ISG20 Restricts BK Polyomavirus Infection and Engages in Reciprocal Regulation with Viral Large T Antigen
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Plasmids
2.3. Stable Cells
2.4. Viruses
2.5. IFN-β Antiviral Assay
2.6. ISG20 Antiviral Assay
2.7. Transient DNA Transfection
2.8. Western Blotting (WB)
2.9. Quantitative Real-Time PCR (qPCR)
2.10. Immunofluorescence Staining and Microscopy
2.11. Co-Immunoprecipitation (Co-IP)
2.12. Cell Viability Assay
2.13. Statistical Analysis
3. Results
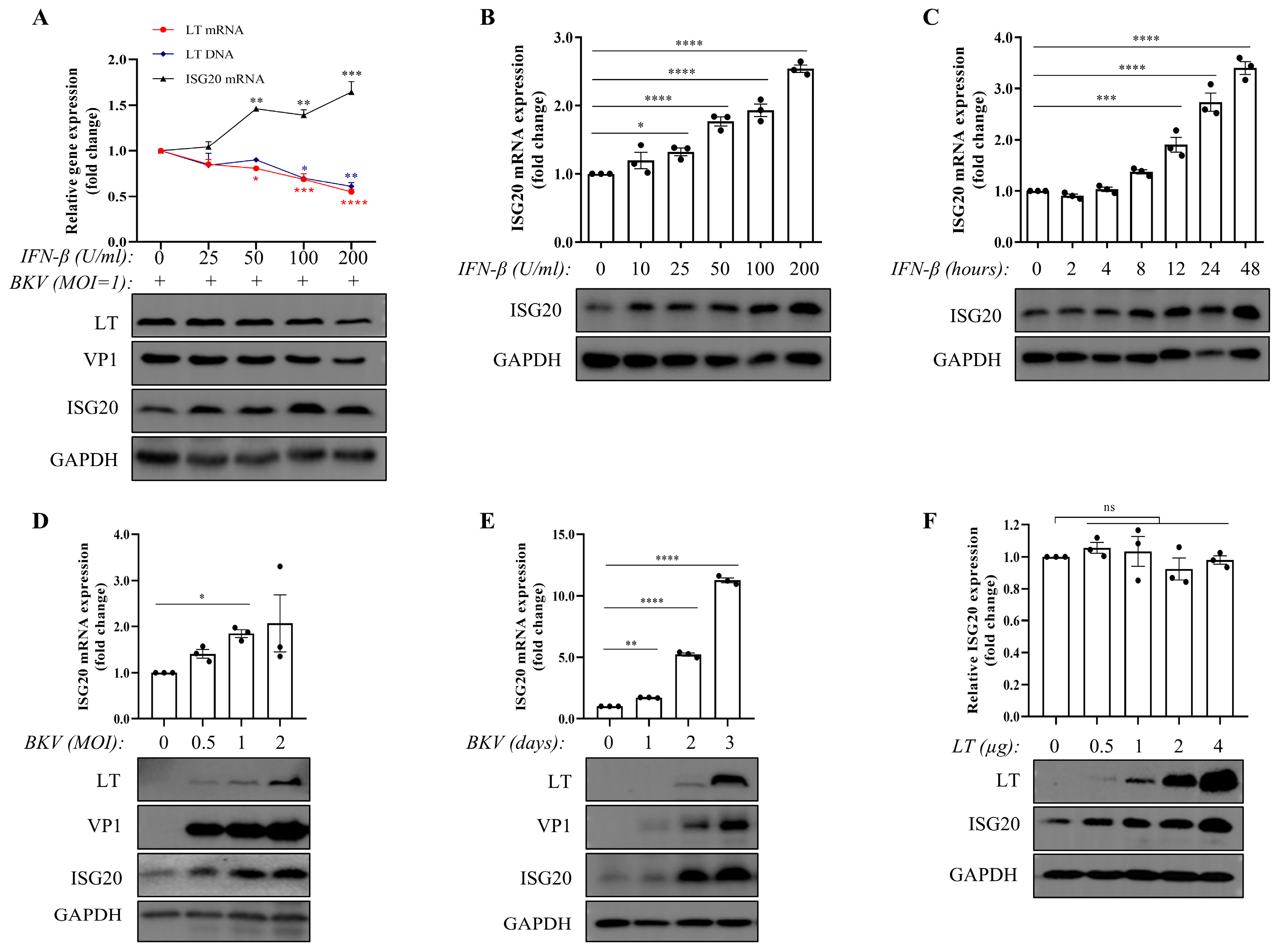
3.1. Both IFN-β and BKPyV Upregulate the Expression of ISG20
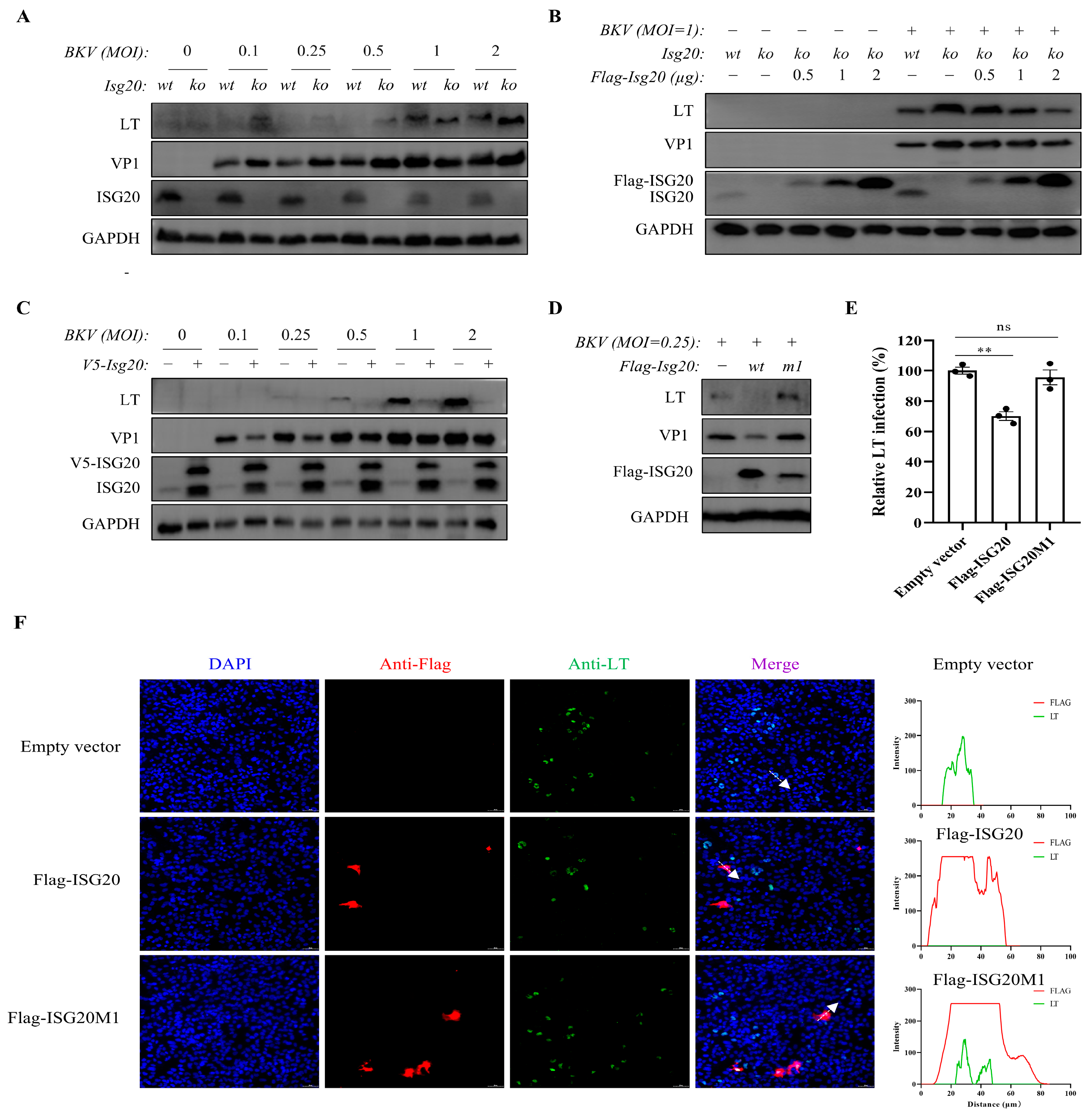
3.2. ISG20 Is an Antiviral Factor Against BKPyV
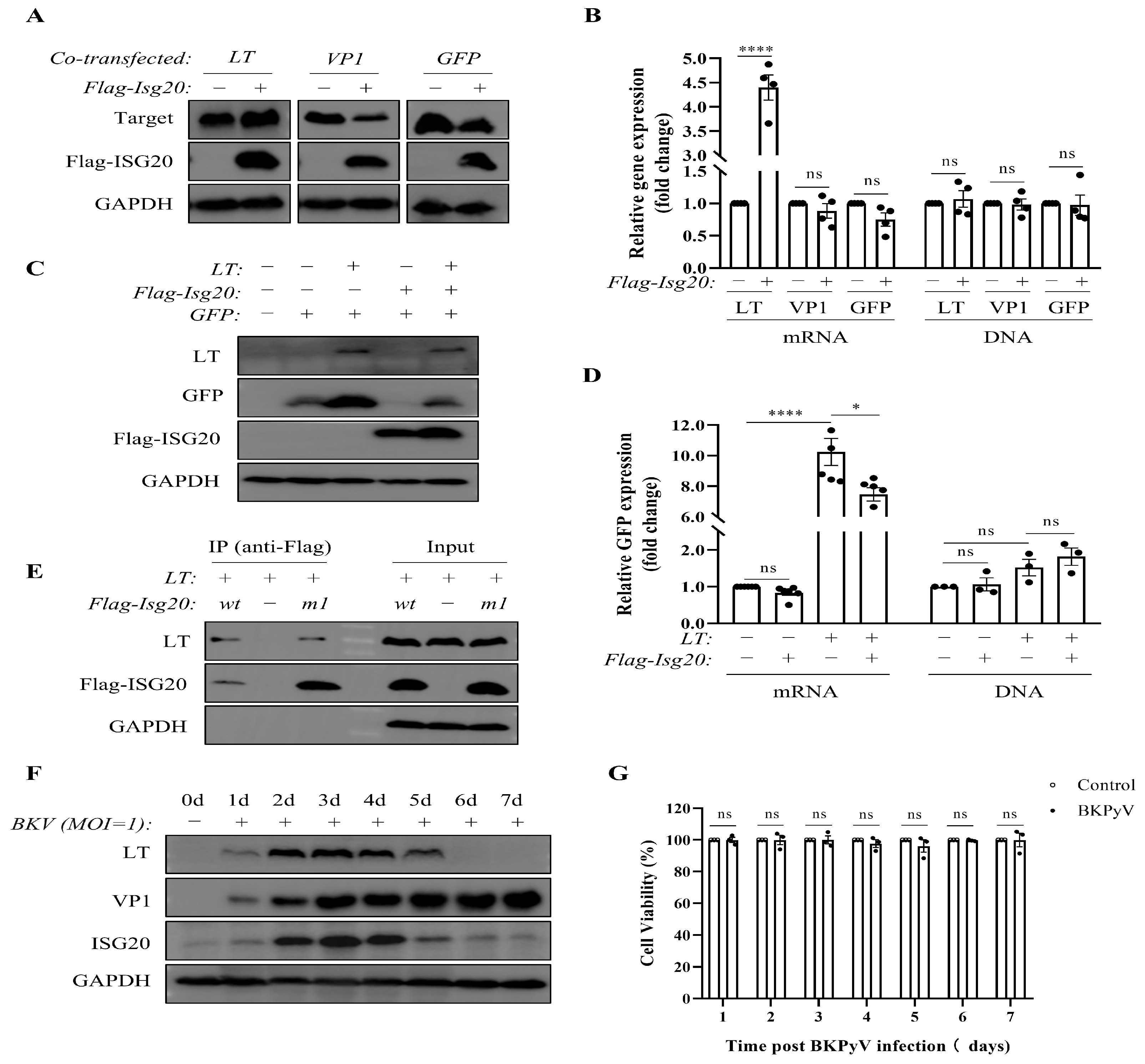
3.3. Dynamic LT-ISG20 Crosstalk Balances Viral Propagation and Host Restriction During BKPyV Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BKPyV | BK polyomavirus |
| WB | Western blot |
| qPCR | Quantitative Polymerase Chain Reaction |
| MOI | Multiplicity of Infection |
| GAPDH | Glyceraldehyde3-phosphate dehydrogenase |
| ECL | Enhanced Chemiluminescence |
| ISG | Interferon-Stimulated Gene |
| IFN | Interferon |
| CO-IP | Co-immunoprecipitation |
| MEM/EBSS | Minimum Essential Medium with Earle’s Balanced Salt Solution |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EGFP | Enhanced Green Fluorescent Protein |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| SEM | Standard Error of the Mean |
| CCK-8 | Cell Counting Kit-8 |
References
- Laine, H.K.; Waterboer, T.; Syrjänen, K.; Grenman, S.; Louvanto, K.; Syrjänen, S. Seroprevalence of polyomaviruses BK and JC in Finnish women and their spouses followed-up for three years. Sci. Rep. 2023, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Al-Talib, M.; Welberry-Smith, M.; Macdonald, A.; Griffin, S. BK Polyomavirus-associated nephropathy—Diagnostic and treatment standard. Nephrol. Dial. Transplant. 2025, 40, 651–661. [Google Scholar] [CrossRef]
