Norditerpene Natural Products from Subterranean Fungi with Anti-Parasitic Activity
Abstract
1. Introduction
2. Materials and Methods
3. Results
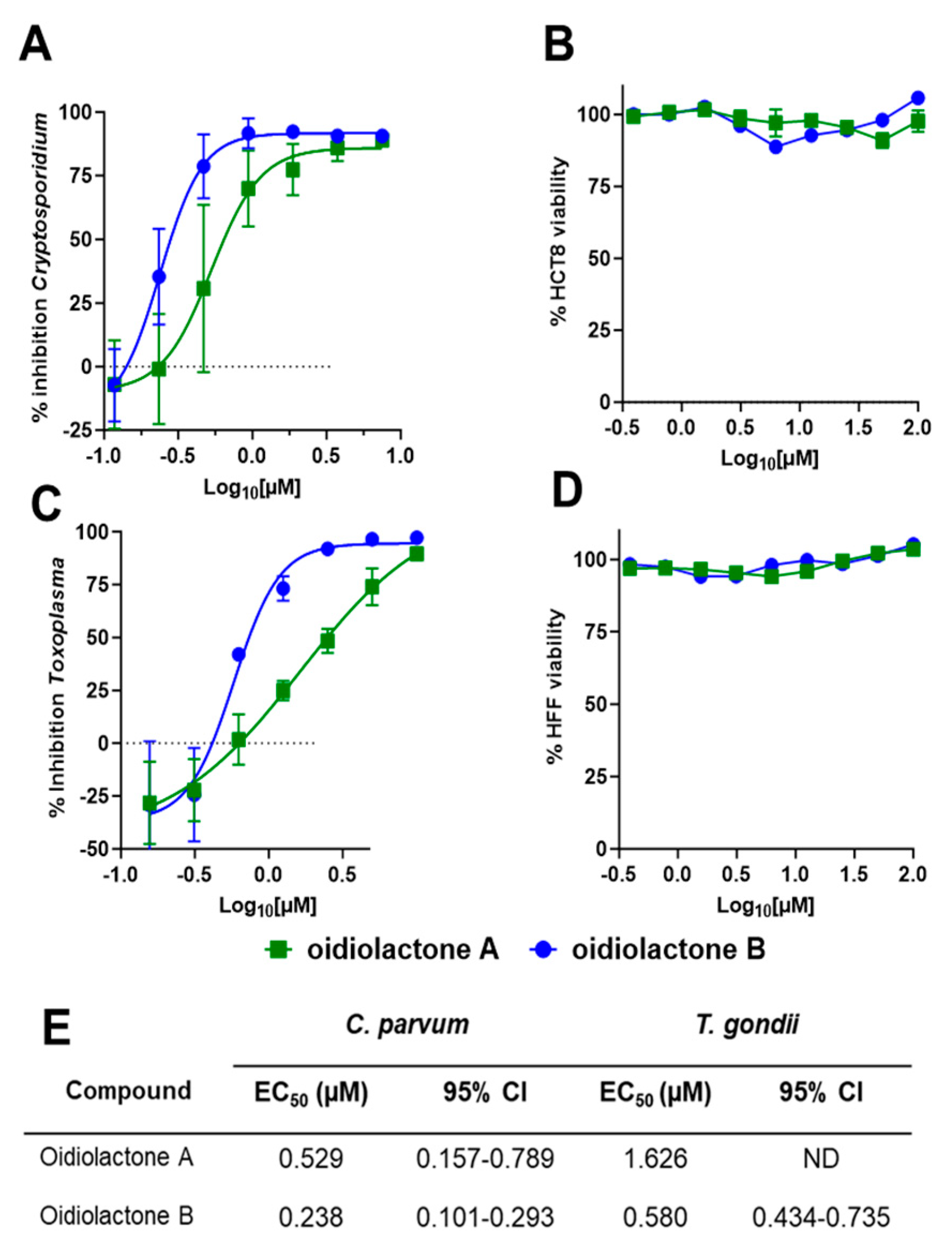
3.1. Two Norditerpene Lactones Exhibit Potent Activity Against C. parvum and T. gondii
3.2. Oidiolactones A and B Exhibit Anti-Apicomplexan Activity Without Toxicity to Host Cells
3.3. Oidiolactone B Exhibits Some Cytotoxicity Towards Sub-Confluent HCT-8 Cells
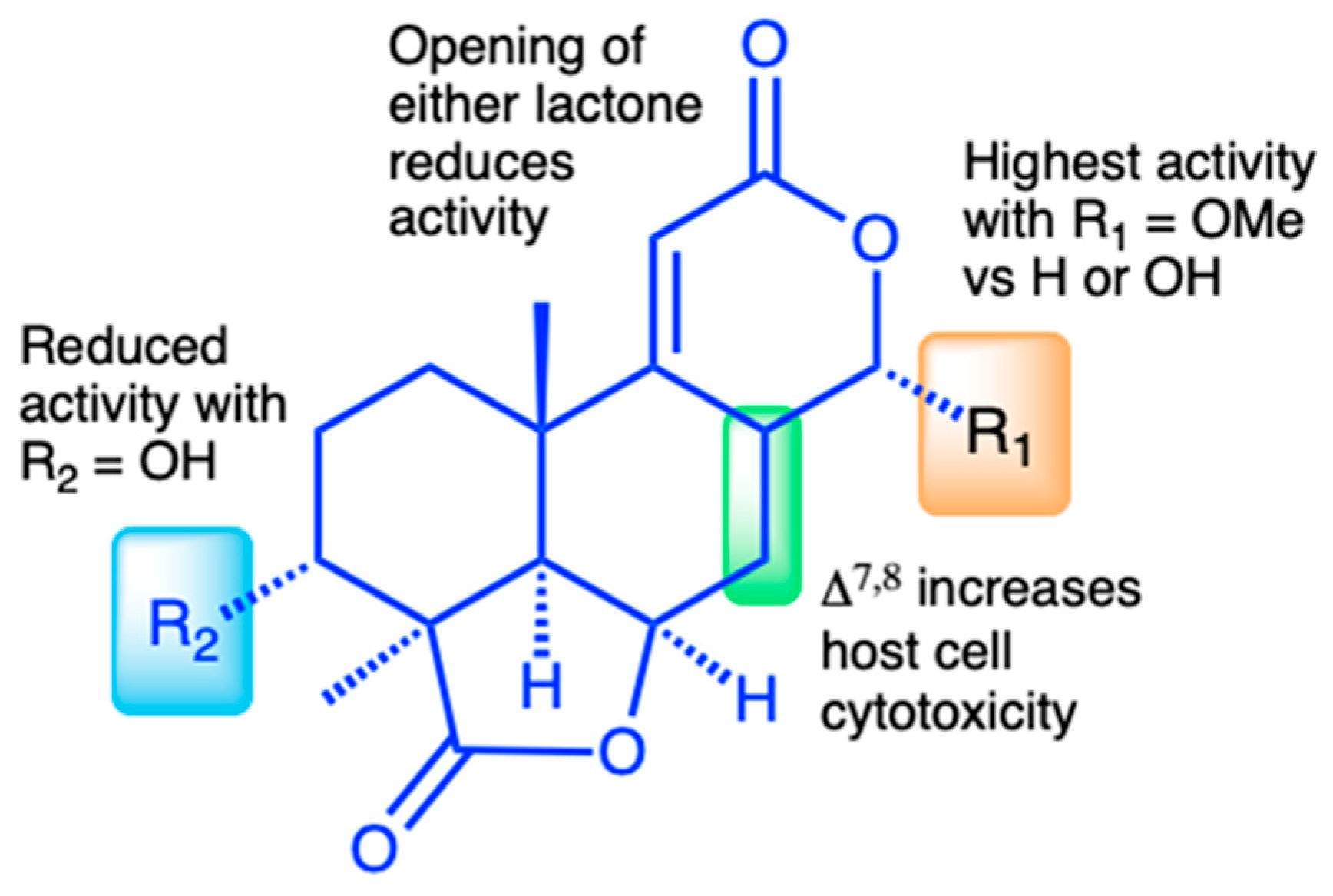
3.4. Structure–Activity Relationship of C. parvum Inhibition and HCT-8 Cytotoxicity
3.5. In Vitro Pharmacokinetics of Oidiolactone A
3.6. Oidiolactone A Inhibits Intracellular C. parvum and T. gondii Replication
3.7. Oidiolactone A Has -Cidal Activity Against Both C. parvum and T. gondii
3.8. Oidiolactone A Inhibits C. parvum Parasites During Asexual Replication and the Transition to Sexual Stages
3.9. Oidiolactone A Does Not Inhibit Excystation or Prevent Invasion of C. parvum Sporozoites into Host Cells
3.10. Oidiolactone A Reduces Infection in IFNγ−/− Mice Infected with Cp-NLuc
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.-Y.; Li, M.-Y.; Qi, Z.-Z.; Fu, M.; Sun, T.-F.; Elsheikha, H.M.; Cong, W. Waterborne Protozoan Outbreaks: An Update on the Global, Regional, and National Prevalence from 2017 to 2020 and Sources of Contamination. Sci. Total Environ. 2022, 806, 150562. [Google Scholar] [CrossRef]
- Ali, M.; Ji, Y.; Xu, C.; Hina, Q.; Javed, U.; Li, K. Food and Waterborne Cryptosporidiosis from a One Health Perspective: A Comprehensive Review. Animals 2024, 14, 3287. [Google Scholar] [CrossRef]
- Tzipori, S.; Ward, H. Cryptosporidiosis: Biology, Pathogenesis and Disease. Microbes Infect. 2002, 4, 1047–1058. [Google Scholar] [CrossRef]
- English, E.D.; Guérin, A.; Tandel, J.; Striepen, B. Live Imaging of the Cryptosporidium parvum Life Cycle Reveals Direct Development of Male and Female Gametes from Type I Meronts. PLoS Biol. 2022, 20, e3001604. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; White, A.C., Jr.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.