted Escherichia coli O157:H7 When Exposed to Reduced pH Achieved with Various Organic Acids. J. Food Prot. 1999, 62, 451–455. [Google Scholar] [CrossRef]
- Jang, S.; Jun, H.-I.; Oh, H.; Jeong, D.-Y.; Song, G.-S. Quality Characteristics of Aronia Vinegar Imparted by Varying Concentrations of Seed Vinegar. J. Korean Soc. Food Sci. Nutr. 2021, 50, 522–530. [Google Scholar] [CrossRef]
- Wu, L.-H.; Lu, Z.-M.; Zhang, X.-J.; Wang, Z.-M.; Yu, Y.-J.; Shi, J.-S.; Xu, Z.-H. Metagenomics Reveals Flavour Metabolic Network of Cereal Vinegar Microbiota. Food Microbiol. 2017, 62, 23–31. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The Regulation of Key Flavor of Traditional Fermented Food by Microbial Metabolism: A Review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich Pathway for Fusel Alcohol Production: A Century of Research on Saccharomyces cerevisiae Metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of Taste-Active Amino Acids, Amino Acid Derivatives and Peptides in Food Fermentations—A Review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.; Sun, Y.; Zhang, Y.; Sun, B.; Chen, H. Determination of the Free Amino Acid, Organic Acid, and Nucleotide in Commercial Vinegars. J. Food Sci. 2017, 82, 1116–1123. [Google Scholar] [CrossRef]
- Callejón, R.M.; Morales, M.L.; Ferreira, A.C.S.; Troncoso, A.M. Defining the Typical Aroma of Sherry Vinegar: Sensory and Chemical Approach. J. Agric. Food Chem. 2008, 56, 8086–8095. [Google Scholar] [CrossRef]
- Liu, C.; Feng, S.; Wu, Q.; Huang, H.; Chen, Z.; Li, S.; Xu, Y. Raw Material Regulates Flavor Formation via Driving Microbiota in Chinese Liquor Fermentation. Front. Microbiol. 2019, 10, 1520. [Google Scholar] [CrossRef]
- Li, S.; Li, P.; Feng, F.; Luo, L.-X. Microbial Diversity and Their Roles in the Vinegar Fermentation Process. Appl. Microbiol. Biotechnol. 2015, 99, 4997–5024. [Google Scholar] [CrossRef]
- Rhee, S.; Lee, J.-E.; Lee, C.-H. Importance of Lactic Acid Bacteria in Asian Fermented Foods. Microb. Cell Factories 2011, 10, S5. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, J.; Gan, H.M.; Yin, W.-F.; Chan, K.-G. Microbial Succession and the Functional Potential during the Fermentation of Chinese Soy Sauce Brine. Front. Microbiol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Tian, T.; Yang, H.; Yang, F.; Li, B.; Sun, J.; Wu, D.; Lu, J. Optimization of Fermentation Conditions and Comparison of Flavor Compounds for Three Fermented Greengage Wines. LWT 2018, 89, 542–550. [Google Scholar] [CrossRef]
- Wang, F.; Song, Y.; Vidyarthi, S.K.; Zhang, R. Physicochemical Properties, and Volatile Compounds of Blackened Jujube Vinegar as Prepared by Optimized Fermentation Process. Int. J. Food Prop. 2022, 25, 288–304. [Google Scholar] [CrossRef]
- Feng, Y.; Su, G.; Zhao, H.; Cai, Y.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Characterisation of Aroma Profiles of Commercial Soy Sauce by Odour Activity Value and Omission Test. Food Chem. 2015, 167, 220–228. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Okamoto, A.; Ohshima, T. Olfactometric Characterization of Aroma Active Compounds in Fermented Fish Paste in Comparison with Fish Sauce, Fermented Soy Paste and Sauce Products. Food Res. Int. 2010, 43, 1027–1040. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative Study of Volatile Compounds in the Fruit of Two Banana Cultivars at Different Ripening Stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Morales, M.L.; Troncoso, A.M.; Silva Ferreira, A.C. Targeting Key Aromatic Substances on the Typical Aroma of Sherry Vinegar. J. Agric. Food Chem. 2008, 56, 6631–6639. [Google Scholar] [CrossRef]
