Genome Mining Reveals Pathways for Terpene Production in Aerobic Endospore-Forming Bacteria Isolated from Brazilian Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Ethics Statement
2.3. Sequencing, Assembly, Annotation, and Data Availability
2.4. Whole Genome-Based Features and Phylogeny
2.5. BGC Predictions
2.6. MEP Pathway Reconstruction
2.7. Detection of Polyprenyl Synthase Enzymes
2.8. Similarity of the Enzyme Set for Terpene Production
2.9. Phylogenetic Tree Reconstruction Based on Terpene Synthase Content
3. Results
3.1. SDF Strain Genome Features
3.2. MEP Pathway Reconstruction
3.3. Detection of Polyprenyl Synthase Enzymes
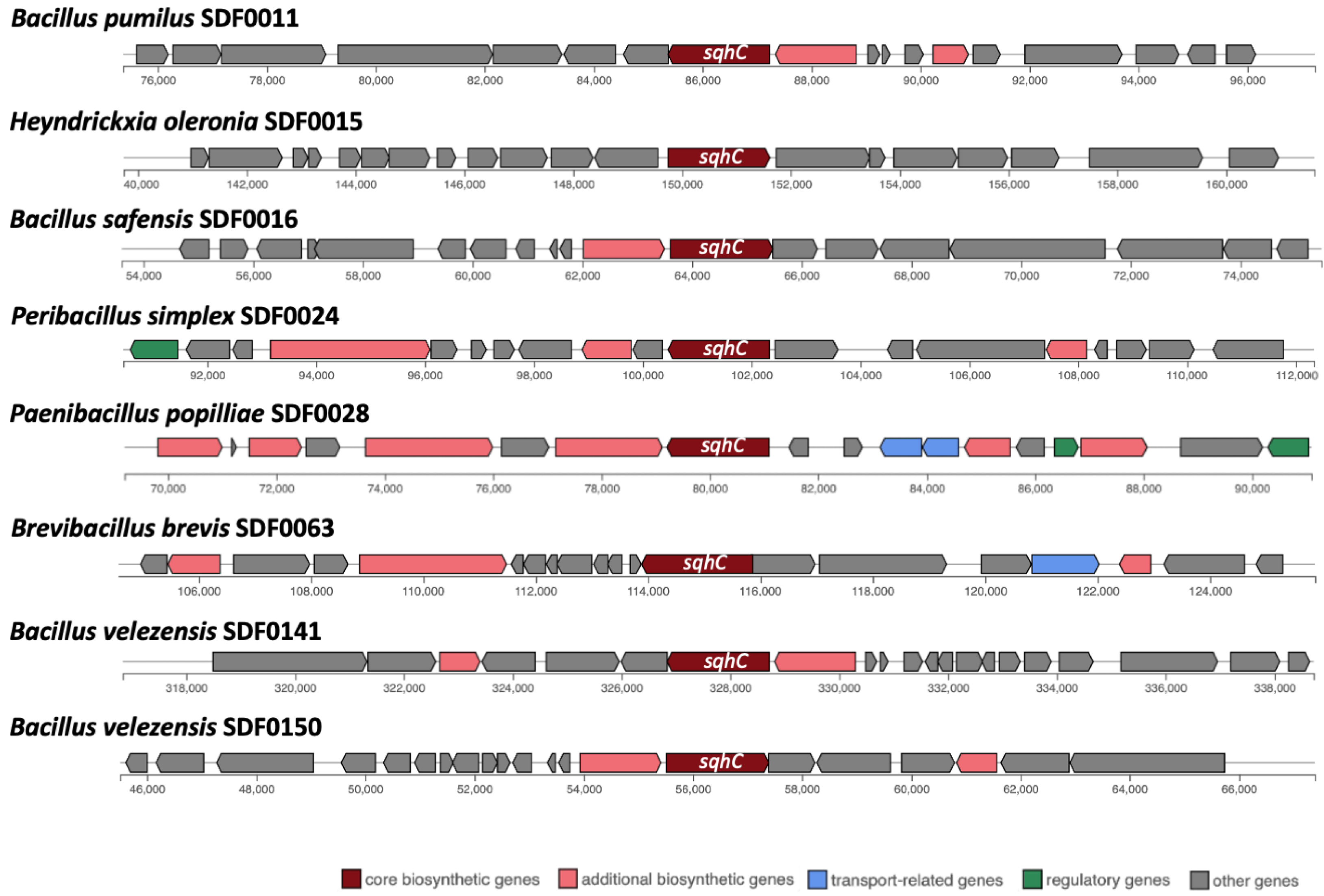
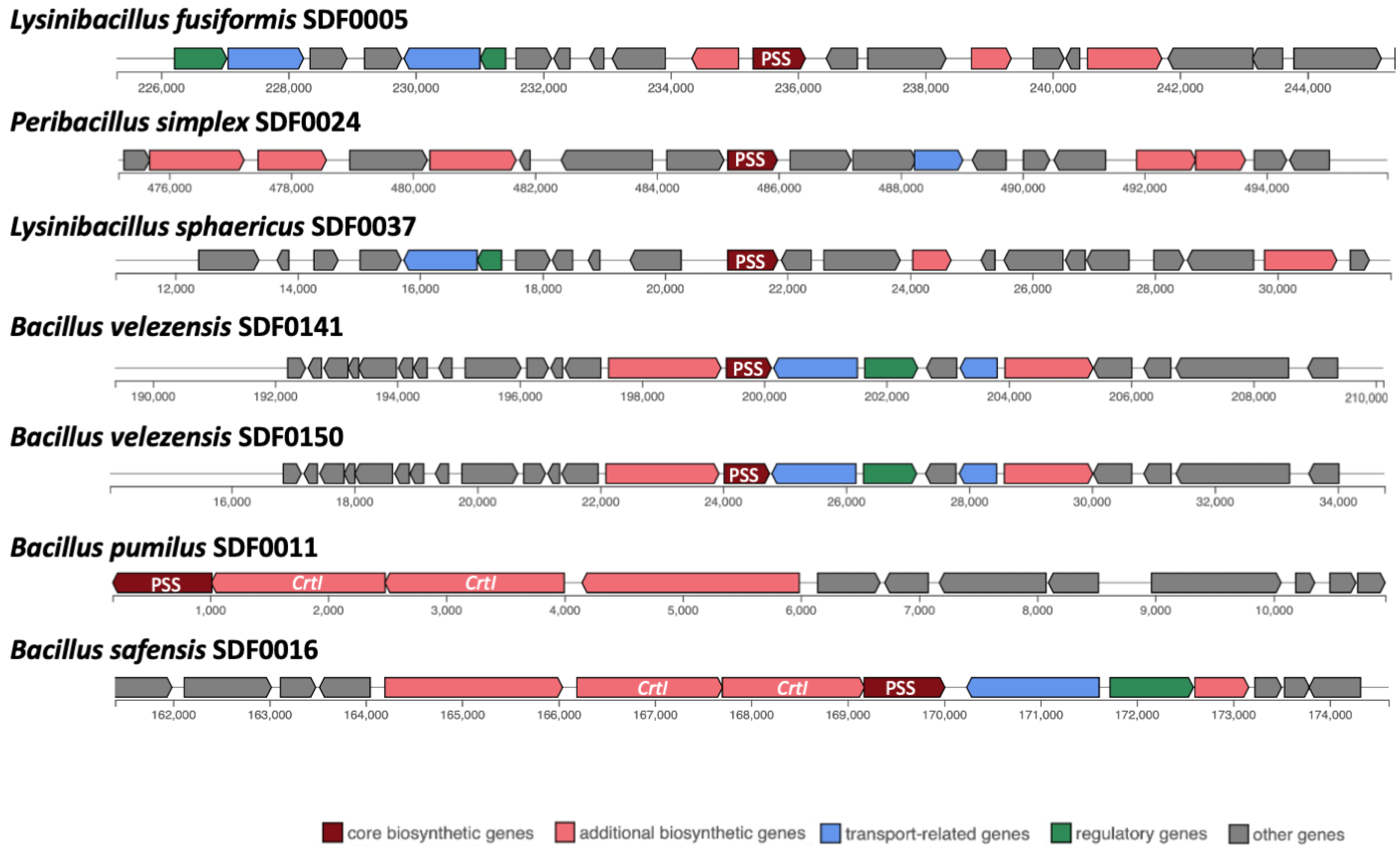
3.4. Prediction of Biosynthetic Gene Clusters Associated with Terpene Synthesis
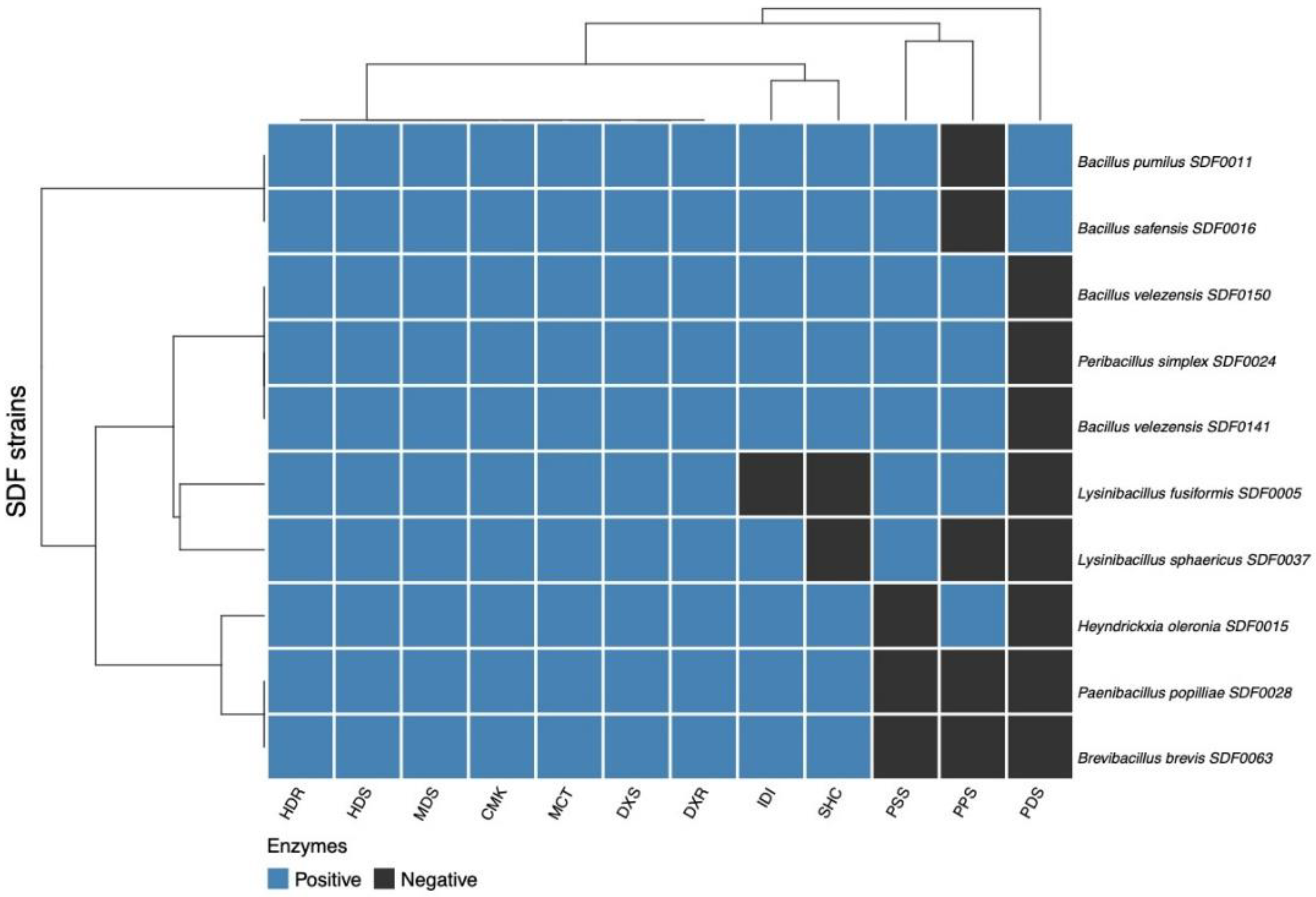
3.5. Distribution of the Enzyme Set for Terpene Production Among the 10 SDF Strains
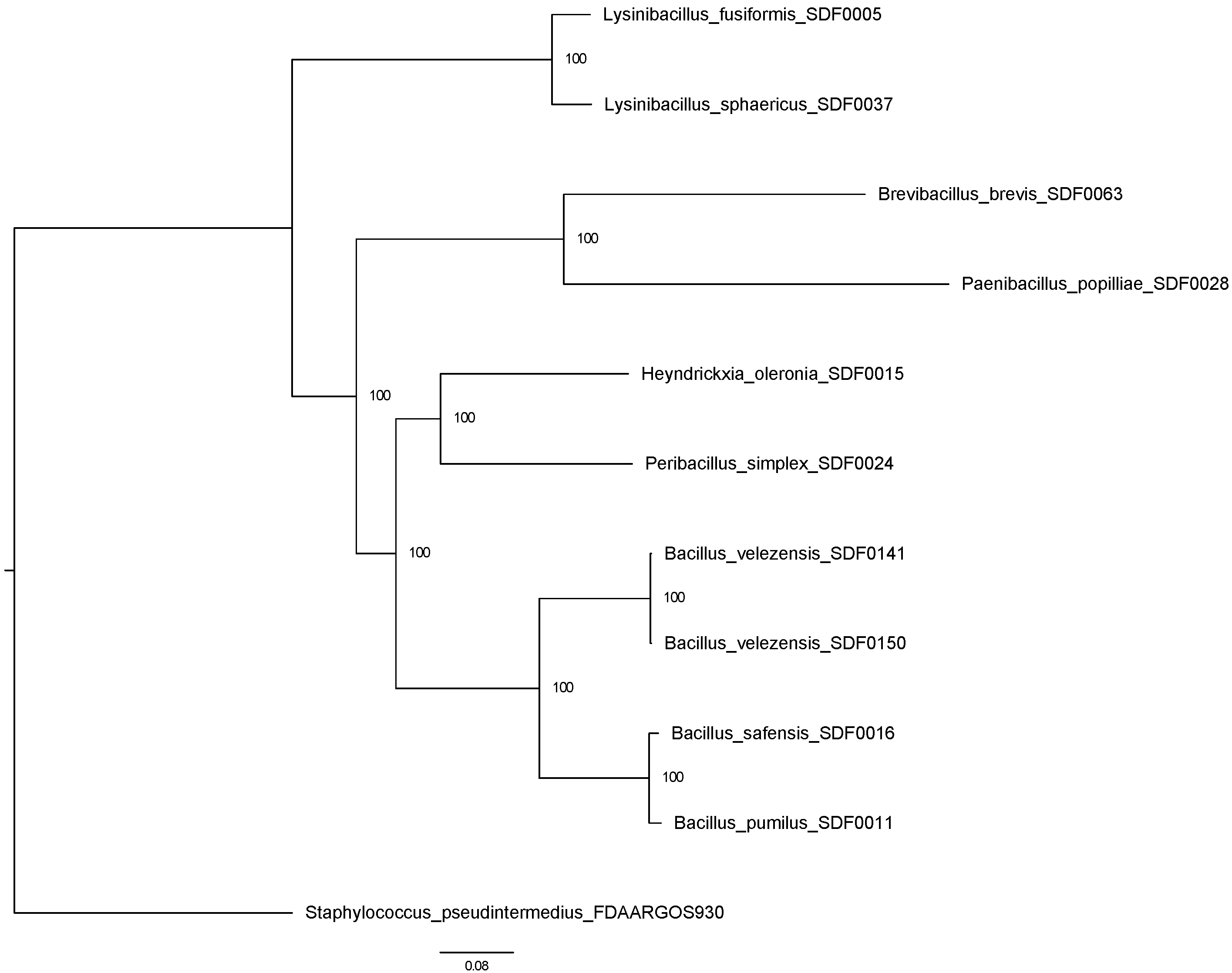
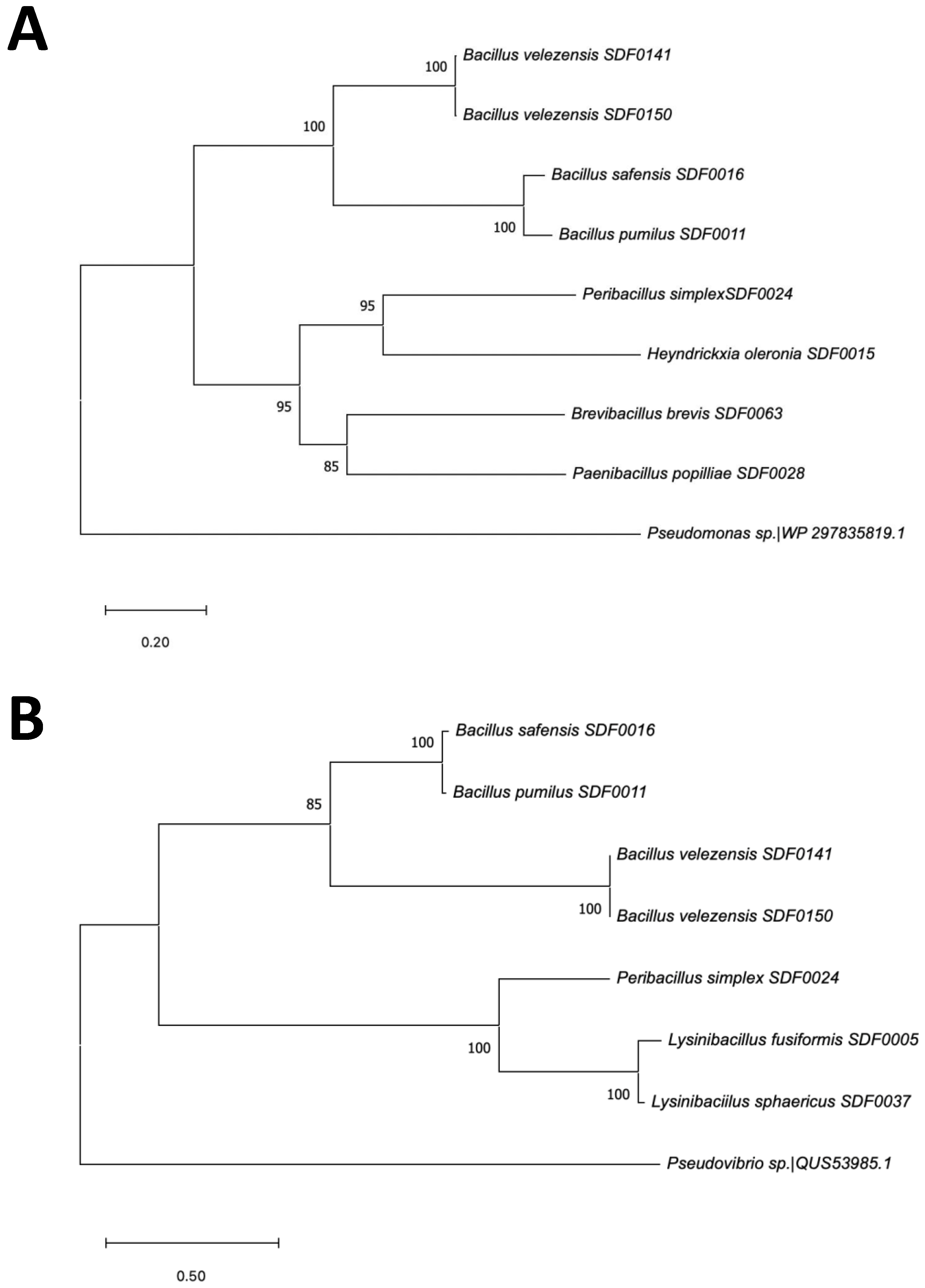
3.6. SDF Strains’ Evolutionary Relationship Based on Two TS Amino Acid Sequences
4. Discussion
4.1. Uncovering Enzymes from the MEP Pathway and the Polyprenyl Synthase Family in the SDF Strains
4.2. Genomic Potential of Selected SDF Strains for Terpene Production
4.3. The Evolutionary Nature of Terpene Production in the SDF Stains
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, B.P.; Rateb, M.E.; Rodriguez-Couto, S.; Polizeli, M.D.L.T.D.M.; Li, W.-J. Microbial Secondary Metabolites: Recent Developments and Technological Challenges. Front. Microbiol. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Al-Taweel, A. Terpenes and Terpenoids; IntechOpen: London, UK, 2018; pp. 1–152. ISBN 978-1-83881-529-5. [Google Scholar]
- Rudolf, J.D.; Aslup, T.; Xu, B.; Li, Z. Bacterial Terpenome. Nat. Prod. Rep. 2021, 38, 905–980. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. 2022, 13, 100217. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Ikeda, H. Terpene Synthases are Widely Distributed in Bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef]
- Pinto-Zevallos, D.M.; Hellén, H.; Hakola, H.; Nouhuys, S.V.; Halopainen, J.K. Induced defenses of Veronica spicata: Variability in herbivore-induced volatile organic compounds. Phytochem. Lett. 2013, 6, 653–656. [Google Scholar] [CrossRef]
- Zhao, D.-D.; Jiang, L.-L.; Li, H.-Y.; Yan, P.-F.; Zhang, Y.-L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Quin, M.B.; Flynn, C.M.; Schimidt-Dannert, C. Traversing the Fungal Terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; Elshamy, A.I.; Aziz, M.; Paré, P.W. Molecular Architecture and Biomedical Leads of Terpenes from Red Sea Marine Invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef]