- Ambalathingal, G.R.; Francis, R.S.; Smyth, M.J.; Smith, C.; Khanna, R. BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies. Clin. Microbiol. Rev. 2017, 30, 503–528. [Google Scholar] [CrossRef]
- Starrett, G.J.; Yu, K.; Golubeva, Y.; Lenz, P.; Piaskowski, M.L.; Petersen, D.; Dean, M.; Israni, A.; Hernandez, B.Y.; Tucker, T.C.; et al. Evidence for virus-mediated oncogenesis in bladder cancers arising in solid organ transplant recipients. Elife 2023, 12, e82690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Imperiale, M.J. A Cell Culture Model of BK Polyomavirus Persistence, Genome Recombination, and Reactivation. mBio 2021, 12, e0235621. [Google Scholar] [CrossRef]
- Bizhani, S.; Afshari, A.; Yaghobi, R. BK Polyomavirus and acute kidney injury in transplant recipients: Signaling pathways and molecular mechanisms. Virol. J. 2025, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Needham, J.M.; Greco, T.M.; Cristea, I.M.; Thompson, S.R. Ribosomal protein S25 promotes cell cycle entry for a productive BK polyomavirus infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2025, 380, 20230390. [Google Scholar] [CrossRef]
- An, P.; Sáenz Robles, M.T.; Duray, A.M.; Cantalupo, P.G.; Pipas, J.M. Human polyomavirus BKV infection of endothelial cells results in interferon pathway induction and persistence. PLoS Pathog. 2019, 15, e1007505. [Google Scholar] [CrossRef]
- Espert, L.; Degols, G.; Gongora, C.; Blondel, D.; Williams, B.R.; Silverman, R.H.; Mechti, N. ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA genomic viruses. J. Biol. Chem. 2003, 278, 16151–16158. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, N.; Woodson, S.E.; Dong, Q.; Wang, J.; Liang, Y.; Rijnbrand, R.; Wei, L.; Nichols, J.E.; Guo, J.T.; et al. Antiviral activities of ISG20 in positive-strand RNA virus infections. Virology 2011, 409, 175–188. [Google Scholar] [CrossRef]