- Lee, S.-W.; Yoon, S.-R.; Kim, G.-R.; Woo, S.-M.; Jeong, Y.-J.; Yeo, S.-H.; Kim, K.-S.; Kwon, J.-H. Effect of Nuruk and Fermentation Method on Organic Acid and Volatile Compounds in Brown Rice Vinegar. Food Sci. Biotechnol. 2012, 21, 453–460. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Durán-Guerrero, E.; Natera-Marín, R.; Castro-Mejías, R.; García-Barroso, C. Characterisation of Commercial Aromatised Vinegars: Phenolic Compounds, Volatile Composition and Antioxidant Activity. J. Sci. Food Agric. 2013, 93, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Chanivet, M.; Es-sbata, I.; Astola, A.; Durán-Guerrero, E.; Castro, R. Thermotolerant Acetic Acid Bacteria in the Production of a Red Wine Vinegar by Surface Culture at Different Temperatures: Volatile and Polyphenolic Composition. Eur. Food Res. Technol. 2024, 250, 2849–2862. [Google Scholar] [CrossRef]
- Es-sbata, I.; Castro, R.; Durán-Guerrero, E.; Zouhair, R.; Astola, A. Production of Prickly Pear (Opuntia ficus-indica) Vinegar in Submerged Culture Using Acetobacter malorum and Gluconobacter oxydans: Study of Volatile and Polyphenolic Composition. J. Food Compos. Anal. 2022, 112, 104699. [Google Scholar] [CrossRef]



| Sample | Organic Acid (mg/100 mL) | Total | ||||
|---|---|---|---|---|---|---|
| Citric Acid | Malic Acid | Succinic Acid | Lactic Acid | Acetic Acid | ||
| BBV12-8 (day 0) | 139.89 ± 0.49 b 1 | 8.32 ± 0.12 b | 25.57 ± 0.87 a | 12.04 ± 0.51 a | 39.79 ± 3.09 b | 225.60 ± 5.08 |
| BBV12-8 (day 10) | 92.48 ± 0.24 d | 6.93 ± 0.04 c | 21.23 ± 0.06 b | 1.84 ± 0.01 b | 408.09 ± 1.45 a | 530.56 ± 1.81 |
| BBV8-22 (day 0) | 140.71 ± 0.35 a | 8.45 ± 0.10 b | 26.00 ± 0.04 a | 12.12 ± 0.05 a | 39.26 ± 0.05 b | 226.54 ± 0.59 |
| BBV8-22 (day 10) | 93.34 ± 0.06 c | 10.45 ± 0.15 a | 21.52 ± 0.83 b | 1.52 ± 0.25 b | 423.53 ± 25.74 a | 550.36 ± 27.02 |
| RT (min) | Compound | VIP 1 | p-Value 2 |
|---|---|---|---|
| 2.72 | Acetic acid, ethyl ester | 1.44 | 5.24 × 10−6 |
| 3.34 | Ethanol | 0.95 | 1.45 × 10−6 |
| 3.71 | Butanoic acid, ethyl ester | 0.84 | 3.39 × 10−3 |
| 3.85 | Acetic acid, propyl ester | 1.29 | 3.50 × 10−7 |
| 4.52 | Isobutyl acetate | 1.24 | 9.04 × 10−9 |
| 6.37 | 2-Methyl-1-propanol | 0.99 | 5.50 × 10−5 |
| 6.91 | 3-Methylbutyl acetate | 1.28 | 2.21 × 10−8 |
| 7.42 | cis-Ocimene | 0.82 | 2.39 × 10−1 |
| 8.78 | dl-Limonene | 0.76 | 4.00 × 10−1 |
| 9.32 | 2-Methyl-1-butanol | 1.09 | 6.46 × 10−7 |
| 9.91 | Hexanoic acid, ethyl ester | 0.92 | 7.69 × 10−6 |
| 11.23 | 3-Methoxy-1,2-propanediol | 0.93 | 3.30 × 10−7 |
| 11.23 | 3-Hydroxy-2-butanone | 0.93 | 3.38 × 10−7 |
| 12.43 | 5-Ethyl-2-nonanol | 0.93 | 1.31 × 10−1 |
| 12.93 | Ethyl 2-hydroxypropanoate | 0.94 | 2.71 × 10−7 |
| 15.39 | Acetic acid | 0.93 | 5.15 × 10−7 |
| 16.44 | Benzaldehyde | 1.02 | 8.76 × 10−5 |
| 17.02 | Isobutyric acid | 0.93 | 7.42 × 10−6 |
