Changes in the Gut Microbiota of Patients After SARS-CoV-2 Infection: What Do We Know?
Abstract
1. Introduction
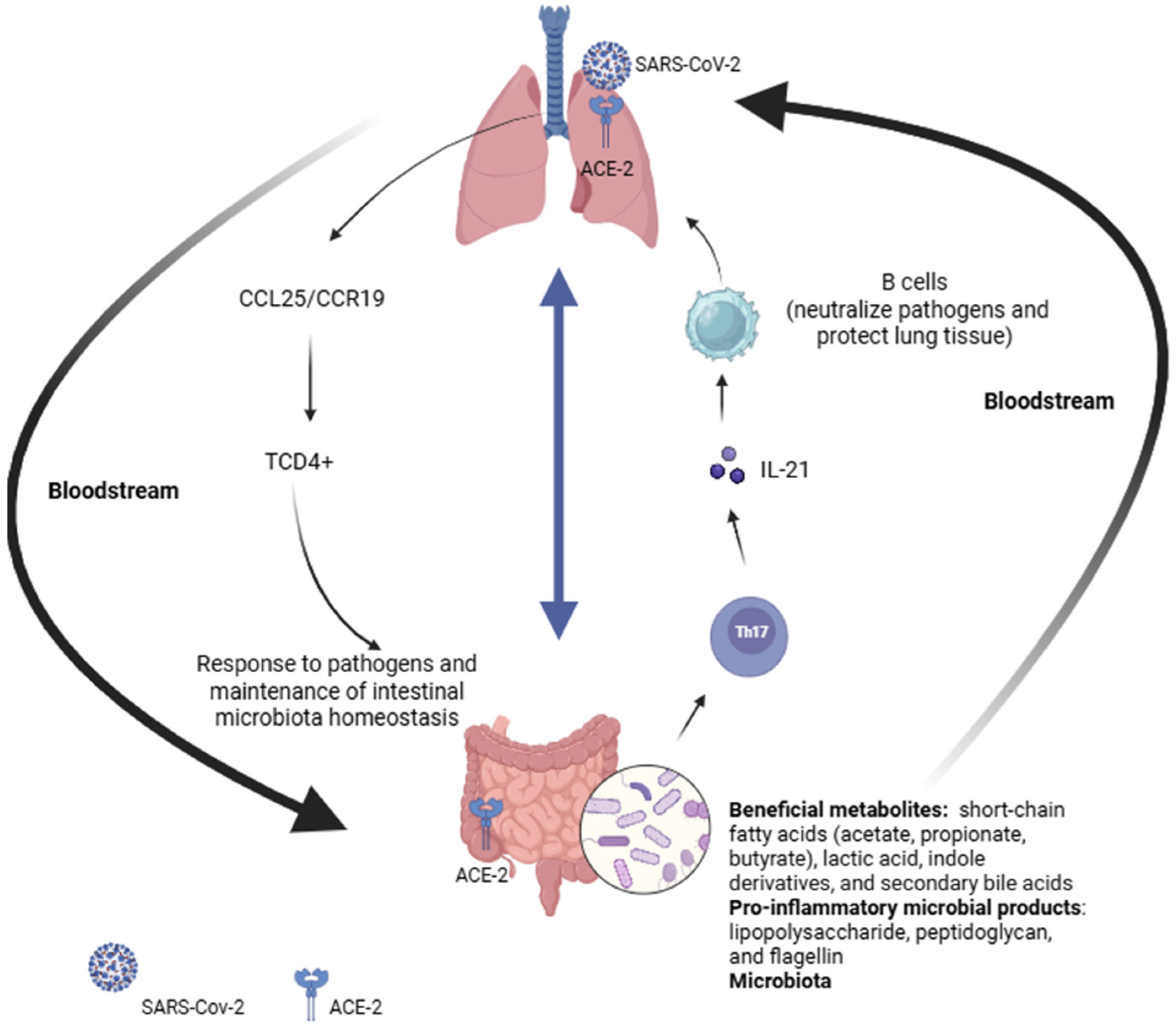
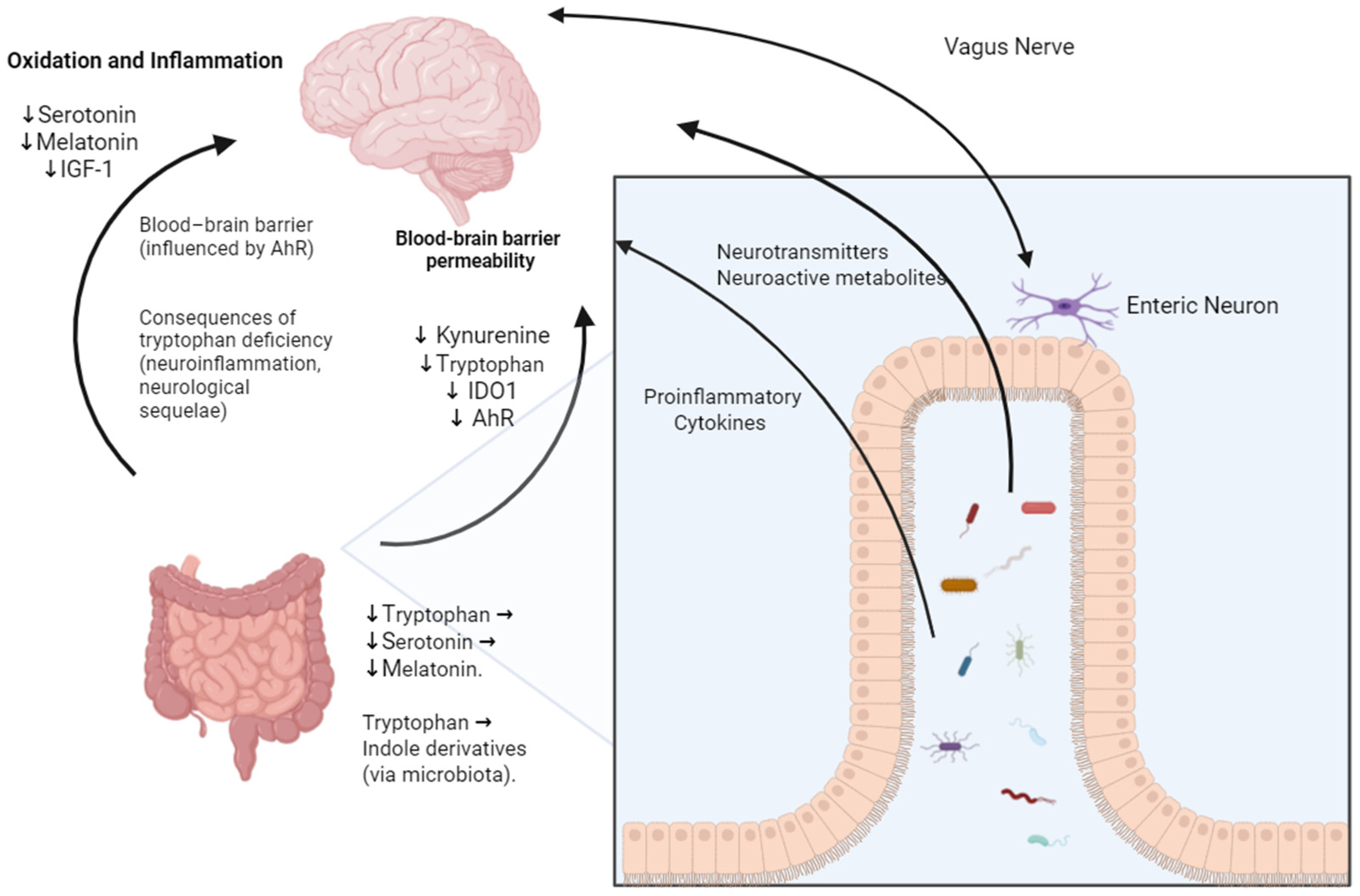
2. Gut–Lung and Gut–Brain Axis
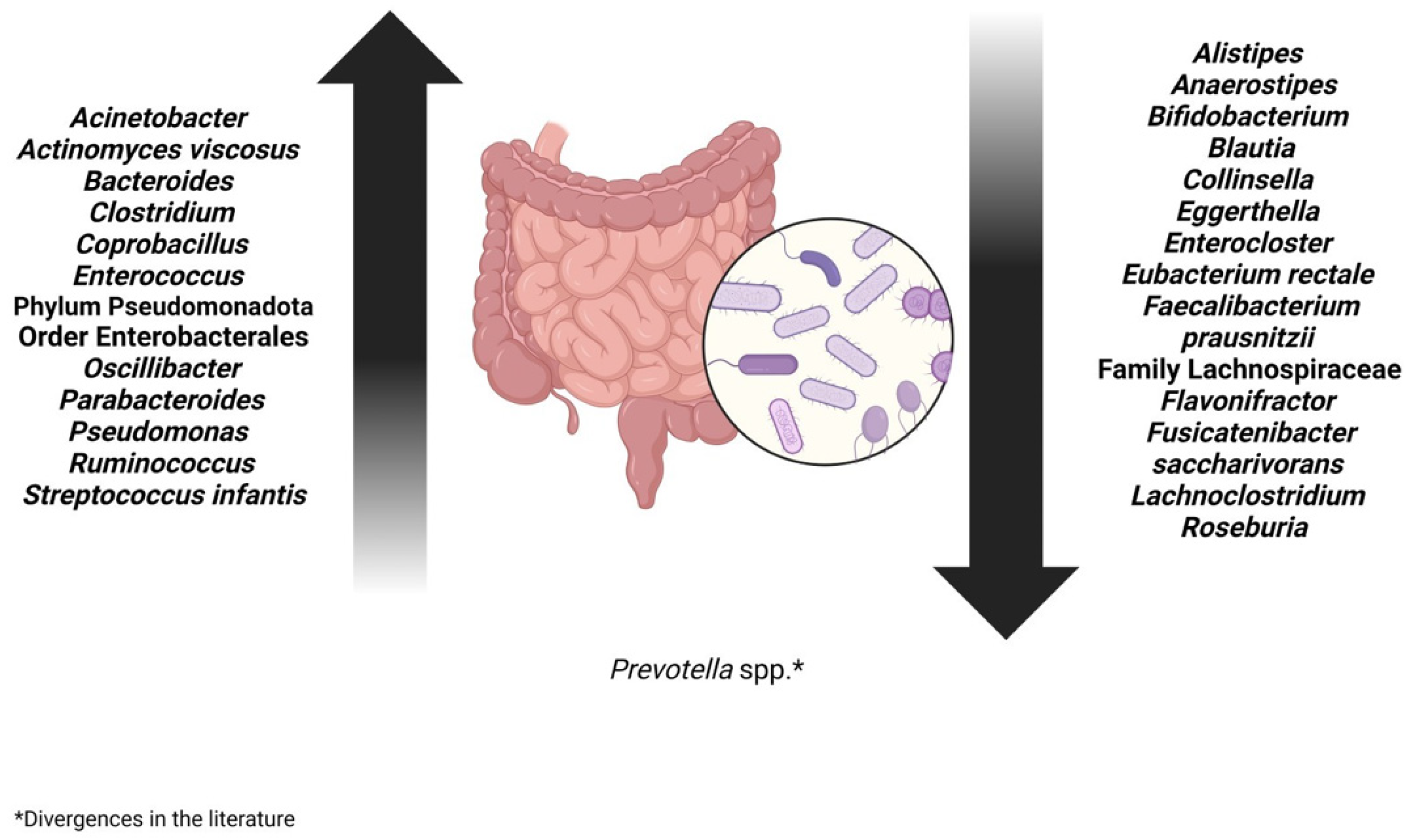
3. Change in the Bacterial Population of the Gut Microbiota After SARS-CoV-2 Infection
4. Treatment Options for Intestinal Dysbiosis in Long-COVID Patients
4.1. Prebiotics
4.2. Probiotics
4.3. Symbiotic and Fecal Microbiota Transplantation (FMT)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan American Health Organization (PAHO). Histórico da Pandemia de COVID-19. 2024. Available online: https://www.paho.org/pt/covid19/historico-da-pandemia-covid-19 (accessed on 26 June 2024).