- Imam, H.; Kim, G.W.; Mir, S.A.; Khan, M.; Siddiqui, A. Interferon-stimulated gene 20 (ISG20) selectively degrades N6-methyladenosine modified Hepatitis B Virus transcripts. PLoS Pathog. 2020, 16, e1008338. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Nguyen, X.N.; Wang, L.; Appourchaux, R.; Zhang, C.; Panthu, B.; Gruffat, H.; Journo, C.; Alais, S.; Qin, J.; et al. The interferon stimulated gene 20 protein (ISG20) is an innate defense antiviral factor that discriminates self versus non-self translation. PLoS Pathog. 2019, 15, e1008093. [Google Scholar] [CrossRef]
- Deymier, S.; Louvat, C.; Fiorini, F.; Cimarelli, A. ISG20: An enigmatic antiviral RNase targeting multiple viruses. FEBS Open Bio 2022, 12, 1096–1111. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.M.; Trobaugh, D.W.; Sun, C.; Lucas, T.M.; Diamond, M.S.; Ryman, K.D.; Klimstra, W.B. The Interferon-Induced Exonuclease ISG20 Exerts Antiviral Activity through Upregulation of Type I Interferon Response Proteins. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Xu, T.; Ruan, H.; Gao, S.; Liu, J.; Liu, Y.; Song, Z.; Cao, Q.; Wang, K.; Bao, L.; Liu, D.; et al. ISG20 serves as a potential biomarker and drives tumor progression in clear cell renal cell carcinoma. Aging 2020, 12, 1808–1827. [Google Scholar] [CrossRef]
- Jia, M.; Li, L.; Chen, R.; Du, J.; Qiao, Z.; Zhou, D.; Liu, M.; Wang, X.; Wu, J.; Xie, Y.; et al. Targeting RNA oxidation by ISG20-mediated degradation is a potential therapeutic strategy for acute kidney injury. Mol. Ther. 2023, 31, 3034–3051. [Google Scholar] [CrossRef]