- Morandini, L.; Caulier, S.; Bragard, C.; Mahillon, J. Bacillus cereus sensu lato Antimicrobial Arsenal: An Overview. Microbiol. Res. 2024, 283, 127697. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Liang, Z.; Zhi, H.; Fang, Z.; Zhang, P. Genetic engineering of yeast, filamentous fungi and bacteria for terpene production and applications in food industry. Food Res. Int. 2021, 147, 110487. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Rico, J.; Gil, J.V.; Orejas, M. De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain. Microb. Cell Factories 2015, 14, 136. [Google Scholar] [CrossRef]
- Lv, H.-W.; Tang, J.-G.; Wei, B.; Zhu, M.-D.; Zhang, H.-W.; Zhou, Z.-B.; Fan, B.-Y.; Wang, H.; Li, X.-N. Bioinformatics assisted construction of the link between biosynthetic gene clusters and secondary metabolites in fungi. Biotechnol. Adv. 2025, 81, 108547. [Google Scholar] [CrossRef] [PubMed]
- Mudbhari, S.; Lofgren, L.; Appidi, M.A.; Vilgalys, R.; Hettich, R.L.; Abraham, P.E. Decoding the chemical language of Suillus fungi: Genome mining and untargeted metabolomics uncover terpene chemical diversity. Msystems 2024, 9, e0122523. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, M.; Kim, S.-H.; Hong, C.-Y.; Ryu, S.-H.; Choi, I.-G. Transcriptomic analysis of the white rot fungus Polyporus brumalis provides insight into sesquiterpene biosynthesis. Microbiol. Res. 2016, 182, 141–149. [Google Scholar] [CrossRef]
- Harirchi, S.; Sar, T.; Ramezani, M.; Aliyu, H.; Etemadifar, Z.; Nojoumi, S.A.; Yazdian, F.; Awasthi, M.K.; Taherzadeh, M.J. Bacillales: From Taxonomy to Biotechnological and Industrial Perspectives. Microorganisms 2022, 10, 2355. [Google Scholar] [CrossRef]
- Put, H.; Gerstmans, H.; Capelle, H.V.; Fauvart, M.; Michiels, J.; Masschelein, J. Bacillus subtilis as a host for natural product discovery and engineering of biosynthetic gene clusters. Nat. Prod. Rep. 2024, 41, 1113–1151. [Google Scholar] [CrossRef]
- Zheng, D.; Ding, N.; Jiang, Y.; Zhang, J.; Ma, J.; Chen, X.; Liu, J.; Han, L.; Huang, X. Albaflavenoid, a New Tricyclic Sesquiterpenoid from Streptomyces violascens. J. Antibiot. 2016, 69, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Fritze, D. Taxonomy of the Genus Bacillus and Related Genera: The Aerobic Endospore-Forming Bacteria. Phytopathology 2004, 94, 1245–1248. [Google Scholar] [CrossRef]