| 17.80 | Caryophyllene | 0.73 | 4.47 × 10−1 |
| 18.99 | Methyl salicylate | 0.71 | 3.02 × 10−1 |
| 19.25 | Acetic acid, 2-phenylethyl ester | 1.05 | 2.60 × 10−7 |
| 19.91 | Benzeneethanol | 0.93 | 1.43 × 10−3 |
| Group | Compound | BBV12-8 (Day 0) | BBV12-8 (Day 10) | BBV8-22 (Day 0) | BBV8-22 (Day 10) |
|---|---|---|---|---|---|
| Esters | Acetic acid, ethyl ester | 54.21 ± 7.08 b 2 | 106.95 ± 9.00 a | 54.73 ± 4.74 b | 18.65 ± 2.14 c |
| Butanoic acid, ethyl ester | 0.09 ± 0.04 | N.D. 1 | 0.09 ± 0.01 | N.D. | |
| Acetic acid, propyl ester | 0.19 ± 0.01 b | 1.04 ± 0.10 a | 0.16 ± 0.03 b | 0.14 ± 0.01 b | |
| Isobutyl acetate | 0.38 ± 0.03 b | 4.87 ± 0.31 a | 0.46 ± 0.02 b | 0.76 ± 0.11 b | |
| 3-Methylbutyl acetate | 2.32 ± 0.19 b | 20.64 ± 1.53 a | 2.30 ± 0.04 b | 2.36 ± 0.30 b | |
| Hexanoic acid, ethyl ester | 0.06 ± 0.01 | N.D. | 0.08 ± 0.01 | N.D. | |
| Ethyl 2-hydroxypropanoate | 0.17 ± 0.02 | N.D. | 0.19 ± 0.01 | N.D. | |
| Acetic acid, 2-phenylethyl ester | N.D. | 0.20 ± 0.02 ** 3 | N.D. | 0.08 ± 0.02 | |
| Alcohols | Ethanol | 31.15 ± 3.74 a | 5.74 ± 0.54 b | 27.82 ± 2.46 a | 0.56 ± 0.14 b |
| 2-Methyl-1-propanol | 1.22 ± 0.13 a | 0.71 ± 0.09 b | 1.08 ± 0.07 a | 0.42 ± 0.01 c | |
| 2-Methyl-1-butanol | 13.50 ± 0.93 a | 8.25 ± 0.46 b | 12.89 ± 0.53 a | 3.36 ± 0.44 c | |
| 5-Ethyl-2-nonanol | 0.11 ± 0.02 ab | 0.17 ± 0.05 a | 0.11 ± 0.01 ab | 0.10 ± 0.00 b | |
| Benzeneethanol | 0.26 ± 0.02 b | 0.22 ± 0.01 bc | 0.31 ± 0.02 a | 0.18 ± 0.03 c | |
| Acids | Acetic acid | 2.13 ± 0.34 b | 26.14 ± 2.52 a | 2.07 ± 0.25 b | 24.51 ± 2.31 a |
| Isobutyric acid | 0.06 ± 0.00 b | 0.15 ± 0.02 a | N.D. | 0.16 ± 0.01 a | |
| Terpenes | cis-Ocimene | 0.18 ± 0.07 a | 0.16 ± 0.07 a | 0.18 ± 0.03 a | 0.08 ± 0.02 a |
| dl-Limonene | 0.16 ± 0.08 a | 0.22 ± 0.08 a | 0.20 ± 0.03 a | 0.11 ± 0.06 a | |
| Caryophyllene | 0.69 ± 0.10 a | 0.85 ± 0.19 a | 0.69 ± 0.15 a | 0.64 ± 0.02 a | |
| Others | 3-Methoxy-1,2-propanediol | 0.29 ± 0.03 | N.D. | 0.29 ± 0.03 | N.D. |
| 3-Hydroxy-2-butanone | N.D. | 1.61 ± 0.17 | N.D. | 1.55 ± 0.14 | |
| Benzaldehyde | 0.05 ± 0.01 b | 0.08 ± 0.02 b | N.D. | 0.13 ± 0.01 a | |
| Methyl salicylate | 1.05 ± 0.57 a | 1.41 ± 0.50 a | 0.70 ± 0.06 a | 0.77 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Jang, H.-w.; Gwon, H.-M. Development of Sequential Fermentation Starters and Comparison of Quality Characteristics for Black Barley Vinegar Production. Microorganisms 2025, 13, 2576. https://doi.org/10.3390/microorganisms13112576
Lee S-Y, Jang H-w, Gwon H-M. Development of Sequential Fermentation Starters and Comparison of Quality Characteristics for Black Barley Vinegar Production. Microorganisms. 2025; 13(11):2576. https://doi.org/10.3390/microorganisms13112576
Chicago/Turabian StyleLee, Soo-Young, Hyun-wook Jang, and Hee-Min Gwon. 2025. "Development of Sequential Fermentation Starters and Comparison of Quality Characteristics for Black Barley Vinegar Production" Microorganisms 13, no. 11: 2576. https://doi.org/10.3390/microorganisms13112576
APA StyleLee, S.-Y., Jang, H.-w., & Gwon, H.-M. (2025). Development of Sequential Fermentation Starters and Comparison of Quality Characteristics for Black Barley Vinegar Production. Microorganisms, 13(11), 2576. https://doi.org/10.3390/microorganisms13112576