- Jia, M.; Han, S.; Li, L.; Fu, Y.; Zhou, D. Interferon-Stimulated Genes: Novel Targets in Renal Pathogenesis. Kidney Dis. 2025, 11, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, Y.; Shi, H.; Hou, Y.; Hu, C.; Zeng, Y.; Wu, G.; Zhu, T. Intracellular Low Iron Exerts Anti-BK Polyomavirus Effect by Inhibiting the Protein Synthesis of Exogenous Genes. Microbiol. Spectr. 2021, 9, e0109421. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, X.; Zhu, T.; Hou, Y.; Shi, Y.; Sun, J.; Wu, N. High-sensitivity BK virus detection system using viewRNA in situ hybridization. Diagn. Microbiol. Infect. Dis. 2025, 112, 116790. [Google Scholar] [CrossRef]
- Schowalter, R.M.; Pastrana, D.V.; Buck, C.B. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog. 2011, 7, e1002161. [Google Scholar] [CrossRef]
- Swaraj, S.; Tripathi, S. Interference without interferon: Interferon-independent induction of interferon-stimulated genes and its role in cellular innate immunity. mBio 2024, 15, e0258224. [Google Scholar] [CrossRef]
- Wang, W.; Xu, L.; Brandsma, J.H.; Wang, Y.; Hakim, M.S.; Zhou, X.; Yin, Y.; Fuhler, G.M.; van der Laan, L.J.; van der Woude, C.J.; et al. Convergent Transcription of Interferon-stimulated Genes by TNF-α and IFN-α Augments Antiviral Activity against HCV and HEV. Sci. Rep. 2016, 6, 25482. [Google Scholar] [CrossRef]
- Googins, M.R.; An, P.; Gauthier, C.H.; Pipas, J.M. Polyomavirus large T antigens: Unraveling a complex interactome. Tumour Virus Res. 2025, 19, 200306. [Google Scholar] [CrossRef]
- de Kort, H.; Heutinck, K.M.; Ruben, J.M.; Ede, V.S.A.; Wolthers, K.C.; Hamann, J.; Ten Berge, I.J.M. Primary Human Renal-Derived Tubular Epithelial Cells Fail to Recognize and Suppress BK Virus Infection. Transplantation 2017, 101, 1820–1829. [Google Scholar] [CrossRef]
- Espert, L.; Degols, G.; Lin, Y.L.; Vincent, T.; Benkirane, M.; Mechti, N. Interferon-induced exonuclease ISG20 exhibits an antiviral activity against human immunodeficiency virus type 1. J. Gen. Virol. 2005, 86, 2221–2229. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, H.; Mao, R.; Mitra, B.; Cai, D.; Yan, R.; Guo, J.T.; Block, T.M.; Mechti, N.; Guo, H. Interferon-inducible ribonuclease ISG20 inhibits hepatitis B virus replication through directly binding to the epsilon stem-loop structure of viral RNA. PLoS Pathog. 2017, 13, e1006296. [Google Scholar] [CrossRef]