- Mandic-Mulec, I.; Prosser, J.I. Diversity of Endospore-forming Bacteria in Soil: Characterisation and Driving Mechanisms. In Endospore-Forming Soil Bacteria, 1st ed.; Logan, N.A., Vos, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 27, pp. 31–59. ISBN 978-3-642-19577-8. [Google Scholar]
- Logan, N.A. Bacillus and relatives in foodborne illness. J. Appl. Microbiol. 2012, 112, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Orem, J.C.; Silva, W.M.C.; Raiol, T.; Magalhães, M.I.; Martins, P.H.; Cavalcante, D.A.; Kruger, R.H.; Brigido, M.M.; De-Souza, M.T. Phylogenetic diversity of aerobic spore-forming Bacillales isolated from Brazilian soils. Int. Microbiol. 2019, 22, 511–520. [Google Scholar] [CrossRef]
- Cavalcante, D.A.; De-Souza, M.T.; Orem, J.C.; Magalhães, M.I.A.; Martins, P.H.; Boone, T.J.; Castillo, J.A.; Driks, A. Ultrastructural analysis of spores from diverse Bacillales species isolated from Brazilian soil. Environ. Microbiol. Rep. 2019, 11, 155–164. [Google Scholar] [CrossRef]
- Martins, P.H.R.; Silva, L.P.; Orem, J.C.; Magalhães, M.I.A.; Cavalcante, D.A.; De-Souza, M.T. Protein profiling as a tool for identifying environmental aerobic endospore-forming bacteria. Open J. Bacteriol. 2020, 4, 1–7. [Google Scholar]
- Martins, P.H.R.; Rabinovitch, L.; Orem, J.C.; Silva, W.M.C.; Mesquita, F.A.; Magalhães, M.I.A.; Cavalcante, D.A.; Vivoni, A.M.; Oliveira, E.J.; Lima, V.C.P.; et al. Biochemical, physiological, and molecular characterisation of a large collection of aerobic endospore-forming bacteria isolated from Brazilian soils. Neotrop. Biol. Conserv. 2023, 18, 53–72. [Google Scholar] [CrossRef]
- Driks, A.; Eichenberger, P. The Bacterial Spore: From Molecules to Systems; ASM Press: Washington, DC, USA, 2016; pp. 1–397. ISBN 9781555816759. [Google Scholar]
- Christie, G.; Setlow, P. Bacillus spore germination: Knowns, unknowns and what we need to learn. Cell. Signal. 2020, 74, 109729. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Yutin, N.; Wolf, Y.I.; Alvarez, R.V.; Koonin, E.V. Conservation and Evolution of the Sporulation Gene Set in Diverse Members of the Firmicutes. J. Bacteriol. 2022, 204, e00079-22. [Google Scholar] [CrossRef]
- Sumi, C.D.; Yang, B.W.; Yeo, I.-C.; Hahm, Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015, 61, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, S.; Krismer, B.; Brötz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Singh, S.P.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as Bio-factories for Antifungal Secondary Metabolites: Innovation Beyond Whole Organism Formulations. Microb. Ecol. 2023, 86, 1–24. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J.; Islam, M.T. Diversity of Secondary Metabolites from Marine Bacillus Species: Chemistry and Biological Activity. Mar. Drugs 2013, 11, 2846–2872. [Google Scholar] [CrossRef]