-M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A Review of the Global Burden, Novel Diagnostics, Therapeutics, and Vaccine Targets for Cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Mmbaga, B.T.; Houpt, E.R. Cryptosporidium and Giardia Infections in Children: A Review. Pediatr. Clin. N. Am. 2017, 64, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Mac Kenzie, W.R.; Hoxie, N.J.; Proctor, M.E.; Gradus, M.S.; Blair, K.A.; Peterson, D.E.; Kazmierczak, J.J.; Addiss, D.G.; Fox, K.R.; Rose, J.B.; et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994, 331, 161–167, Erratum in N. Engl. J. Med. 1994, 331, 1035. [Google Scholar] [CrossRef] [PubMed]
- Bourli, P.; Eslahi, A.V.; Tzoraki, O.; Karanis, P. Waterborne Transmission of Protozoan Parasites: A Review of Worldwide Outbreaks—An Update 2017–2022. J. Water Health 2023, 21, 1421–1447. [Google Scholar] [CrossRef]
- Wang, R.-J.; Li, J.-Q.; Chen, Y.-C.; Zhang, L.-X.; Xiao, L.-H. Widespread Occurrence of Cryptosporidium Infections in Patients with HIV/AIDS: Epidemiology, Clinical Feature, Diagnosis, and Therapy. Acta Trop. 2018, 187, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Abubakar, I.I.; Aliyu, S.H.; Arumugam, C.; Hunter, P.R.; Usman, N. Prevention and Treatment of Cryptosporidiosis in Immunocompromised Patients. Cochrane Libr. 2007, 1, 1–25. [Google Scholar] [CrossRef]
- Tam, P.; Arnold, S.L.M.; Barrett, L.K.; Chen, C.R.; Conrad, T.M.; Douglas, E.; Gordon, M.A.; Hebert, D.; Henrion, M.; Hermann, D.; et al. Clofazimine for Treatment of Cryptosporidiosis in Human Immunodeficiency Virus Infected Adults: An Experimental Medicine, Randomized, Double-Blind, Placebo-Controlled Phase 2a Trial. Clin. Infect. Dis. 2021, 73, 183–191. [Google Scholar]
- Khan, S.M.; Witola, W.H. Past, Current, and Potential Treatments for Cryptosporidiosis in Humans and Farm Animals: A Comprehensive Review. Front. Cell. Infect. Microbiol. 2023, 13, 1115522. [Google Scholar] [CrossRef]
- Love, M.S.; Choy, R.K.M. Emerging Treatment Options for Cryptosporidiosis. Curr. Opin. Infect. Dis. 2021, 34, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.J.; Innes, E.A.; Morrison, L.J.; Katzer, F.; Wells, B. Long-Term Production Effects of Clinical Cryptosporidiosis in Neonatal Calves. Int. J. Parasitol. 2020, 50, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Santín, M. Clinical and Subclinical Infections with Cryptosporidium in Animals. N. Z. Vet. J. 2013, 61, 1–10. [Google Scholar] [CrossRef]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine Cryptosporidiosis: Impact, Host-Parasite Interaction and Control Strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Christensen, S.B. Natural Products That Changed Society. Biomedicines 2021, 9, 472. [Google Scholar] [CrossRef]