- World Health Organization (WHO). World Health Organization COVID-19 Deaths/WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/deaths?n=o (accessed on 26 June 2024).
- Moreno-Corona, N.C.; López-Ortega, O.; Pérez-Martínez, C.A.; Martínez-Castillo, M.; De Jesús-González, L.A.; León-Reyes, G.; León-Juárez, M. Dynamics of the Microbiota and Its Relationship with Post-COVID-19 Syndrome. Int. J. Mol. Sci. 2023, 24, 14822. [Google Scholar] [CrossRef]
- De, R.; Dutta, S. Role of the Microbiome in the Pathogenesis of COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 736397. [Google Scholar] [CrossRef]
- Gang, J.; Wang, H.; Xue, X.; Zhang, S. Microbiota and COVID-19: Long-Term and Complex Influencing Factors. Front. Microbiol. 2022, 13, 963488. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. Int. J. Mol. Sci. 2023, 24, 17198. [Google Scholar] [CrossRef]
- Ashktorab, H.; Challa, S.R.; Singh, G.; Nanduri, S.; Ibrahim, M.; Martirosyan, Z.; Whitsell, P.; Chirumamilla, L.G.; Shayegh, N.; Watson, K.; et al. Gastrointestinal Manifestations and Their Association with Neurologic and Sleep Problems in Long COVID-19 Minority Patients: A Prospective Follow-Up Study. Dig. Dis. Sci. 2023, 69, 562–569. [Google Scholar] [CrossRef]
- Giovanetti, M.; Pannella, G.; Altomare, A.; Rocchi, G.; Guarino, M.; Ciccozzi, M.; Riva, E.; Gherardi, G. Exploring the Interplay Between COVID-19 and Gut Health: The Potential Role of Prebiotics and Probiotics in Immune Support. Viruses 2024, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wu, X.; Wen, W.; Lan, P. Gut Microbiome Alterations in COVID-19. Genom. Proteom. Bioinform. 2021, 19, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Suskun, C.; Kilic, O.; Yilmaz Ciftdogan, D.; Guven, S.; Karbuz, A.; Ozkaya Parlakay, A.; Kara, Y.; Kacmaz, E.; Sahin, A.; Boga, A.; et al. Intestinal Microbiota Composition of Children with Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Multisystem Inflammatory Syndrome (MIS-C). Eur. J. Pediatr. 2022, 181, 3175–3191. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Mills, N.L.; Winter, A.J.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. True prevalence of long-COVID in a nationwide, population cohort study. Nat. Commun. 2023, 14, 7892. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef]
- Chatterjee, P.; Garcia, M.A.; Cote, J.A.; Yun, K.; Legerme, G.P.; Habib, R.; Tripepi, M.; Young, C.; Kulp, D.; Dyall-Smith, M.; et al. Involvement of ArlI, ArlJ, and CirA in Archaeal Type IV Pilin-Mediated Motility Regulation. J. Bacteriol. 2024, 206, e0008924. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Piccoli, G.; Stocchi, V.; Sestili, P. Gut Microbiota Status in COVID-19: An Unrecognized Player? Front. Cell. Infect. Microbiol. 2020, 10, 576551. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Junior, A.S.; Borgonovi, T.F.; Salis, L.V.V.; Leite, A.Z.; Dantas, A.S.; De Salis, G.V.V.; Cruz, G.N.F.; De Oliveira, L.F.V.; Gomes, E.; Penna, A.L.B.; et al. Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public Health 2022, 19, 10189. [Google Scholar] [CrossRef] [PubMed]
- Rueda Ruzafa, L.; Cedillo, J.L.; Hone, A.J. Nicotinic Acetylcholine Receptor Involvement in Inflammatory Bowel Disease and Interactions with Gut Microbiota. Int. J. Environ. Res. Public Health 2021, 18, 1189. [Google Scholar] [CrossRef]