- Falqueto, S.A.; Pitaluga, B.F.; Sousa, J.R.; Targanski, S.K.; Campos, M.G.; Mendes, T.A.O.; Silva, G.F.; Silva, D.H.S.; Soares, M.A. Bacillus spp. metabolites are effective in eradicating Aedes aegypti (Diptera: Culicidae) larvae with low toxicity to non-target species. J. Invertebr. Pathol. 2021, 179, 107525. [Google Scholar] [CrossRef]
- Mesquita, F.A.; Silva, W.M.C.; De-Souza, M.T. In silico Analysis of the Genomic Potential for the Production of Specialized Metabolites of Ten Strains of the Bacillales Order Isolated from the Soil of the Federal District, Brazil. In Advances in Bioinformatics and Computational Biology, 1st ed.; Scherer, N.M., Melo-Minardi, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 13523, pp. 158–163. ISBN 978-3-031-21175-1. [Google Scholar]
- Coil, D.; Jospin, G.; Darling, A.M. A5-miseq: An updated pipeline to assemble microbial genomes from Illumina Miseq data. Bioinformatics 2015, 31, 587–589. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acid Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Setubal, J.C.; Almeida, N.F.; Wattam, A.R. Comparative Genomics for Prokaryotes. Methods Mol. Biol. 2018, 1704, 55–78. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stoeckert, J., Jr.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Bogdanove, A.J.; Koebnik, R.; Lu, H.; Furutani, A.; Angiuoli, S.V.; Patil, P.B.; Sluys, M.A.V.; Ryan, R.P.; Meyer, D.F.; Han, S.-W.; et al. Two New Complete Genome Sequences Offer Insight into Host and Tissue Specificity of Plant Pathogenic Xanthomonas spp. J. Bacteriol. 2011, 193, 5450–5464. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Hummel, M.; Edelmann, D.; Kopp-Schneider, A. Clustering of samples and variables with mixed-type data. PLoS ONE 2017, 12, e0188274. [Google Scholar] [CrossRef]
- Medema, M.H.; Kottmann, R.; Yilmaz, P. Minimum Information about a Biosynthetic Gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef]
- Köcher, S.; Breitenbach, J.; Müller, V.; Sandmann, G. Structure, function and biosynthesis of carotenoids in the moderately halophilic bacterium Halobacillus halophilus. Arch. Microbiol. 2009, 191, 95–104. [Google Scholar] [CrossRef]