- Tu, Y. The Discovery of Artemisinin (Qinghaosu) and Gifts from Chinese Medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.L.; Monaghan, R.L.; Olson, G.; Putter, I.; et al. Avermectins, New Family of Potent Anthelmintic Agents: Producing Organism and Fermentation. Antimicrob. Agents Chemother. 1979, 15, 361–367. [Google Scholar] [CrossRef]
- Chabala, J.C.; Mrozik, H.; Tolman, R.L.; Eskola, P.; Lusi, A.; Peterson, L.H.; Woods, M.F.; Fisher, M.H.; Campbell, W.C.; Egerton, J.R.; et al. Ivermectin, a New Broad-Spectrum Antiparasitic Agent. J. Med. Chem. 1980, 23, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, C. Natural Products as Source of Therapeutics against Parasitic Diseases. Angew. Chem. Int. Ed. Engl. 2015, 54, 14622–14624. [Google Scholar] [CrossRef]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural Products as a Source for Treating Neglected Parasitic Diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef]
- Rusman, Y.; Wilson, M.B.; Williams, J.M.; Held, B.W.; Blanchette, R.A.; Anderson, B.N.; Lupfer, C.R.; Salomon, C.E. Antifungal Norditerpene Oidiolactones from the Fungus Oidiodendron truncatum, a Potential Biocontrol Agent for White-Nose Syndrome in Bats. J. Nat. Prod. 2020, 83, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Tan, R.; Herald, D.L.; Hamblin, J.; Pettit, R.K. Antineoplastic Agents. 488. Isolation and Structure of Yukonin from a Yukon Territory Fungus. J. Nat. Prod. 2003, 66, 276–278. [Google Scholar] [CrossRef]
- Cotto-Rosario, A.; Miller, E.Y.D.; Fumuso, F.G.; Clement, J.A.; Todd, M.J.; O’Connor, R.M. The Marine Compound Tartrolon E Targets the Asexual and Early Sexual Stages of Cryptosporidium parvum. Microorganisms 2022, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.S.; Donald, R.G.; Morrissette, N.S.; Moulton, A.L. Molecular Tools for Genetic Dissection of the Protozoan Parasite Toxoplasma gondii. Methods Cell Biol. 1994, 45, 27–63. [Google Scholar]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Nardella, F.; Jiang, T.; Wang, L.; Bohmer, M.J.; Chakraborty, S.; Okombo, J.; Calla, J.; Silva, T.M.; Pazicky, S.; Che, J.; et al. Plasmodium falciparum Protein Kinase 6 and Hemozoin Formation Are Inhibited by a Type II Human Kinase Inhibitor Exhibiting Antimalarial Activity. Cell Chem. Biol. 2025, 32, 926–941.e23. [Google Scholar] [CrossRef]