- Leong, C.R.; Funami, K.; Oshiumi, H.; Mengao, D.; Takaki, H.; Matsumoto, M.; Aly, H.H.; Watashi, K.; Chayama, K.; Seya, T. Interferon-stimulated gene of 20 kDa protein (ISG20) degrades RNA of hepatitis B virus to impede the replication of HBV in vitro and in vivo. Oncotarget 2016, 7, 68179–68193. [Google Scholar] [CrossRef]
- Stadler, D.; Kächele, M.; Jones, A.N.; Hess, J.; Urban, C.; Schneider, J.; Xia, Y.; Oswald, A.; Nebioglu, F.; Bester, R.; et al. Interferon-induced degradation of the persistent hepatitis B virus cccDNA form depends on ISG20. EMBO Rep. 2021, 22, e49568. [Google Scholar] [CrossRef]
- Starrett, G.J.; Serebrenik, A.A.; Roelofs, P.A.; McCann, J.L.; Verhalen, B.; Jarvis, M.C.; Stewart, T.A.; Law, E.K.; Krupp, A.; Jiang, M.; et al. Polyomavirus T Antigen Induces APOBEC3B Expression Using an LXCXE-Dependent and TP53-Independent Mechanism. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; Geoghegan, E.M.; Pastrana, D.V.; Smola, S.; Feld, P.; Sauter, M.; Lohse, S.; Ramesh, M.; Lim, E.S.; Wang, D.; et al. Characterization of BK Polyomaviruses from Kidney Transplant Recipients Suggests a Role for APOBEC3 in Driving In-Host Virus Evolution. Cell Host Microbe 2018, 23, 628–635.e7. [Google Scholar] [CrossRef] [PubMed]
- Giacobbi, N.S.; Gupta, T.; Coxon, A.T.; Pipas, J.M. Polyomavirus T antigens activate an antiviral state. Virology 2015, 476, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Reus, J.B.; Trivino-Soto, G.S.; Wu, L.I.; Kokott, K.; Lim, E.S. SV40 Large T Antigen Is Not Responsible for the Loss of STING in 293T Cells but Can Inhibit cGAS-STING Interferon Induction. Viruses 2020, 12, 137. [Google Scholar] [CrossRef]