- Xu, X.; Kóvacs, A.T. How to identify and quantify the members of the Bacillus genus? Environ. Microbiol. 2024, 26, e16593. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Patel, S. Robust Demarcation of the Family Caryophanaceae (Planococcaceae) and Its Different Genera Including Three Novel Genera Based on Phylogenomics and Highly Specific Molecular Signatures. Front. Microbiol. 2020, 10, 2821. [Google Scholar] [CrossRef] [PubMed]
- Chuvochina, M.; Mussig, A.J.; Chaumeil, P.-A.; Skarkshewski, A.; Rinke, C.; Parks, D.H.; Hugenholtz, P. Proposal of names for 329 higher taxa defined in the Genome Taxonomy Database under two prokaryotic codes. FEMS Microbiol. Lett. 2023, 370, fnad071. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
- Patel, S.; Gupta, R.S. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: Proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. [Google Scholar] [CrossRef]
- Zeng, L.; Dehesh, K. The eukaryotic MEP-pathway genes are evolutionarily conserved and originated from Chlaymidia and cyanobacteria. BMC Genom. 2022, 22, 137. [Google Scholar] [CrossRef]
- Kaneko, T.; Tabata, S. Complete Genome Structure of the Unicellular Cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 1997, 11, 1171–1176. [Google Scholar] [CrossRef]
- Dairi, T. Studies on biosynthetic genes and enzymes of isoprenoids produced by actimomycetes. J. Antibiot. 2005, 58, 227–243. [Google Scholar] [CrossRef]
- Nakashima, T.; Inoue, T.; Oka, A.; Nishino, T.; Osumi, T.; Hata, S. Cloning, expression, and characterisation of cDNAs encoding Arabidopsis thaliana squalene synthase. Proc. Natl. Acad. Sci. USA 1995, 98, 2328–2332. [Google Scholar] [CrossRef]
- Tansey, T.R.; Shechter, I. Squalene synthase: Structure and regulation. Prog. Nucleic Acid Res. Mol. Biol. 2000, 65, 157–195. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene Stimulates a Key Innate Cell to Foster Wound Healing and Tissue Repair. Evid. -Based Complement. Altern. Med. 2018, 2018, 9473094. [Google Scholar] [CrossRef]
- Song, Y.; Guan, Z.; Merkerk, R.V.; Pramastya, H.; Abdallah, I.I.; Setroikromo, R.; Quax, W.J. Production of Squalene in Bacillus subtilis by Squalene Synthase Screening and Metabolic Engineering. J. Agric. Food Chem. 2020, 68, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Siedenburg, G.; Jendrossek, D. Squalene-Hopene Cyclases. Appl. Environ. Microbiol. 2011, 77, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Begum, F.; Rabaan, A.A.; Aljeldah, M.; Al Shammari, B.R.; Alawfi, A.; Alshengeti, A.; Sulaiman, T.; Khan, A. Classification and Multifaceted Potential of Secondary Metabolites Produced by Bacillus subtilis Group: A Comprehensive Review. Molecules 2023, 28, 927. [Google Scholar] [CrossRef]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant-bacteria interactions. Nat. Rev. Microbiol. 