- Michaels, S.A.; Shih, H.-W.; Zhang, B.; Navaluna, E.D.; Zhang, Z.; Ranade, R.M.; Gillespie, J.R.; Merritt, E.A.; Fan, E.; Buckner, F.S.; et al. Methionyl-TRNA Synthetase Inhibitor Has Potent in Vivo Activity in a Novel Giardia lamblia Luciferase Murine Infection Model. J. Antimicrob. Chemother. 2020, 75, 1218–1227. [Google Scholar] [CrossRef]
- Hulverson, M.A.; Michaels, S.A.; Lee, J.W.; Wendt, K.L.; Tran, L.T.; Choi, R.; Van Voorhis, W.C.; Cichewicz, R.H.; Ojo, K.K. Identification of Fungus-Derived Natural Products as New Antigiardial Scaffolds. Microbiol. Spectr. 2023, 11, e0064723. [Google Scholar] [CrossRef]
- Bone Relat, R.M.; Winder, P.L.; Bowden, G.D.; Guzmán, E.A.; Peterson, T.A.; Pomponi, S.A.; Roberts, J.C.; Wright, A.E.; O’Connor, R.M. High-Throughput Screening of a Marine Compound Library Identifies Anti-Cryptosporidium Activity of Leiodolide A. Mar. Drugs 2022, 20, 240. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Nepveux V, F.J.; Abenoja, J.; Bowden, G.; Reis, P.; Beaushaw, J.; Bone Relat, R.M.; Driskell, I.; Gimenez, F.; Riggs, M.W.; et al. A Symbiotic Bacterium of Shipworms Produces a Compound with Broad Spectrum Anti-Apicomplexan Activity. PLoS Pathog. 2020, 16, e1008600. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef]
- Michaels, S.A.; Hulverson, M.A.; Whitman, G.R.; Tran, L.T.; Choi, R.; Fan, E.; McNamara, C.W.; Love, M.S.; Ojo, K.K. Repurposing the Kinase Inhibitor Mavelertinib for Giardiasis Therapy. Antimicrob. Agents Chemother. 2022, 66, e0001722. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Kim, K.; Khan, F.; Ward, H.D. Expression of Cpgp40/15 in Toxoplasma gondii: A Surrogate System for the Study of Cryptosporidium Glycoprotein Antigens. Infect. Immun. 2003, 71, 6027–6034. [Google Scholar] [CrossRef]
- Jumani, R.S.; Hasan, M.M.; Stebbins, E.E.; Donnelly, L.; Miller, P.; Klopfer, C.; Bessoff, K.; Teixeira, J.E.; Love, M.S.; McNamara, C.W.; et al. A Suite of Phenotypic Assays to Ensure Pipeline Diversity When Prioritizing Drug-like Cryptosporidium Growth Inhibitors. Nat. Commun. 2019, 10, 1862. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Wanyiri, J.W.; Cevallos, A.M.; Priest, J.W.; Ward, H.D. Cryptosporidium parvum Glycoprotein Gp40 Localizes to the Sporozoite Surface by Association with Gp15. Mol. Biochem. Parasitol. 2007, 156, 80–83. [Google Scholar] [CrossRef]