- Fallatah, D.I.; Christmas, S.; Binshaya, A. Adaptive immune response and evasion strategies of BK virus. Transpl. Rev. 2025, 39, 100959. [Google Scholar] [CrossRef]
- Duggal, N.K.; Emerman, M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat. Rev. Immunol. 2012, 12, 687–695. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, G.; Zhu, L.; Zhao, Z.; Liu, Y.; Han, W.; Zhang, X.; Zhang, Y.; Xiong, T.; Zeng, H.; et al. HIV-1 Vif suppresses antiviral immunity by targeting STING. Cell Mol. Immunol. 2022, 19, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Schierhorn, K.L.; Jolmes, F.; Bespalowa, J.; Saenger, S.; Peteranderl, C.; Dzieciolowski, J.; Mielke, M.; Budt, M.; Pleschka, S.; Herrmann, A.; et al. Influenza A Virus Virulence Depends on Two Amino Acids in the N-Terminal Domain of Its NS1 Protein To Facilitate Inhibition of the RNA-Dependent Protein Kinase PKR. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Hu, C.; Shi, Y.; Zhou, X.; Zhu, T.; Wu, N. ISG20 Restricts BK Polyomavirus Infection and Engages in Reciprocal Regulation with Viral Large T Antigen. Microorganisms 2025, 13, 2540. https://doi.org/10.3390/microorganisms13112540
Hou Y, Hu C, Shi Y, Zhou X, Zhu T, Wu N. ISG20 Restricts BK Polyomavirus Infection and Engages in Reciprocal Regulation with Viral Large T Antigen. Microorganisms. 2025; 13(11):2540. https://doi.org/10.3390/microorganisms13112540
Chicago/Turabian StyleHou, Yumin, Chunlan Hu, Yejing Shi, Xiaohui Zhou, Tongyu Zhu, and Nannan Wu. 2025. "ISG20 Restricts BK Polyomavirus Infection and Engages in Reciprocal Regulation with Viral Large T Antigen" Microorganisms 13, no. 11: 2540. https://doi.org/10.3390/microorganisms13112540
APA StyleHou, Y., Hu, C., Shi, Y., Zhou, X., Zhu, T., & Wu, N. (2025). ISG20 Restricts BK Polyomavirus Infection and Engages in Reciprocal Regulation with Viral Large T Antigen. Microorganisms, 13(11), 2540. https://doi.org/10.3390/microorganisms13112540