2018, 16, 304–315. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Espinosa, V.M.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and β-Carotene Extraction from Tomato Processing Waste Using Supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnological production pf lycopene by microorganisms. Appl. Microbiol. Biotechnol. 2020, 104, 10307–10324. [Google Scholar] [CrossRef]
- Zou, D.; Ye, C.; Min, Y.; Li, L. Production of a novel lycopene-rich soybean food by fermentation with Bacillus amyloliquefaciens. Food Sci. Technol. 2022, 153, 112551. [Google Scholar] [CrossRef]
- Luo, H.; Bao, Y.; Zhu, P. Development of a novel functional yogurt rich in lycopene by Bacillus subtilis. Food Chem. 2023, 407, 135142. [Google Scholar] [CrossRef]
- Osawa, A.; Iki, K.; Sandmann, G.; Shindo, K. Isolation and identification of 4,4′-diapolycopene-4,4′-dioc acid produced by Bacillus firmus GB1 and its singlet oxygen quenching activity. J. Oleo Sci. 2013, 62, 955–960. [Google Scholar] [CrossRef]
- Werner, W. Botanische Beschreinbung Häufiger am Buttersäureabbau Beteiligter Sporenbildender Bakteriensspezies; Fischer: Frankfurt, German, 1933. [Google Scholar]
- Hwang, C.Y.; Cho, E.-S.; Yoon, D.J.; Cha, I.-T.; Jung, D.-H.; Nam, Y.-D.; Park, S.-L.; Lim, S.-I.; Seo, M.-J. Genomic and Physiological Characterisation of Metabacillus flavus sp. nov., a Novel Carotenoid-Producing Bacilli Isolated from Korean Marine Mud. Microorganisms 2022, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Vattekkatte, A.; Garms, S.; Brandt, W.; Boland, W. Enhanced structural diversity in terpenoid biosynthesis: Enzymes, substrates and cofactors. Org. Biomol. Chem. 2018, 16, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, R.; Meirinhos-Soares, L.; Carriço, J.A.; Pintado, M.; Peixe, L.V. Phylogenetic and clonality analysis of Bacillus pumilus isolates uncovered a highly heterogeneous population of different closely related species and clones. FEMS Microbiol. Ecol. 2014, 90, 689–698. [Google Scholar] [CrossRef] [PubMed]
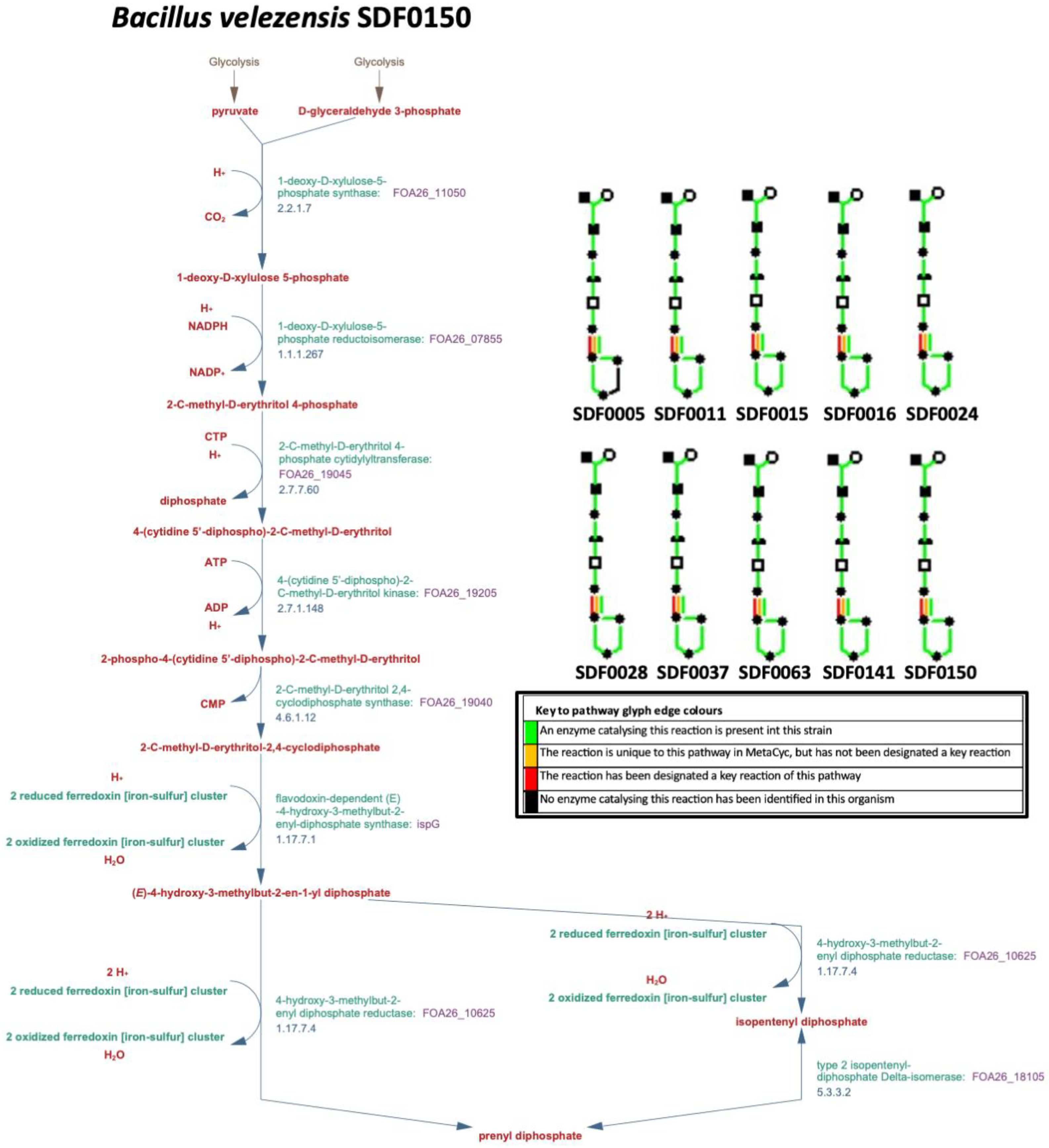
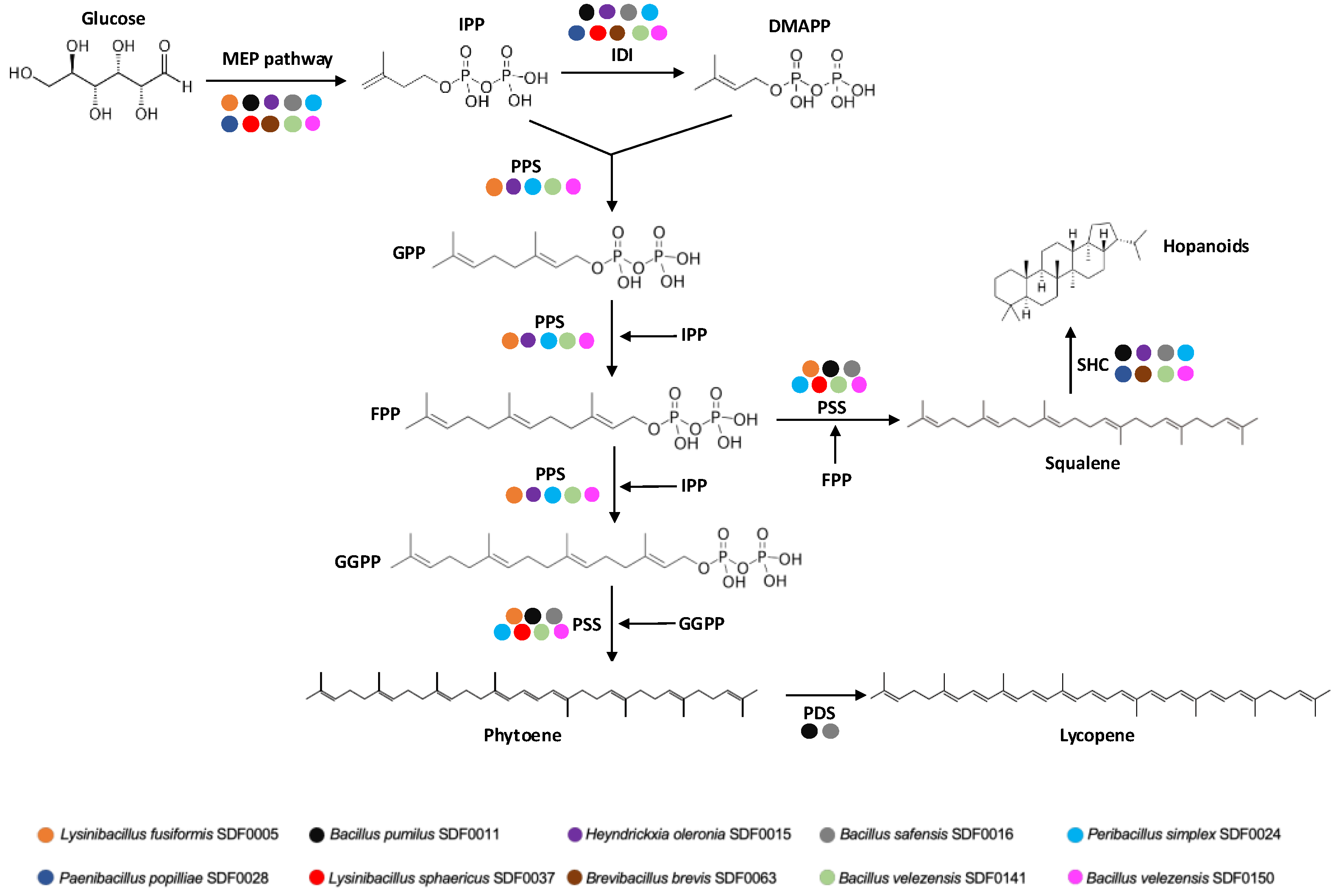
| Substrate | Enzyme Code * | Enzyme Name (Abbreviation) | Product (Abbreviation) |
|---|---|---|---|
| Pyruvate and G3P | 2.2.1.7 | 1-deoxy-D-xylulose-5-phosphate synthase (DXS) | 1-deoxy-D-xylulose-5-phosphate (DXP) |
| DXP and NADPH | 1.1.1.267 | DXP reductorisomerase (DXR) | methylerythritol-phosphate (MEP) |
| MEP | 2.7.7.60 | MEP cytidylyltransferase (MCT) | 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol (CD-ME) |