- Verma, A.; Bhagchandani, T.; Rai, A.; Nikita; Sardarni, U.K.; Bhavesh, N.S.; Gulati, S.; Malik, R.; Tandon, R. Short-Chain Fatty Acid (SCFA) as a Connecting Link Between Microbiota and Gut-Lung Axis—A Potential Therapeutic Intervention to Improve Lung Health. ACS Omega 2024, 9, 14648–14671. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Zuo, T.; Yeoh, Y.K.; Liu, Q.; Zhang, L.; Zhan, H.; Lu, W.; Xu, W.; Lui, G.C.Y.; et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients with COVID-19. Gastroenterology 2022, 162, 548–561.e4. [Google Scholar] [CrossRef]
- Włodarczyk, J.; Czerwiński, B.; Fichna, J. Short-chain fatty acids-microbiota crosstalk in the coronavirus disease (COVID-19). Pharmacol. Rep. 2022, 74, 1198–1207. [Google Scholar] [CrossRef]
- Ngo, V.L.; Gewirtz, A.T. Microbiota as a Potentially-Modifiable Factor Influencing COVID-19. Curr. Opin. Virol. 2021, 49, 21–26. [Google Scholar] [CrossRef]
- Karlsson, A.C.; Humbert, M.; Buggert, M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020, 5, eabe8063. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Yamamoto, S.; Saito, M.; Tamura, A.; Prawisuda, D.; Mizutani, T.; Yotsuyanagi, H. The Human Microbiome and COVID-19: A Systematic Review. PLoS ONE 2021, 16, e0253293. [Google Scholar] [CrossRef]
- Zrelli, S.; Amairia, S.; Zrelli, M. Respiratory syndrome coronavirus-2 response: Microbiota as Lactobacilli could make the difference. J. Med. Virol. 2021, 93, 3288–3293. [Google Scholar] [CrossRef]
- Ma, P.J.; Wang, M.M.; Wang, Y. Gut microbiota: A new insight into lung diseases. Biomed. Pharmacother. 2022, 155, 113810. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Ianiro, G.; Coppola, G.; Santopaolo, F.; Abbate, V.; Bianco, D.M.; Del Zompo, F.; De Matteis, G.; Leo, M.; Nesci, A.; et al. Improved Gut Microbiota Features After the Resolution of SARS-CoV-2 Infection. Gut Pathog. 2021, 13, 62. [Google Scholar] [CrossRef]
- Ancona, G.; Alagna, L.; Alteri, C.; Palomba, E.; Tonizzo, A.; Pastena, A.; Muscatello, A.; Gori, A.; Bandera, A. Gut and Airway Microbiota Dysbiosis and Their Role in COVID-19 and Long-COVID. Front. Immunol. 2023, 14, 1080043. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, A.; Noor, R. Association of Gut Microbiota with Inflammatory Bowel Disease and COVID-19 Severity: A Possible Outcome of the Altered Immune Response. Curr. Microbiol. 2022, 79, 184. [Google Scholar] [CrossRef]
- Meringer, H.; Wang, A.; Mehandru, S. The Pathogenesis of Gastrointestinal, Hepatic, and Pancreatic Injury in Acute and Long Coronavirus Disease 2019 Infection. Gastroenterol. Clin. N. Am. 2023, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, S.; Merad, M. Pathological Sequelae of Long-Haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Lau, H.C.-H.; Ng, S.C.; Yu, J. Targeting the Gut Microbiota in Coronavirus Disease 2019: Hype or Hope? Gastroenterology 2022, 162, 9–16. [Google Scholar] [CrossRef]
- Vignesh, R.; Swathirajan, C.R.; Tun, Z.H.; Rameshkumar, M.R.; Solomon, S.S.; Balakrishnan, P. Could Perturbation of Gut Microbiota Possibly Exacerbate the Severity of COVID-19 via Cytokine Storm? Front. Immunol. 2021, 11, 607734. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Tuganbaev, T.; Skelly, A.N.; Honda, K. T Cell Responses to the Microbiota. Annu. Rev. Immunol. 2022, 40, 559–587. [Google Scholar] [CrossRef]