- Vinayak, S.; Pawlowic, M.C.; Sateriale, A.; Brooks, C.F.; Studstill, C.J.; Bar-Peled, Y.; Cipriano, M.J.; Striepen, B. Genetic Modification of the Diarrhoeal Pathogen Cryptosporidium parvum. Nature 2015, 523, 477–480. [Google Scholar] [CrossRef]
- Chen, X.; Murawski, A.; Patel, K.; Crespi, C.L.; Balimane, P.V. A Novel Design of Artificial Membrane for Improving the PAMPA Model. Pharm. Res. 2008, 25, 1511–1520. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Wang, P.-H.; Tu, Y.-S.; Tseng, Y.J. PgpRules: A Decision Tree Based Prediction Server for P-Glycoprotein Substrates and Inhibitors. Bioinformatics 2019, 35, 4193–4195. [Google Scholar] [CrossRef]
- Bahar, F.G.; Ohura, K.; Ogihara, T.; Imai, T. Species Difference of Esterase Expression and Hydrolase Activity in Plasma. J. Pharm. Sci. 2012, 101, 3979–3988. [Google Scholar] [CrossRef]
- Funkhouser-Jones, L.J.; Ravindran, S.; Sibley, L.D. Defining Stage-Specific Activity of Potent New Inhibitors of Cryptosporidium Parvum Growth in Vitro. mBio 2020, 11, 10-1128. [Google Scholar] [CrossRef]
- Pane, S.; Putignani, L. Cryptosporidium: Still Open Scenarios. Pathogens 2022, 11, 515. [Google Scholar] [CrossRef]
- Gorla, S.K.; McNair, N.N.; Yang, G.; Gao, S.; Hu, M.; Jala, V.R.; Haribabu, B.; Striepen, B.; Cuny, G.D.; Mead, J.R.; et al. Validation of IMP Dehydrogenase Inhibitors in a Mouse Model of Cryptosporidiosis. Antimicrob. Agents Chemother. 2014, 58, 1603–1614. [Google Scholar] [CrossRef]
- Castellanos-Gonzalez, A.; White, A.C., Jr.; Ojo, K.K.; Vidadala, R.S.R.; Zhang, Z.; Reid, M.C.; Fox, A.M.W.; Keyloun, K.R.; Rivas, K.; Irani, A.; et al. A Novel Calcium-Dependent Protein Kinase Inhibitor as a Lead Compound for Treating Cryptosporidiosis. J. Infect. Dis. 2013, 208, 1342–1348. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, H.; Fritzler, J.M.; Rider, S.D., Jr.; Xiang, L.; McNair, N.N.; Mead, J.R.; Zhu, G. Amelioration of Cryptosporidium parvum Infection in Vitro and in Vivo by Targeting Parasite Fatty Acyl-Coenzyme A Synthetases. J. Infect. Dis. 2014, 209, 1279–1287. [Google Scholar] [CrossRef]