| CD-ME and ATP | 2.7.1.148 | CD-ME kinase (CMK) | 4-difosfocitidil-2-C-metil-Deritritol 2-fosfato (CD-MEP) |
| CD-MEP | 4.6.1.12 | 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase (MDS) | 2C-methyl-D-erythritol-2,4-cyclodiphosphate (MEC) |
| MEC and NADPH | 1.17.7.3 | 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase (HDS) | 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HMBPP) |
| HMBPP and NADPH | 1.17.7.4 | HMBPP reductase (HDR) | isopentenyl pyrophosphate (IPP) |
| IPP | 5.3.3.2 | isopentenyl diphosphate isomerase (IDI) | dimethylallyl pyrophosphate (DMAPP) |
| IPP and DMAPP | 2.5.1.1 | GPP synthase (GPPS) ** | geranyl diphosphate (GPP) |
| GPP and IPP | 2.5.1.10 | FPP synthase (FPPS) ** | farnesyl diphosphate (FPP) |
| FPP and IPP | 2.5.1.29 | GGPP synthase (GGPPS) ** | geranylgeranyl diphosphate (GGPP) |
| Strain | Size (bp) | Scaffold | N50 (bp) | GC Content (%) | CDS # | Protein Coding Regions | Pseudo Genes (Total) | rRNA Genes (5S; 16S; 23S) | tRNA Genes | GenBank Accession |
|---|---|---|---|---|---|---|---|---|---|---|
| Lysinibacillus fusiformis SDF0005 | 4,472,771 | 24 | 392,231 | 37.6 | 4369 | 4328 | 41 | 13; 7; 7 | 85 | * VKHW00000000.1 |
| Bacillus pumilus SDF0011 | 3,686,817 | 56 | 143,274 | 41.2 | 3688 | 3617 | 71 | 7; 3; 2 | 73 | * VKHY00000000.1 |
| Heyndrickxia oleronia SDF0015 | 5,267,437 | 75 | 151,790 | 34.7 | 5127 | 5018 | 109 | 10; 14; 7 | 129 | * VKHZ00000000.1 |
| Bacillus safensis SDF0016 | 3,674,191 | 25 | 484,434 | 41.6 | 3688 | 3640 | 48 | 4; 1; 1 | 74 | SADW00000000.1 |
| Peribacillus simplex SDF0024 | 5,376,271 | 45 | 497,961 | 40.2 | 5204 | 5007 | 197 | 14; 7; 6 | 81 | * VKHX00000000.1 |
| Paenibacillus popilliae SDF0028 | 6,580,875 | 39 | 611,008 | 46.5 | 5684 | 5519 | 165 | 2; 2; 3 | 62 | SADY00000000.1 |
| Lysinibacillus sphaericus SDF0037 | 5,122,785 | 71 | 215,682 | 36.5 | 4869 | 4643 | 226 | 5; 7; 2 | 71 | SADV00000000.1 |
| Brevibacillus brevis SDF0063 | 6,239,737 | 31 | 471,412 | 47.3 | 5789 | 5602 | 187 | 1; 16; 9 | 89 | SADX00000000.1 |
| Bacillus velezensis SDF0141 | 3,945,527 | 15 | 962,078 | 46.4 | 3887 | 3780 | 107 | 8; 3; 2 | 78 | * VKIB00000000.1 |
| Bacillus velezensis SDF0150 | 3,927,067 | 21 | 271,062 | 46.4 | 3870 | 3763 | 107 | 8; 6; 2 | 82 | * VKIC00000000.1 |
| Strain | Occurrence | Identity (%) * | Reference Species | GeneBank Reference Sequence |
|---|---|---|---|---|
| Lysinibacillus fusiformis SDF0005 | + | 99.66 | Lysinibacillus fusiformis | KAB0443654.1 |
| Bacillus pumilus SDF0011 | − | NA | NA | NA |