- Zhou, B.; Pang, X.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut Microbiota in COVID-19: New Insights from Inside. Gut Microbes 2023, 15, 2201157. [Google Scholar] [CrossRef] [PubMed]
- Wais, T.; Hasan, M.; Rai, V.; Agrawal, D.K. Gut-Brain Communication in COVID-19: Molecular Mechanisms, Mediators, Biomarkers, and Therapeutics. Expert Rev. Clin. Immunol. 2022, 18, 947–960. [Google Scholar] [CrossRef] [PubMed]
- He, K.-Y.; Lei, X.-Y.; Zhang, L.; Wu, D.-H.; Li, J.; Lü, L.Y.; Laila, U.E.; Cui, C.-Y.; Xu, Z.; Jian, Y.P. Development and Management of Gastrointestinal Symptoms in Long-Term COVID-19. Front. Microbiol. 2023, 14, 1278479. [Google Scholar] [CrossRef] [PubMed]
- Hamrefors, V.; Kahn, F.; Holmqvist, M.; Carlson, K.; Varjus, R.; Gudjonsson, A.; Fedorowski, A.; Ohlsson, B. Gut Microbiota Composition Is Altered in Postural Orthostatic Tachycardia Syndrome and Post-Acute COVID-19 Syndrome. Sci. Rep. 2024, 14, 3389. [Google Scholar] [CrossRef]
- Nayak, B.N.; Singh, R.B.; Buttar, H.S. Chapter 51—Biochemical and Dietary Functions of Tryptophan and Its Metabolites in Human Health. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: London, UK, 2022; pp. 783–798. [Google Scholar] [CrossRef]
- Blackett, J.W.; Sun, Y.; Purpura, L.; Margolis, K.G.; Elkind, M.S.V.; O’Byrne, S.; Wainberg, M.; Abrams, J.A.; Wang, H.H.; Chang, L.; et al. Decreased Gut Microbiome Tryptophan Metabolism and Serotonergic Signaling in Patients with Persistent Mental Health and Gastrointestinal Symptoms After COVID-19. Clin. Transl. Gastroenterol. 2022, 13, e00524. [Google Scholar] [CrossRef]
- Michaelis, S.; Zelzer, S.; Schnedl, W.J.; Baranyi, A.; Meinitzer, A.; Enko, D. Assessment of tryptophan and kynurenine as prognostic markers in patients with SARS-CoV-2. Clin. Chim. Acta 2022, 525, 29–33. [Google Scholar] [CrossRef]
- Li, J.; Jing, Q.; Li, J.; Hua, M.; Di, L.; Song, C.; Huang, Y.; Wang, J.; Chen, C.; Wu, A.R. Assessment of Microbiota in the Gut and Upper Respiratory Tract Associated with SARS-CoV-2 Infection. Microbiome 2023, 11, 38. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Ghoshal, U. Gastrointestinal Involvement in Post-Acute Coronavirus Disease (COVID)-19 Syndrome. Curr. Opin. Infect. Dis. 2023, 36, 366–370. [Google Scholar] [CrossRef]
- Brīvība, M.; Silamiķele, L.; Birzniece, L.; Ansone, L.; Megnis, K.; Silamiķelis, I.; Pelcmane, L.; Borisova, D.; Rozenberga, M.; Jagare, L.; et al. Gut Microbiome Composition and Dynamics in Hospitalized COVID-19 Patients and Patients with Post-Acute COVID-19 Syndrome. Int. J. Mol. Sci. 2024, 25, 567. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.-Y.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 Faecal Viral Activity in Association with Gut Microbiota Composition in Patients with COVID-19. Gut 2020, 70, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Infection, Dysbiosis and Inflammation Interplay in the COVID Era in Children. Int. J. Mol. Sci. 2023, 24, 10874. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Okin, D.; Drew, D.A.; Battista, V.M.; Jesudasen, S.J.; Kuntz, T.M.; Bhosle, A.; Thompson, K.N.; Reinicke, T.; Lo, C.-H.; et al. Metagenomic Assessment of Gut Microbial Communities and Risk of Severe COVID-19. Genome Med. 2023, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nishiwaki, H.; Hamaguchi, T.; Ito, M.; Ueyama, J.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ohno, K. Intestinal Collinsella May Mitigate Infection and Exacerbation of COVID-19 by Producing Ursodeoxycholate. PLoS ONE 2021, 16, e0260451. [Google Scholar] [CrossRef]