- Ndao, M.; Nath-Chowdhury, M.; Sajid, M.; Marcus, V.; Mashiyama, S.T.; Sakanari, J.; Chow, E.; Mackey, Z.; Land, K.M.; Jacobson, M.P.; et al. A Cysteine Protease Inhibitor Rescues Mice from a Lethal Cryptosporidium parvum Infection. Antimicrob. Agents Chemother. 2013, 57, 6063–6073. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Vinayak, S.; Zambriski, J.A.; Chao, A.T.; Sy, T.; Noble, C.G.; Bonamy, G.M.C.; Kondreddi, R.R.; Zou, B.; Gedeck, P.; et al. A Cryptosporidium PI(4)K Inhibitor Is a Drug Candidate for Cryptosporidiosis. Nature 2017, 546, 376–380. [Google Scholar] [CrossRef]
- Jumani, R.S.; Bessoff, K.; Love, M.S.; Miller, P.; Stebbins, E.E.; Teixeira, J.E.; Campbell, M.A.; Meyers, M.J.; Zambriski, J.A.; Nunez, V.; et al. A Novel Piperazine-Based Drug Lead for Cryptosporidiosis from the Medicines for Malaria Venture Open-Access Malaria Box. Antimicrob. Agents Chemother. 2018, 62, e01505-17. [Google Scholar] [CrossRef]
- Hulverson, M.A.; Vinayak, S.; Choi, R.; Schaefer, D.A.; Castellanos-Gonzalez, A.; Vidadala, R.S.R.; Brooks, C.F.; Herbert, G.T.; Betzer, D.P.; Whitman, G.R.; et al. Bumped-Kinase Inhibitors for Cryptosporidiosis Therapy. J. Infect. Dis. 2017, 215, 1275–1284, Erratum in J. Infect. Dis. 2018, 217, 340. https://doi.org/10.1093/infdis/jix611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Love, M.S.; Beasley, F.C.; Jumani, R.S.; Wright, T.M.; Chatterjee, A.K.; Huston, C.D.; Schultz, P.G.; McNamara, C.W. A High-Throughput Phenotypic Screen Identifies Clofazimine as a Potential Treatment for Cryptosporidiosis. PLoS Negl. Trop. Dis. 2017, 11, e0005373. [Google Scholar] [CrossRef]
- Huston, C.D. The Clofazimine for Treatment of Cryptosporidiosis in HIV-Infected Adults (CRYPTOFAZ) and Lessons Learned for Anticryptosporidial Drug Development. Clin. Infect. Dis. 2021, 73, 192–194. [Google Scholar] [CrossRef]
- Del Campo, J.; Heger, T.J.; Rodríguez-Martínez, R.; Worden, A.Z.; Richards, T.A.; Massana, R.; Keeling, P.J. Assessing the Diversity and Distribution of Apicomplexans in Host and Free-Living Environments Using High-Throughput Amplicon Data and a Phylogenetically Informed Reference Framework. Front. Microbiol. 2019, 10, 2373, Erratum in: Front. Microbiol. 2020, 11, 576322. https://doi.org/10.3389/fmicb.2020.576322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- John, M.; Krohn, K.; Flörke, U.; Aust, H.-J.; Draeger, S.; Schulz, B. Biologically Active Secondary Metabolites from Fungi. 12. Oidiolactones A−F, Labdane Diterpene Derivatives Isolated from Oidiodendron truncata. J. Nat. Prod. 1999, 62, 1218–1221. [Google Scholar] [CrossRef]