| Heyndrickxia oleronia SDF0015 | + | 67.86 | Bacillus pumilus | WP_268443628.1 |
| Bacillus safensis SDF0016 | − | NA | NA | NA |
| Peribacillus simplex SDF0024 | + | 98.65 | Peribacillus sp. | WP_241589686.1 |
| Paenibacillus popilliae SDF0028 | − | NA | NA | NA |
| Lysinibacillus sphaericus SDF0037 | − | NA | NA | NA |
| Brevibacillus brevis SDF0063 | − | NA | NA | NA |
| Bacillus velezensis SDF0141 | + | 100 | Bacillus velezensis | ASK59031.1 |
| Bacillus velezensis SDF0150 | + | 99.65 | Bacillus velezensis | QWC45887.1 |
| Strain | Gene/TS Enzyme | ||
|---|---|---|---|
| sqhC/SHC | Phytoene and/or Squalene Synthase Family Gene/PSS | crti/PDS | |
| Lysinibacillus fusiformis SDF0005 | − | + | − |
| Bacillus pumilus SDF0011 | + | + | + |
| Heyndrickxia oleronia SDF0015 | + | − | − |
| Bacillus safensis SDF0016 | + | + | + |
| Peribacillus simplex SDF0024 | + | + | − |
| Paenibacillus popilliae SDF0028 | + | − | − |
| Lysinibacillus sphaericus SDF0037 | − | + | − |
| Brevibacillus brevis SDF0063 | + | − | − |
| Bacillus velezensis SDF0141 | + | + | − |
| Bacillus velezensis SDF0150 | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, F.d.A.; Silva, W.M.C.d.; Raiol, T.; Brigido, M.d.M.; Almeida, N.F.d.; Fuga, B.; Cavalcante, D.d.A.; De-Souza, M.T. Genome Mining Reveals Pathways for Terpene Production in Aerobic Endospore-Forming Bacteria Isolated from Brazilian Soils. Microorganisms 2025, 13, 2528. https://doi.org/10.3390/microorganisms13112528
Mesquita FdA, Silva WMCd, Raiol T, Brigido MdM, Almeida NFd, Fuga B, Cavalcante DdA, De-Souza MT. Genome Mining Reveals Pathways for Terpene Production in Aerobic Endospore-Forming Bacteria Isolated from Brazilian Soils. Microorganisms. 2025; 13(11):2528. https://doi.org/10.3390/microorganisms13112528
Chicago/Turabian StyleMesquita, Felipe de Araujo, Waldeyr Mendes Cordeiro da Silva, Taina Raiol, Marcelo de Macedo Brigido, Nalvo Franco de Almeida, Bruna Fuga, Danilo de Andrande Cavalcante, and Marlene Teixeira De-Souza. 2025. "Genome Mining Reveals Pathways for Terpene Production in Aerobic Endospore-Forming Bacteria Isolated from Brazilian Soils" Microorganisms 13, no. 11: 2528. https://doi.org/10.3390/microorganisms13112528
APA StyleMesquita, F. d. A., Silva, W. M. C. d., Raiol, T., Brigido, M. d. M., Almeida, N. F. d., Fuga, B., Cavalcante, D. d. A., & De-Souza, M. T. (2025). Genome Mining Reveals Pathways for Terpene Production in Aerobic Endospore-Forming Bacteria Isolated from Brazilian Soils. Microorganisms, 13(11), 2528. https://doi.org/10.3390/microorganisms13112528