- Zhang, D.; Weng, S.; Xia, C.; Ren, Y.; Han, X.; Xu, Y.; Yang, X.; Wu, R.; Peng, L.; Sun, L.; et al. Gastrointestinal Symptoms of Long COVID-19 Related to the Ectopic Colonization of Specific Bacteria That Move Between the Upper and Lower Alimentary Tract and Alterations in Serum Metabolites. BMC Med. 2023, 21, 264. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, Z. COVID-2019-Associated Overexpressed Prevotella Proteins Mediated Host–Pathogen Interactions and Their Role in Coronavirus Outbreak. Bioinformatics 2020, 36, 4065–4069. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Singh, Y.; Hassan Almalki, W.; Rawat, S.; Afzal, O.; Alfawaz Altamimi, A.S.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Singh, S.K.; et al. Gut Microbiota Disruption in COVID-19 or Post-COVID Illness Association with Severity Biomarkers: A Possible Role of Pre/Pro-Biotics in Manipulating Microflora. Chem. Biol. Interact. 2022, 358, 109898. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.; Cao, Z.; Pan, D.; Zhu, Y.; Wang, S.; Liu, D.; Song, Z.; Wei, J.; Ruan, Y.; et al. The Volatile and Heterogeneous Gut Microbiota Shifts of COVID-19 Patients over the Course of a Probiotics-Assisted Therapy. Clin. Transl. Med. 2021, 11, e643. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 389. [Google Scholar] [CrossRef]
- Mak, J.W.Y.; Chan, F.K.L.; Ng, S.C. Probiotics and COVID-19: One Size Does Not Fit All. Lancet Gastroenterol. Hepatol. 2020, 5, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu Y Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic Improves Symptomatic and Viral Clearance in COVID-19 Outpatients: A Randomized, Quadruple-Blinded, Placebo-Controlled Trial. Gut Microbes 2022, 14, e2018899. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, I.d.S.A.; Cavalcante, C.d.S.; Rubens, R.S.; Castro, L.N.P.d.F.; Nóbrega, Y.K.d.M.; Dalmolin, T.V. Changes in the Gut Microbiota of Patients After SARS-CoV-2 Infection: What Do We Know? Microorganisms 2025, 13, 2529. https://doi.org/10.3390/microorganisms13112529
Arruda IdSA, Cavalcante CdS, Rubens RS, Castro LNPdF, Nóbrega YKdM, Dalmolin TV. Changes in the Gut Microbiota of Patients After SARS-CoV-2 Infection: What Do We Know? Microorganisms. 2025; 13(11):2529. https://doi.org/10.3390/microorganisms13112529
Chicago/Turabian StyleArruda, Isabel de Souza Andrade, Caio da Silva Cavalcante, Rebeca Siqueira Rubens, Larissa Nava Pinto de Faria Castro, Yanna Karla de Medeiros Nóbrega, and Tanise Vendruscolo Dalmolin. 2025. "Changes in the Gut Microbiota of Patients After SARS-CoV-2 Infection: What Do We Know?" Microorganisms 13, no. 11: 2529. https://doi.org/10.3390/microorganisms13112529
APA StyleArruda, I. d. S. A., Cavalcante, C. d. S., Rubens, R. S., Castro, L. N. P. d. F., Nóbrega, Y. K. d. M., & Dalmolin, T. V. (2025). Changes in the Gut Microbiota of Patients After SARS-CoV-2 Infection: What Do We Know? Microorganisms, 13(11), 2529. https://doi.org/10.3390/microorganisms13112529



_Di_Marco.png)