- Herath, H.M.T.B.; Herath, W.H.M.W.; Carvalho, P.; Khan, S.I.; Tekwani, B.L.; Duke, S.O.; Tomaso-Peterson, M.; Nanayakkara, N.P.D. Biologically Active Tetranorditerpenoids from the Fungus Sclerotinia homoeocarpa Causal Agent of Dollar Spot in Turfgrass. J. Nat. Prod. 2009, 72, 2091–2097. [Google Scholar] [CrossRef]
- Deng, Z.; Sheng, F.; Yang, S.-Y.; Liu, Y.; Zou, L.; Zhang, L.-L. A Comprehensive Review on the Medicinal Usage of Podocarpus Species: Phytochemistry and Pharmacology. J. Ethnopharmacol. 2023, 310, 116401. [Google Scholar] [CrossRef]
- González-Coloma, A.; Reina, M.; Medinaveitia, A.; Guadaño, A.; Santana, O.; Martínez-Díaz, R.; Ruiz-Mesía, L.; Alva, A.; Grandez, M.; Díaz, R.; et al. Structural Diversity and Defensive Properties of Norditerpenoid Alkaloids. J. Chem. Ecol. 2004, 30, 1393–1408. [Google Scholar] [CrossRef]
- Barrero, A.F.; Quilez Del Moral, J.F.; Mar Herrador, M. Podolactones: A Group of Biologically Active Norditerpenoids. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 28, pp. 453–516. [Google Scholar]
- Kubo, I.; Sutisna, M.; Kah-Siew, T. Effects of Nagilactones on the Growth of Lettuce Seedlings. Phytochemistry 1991, 30, 455–456. [Google Scholar] [CrossRef]
- Manjunatha, U.H.; Lakshminarayana, S.B.; Jumani, R.S.; Chao, A.T.; Young, J.M.; Gable, J.E.; Knapp, M.; Hanna, I.; Galarneau, J.-R.; Cantwell, J.; et al. Cryptosporidium PI(4)K Inhibitor EDI048 Is a Gut-Restricted Parasiticidal Agent to Treat Paediatric Enteric Cryptosporidiosis. Nat. Microbiol. 2024, 9, 2817–2835. [Google Scholar] [CrossRef]
- Caldwell, N.; Peet, C.; Miller, P.; Colon, B.L.; Taylor, M.G.; Cocco, M.; Dawson, A.; Lukac, I.; Teixeira, J.E.; Robinson, L.; et al. Cryptosporidium Lysyl-TRNA Synthetase Inhibitors Define the Interplay between Solubility and Permeability Required to Achieve Efficacy. Sci. Transl. Med. 2024, 16, eadm8631. [Google Scholar] [CrossRef]
- Arnold, S.L.M.; Choi, R.; Hulverson, M.A.; Schaefer, D.A.; Vinayak, S.; Vidadala, R.S.R.; McCloskey, M.C.; Whitman, G.R.; Huang, W.; Barrett, L.K.; et al. Necessity of Bumped Kinase Inhibitor Gastrointestinal Exposure in Treating Cryptosporidium Infection. J. Infect. Dis. 2017, 216, 55–63. [Google Scholar] [CrossRef]
- Amin, M.L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, DTI-S12519. [Google Scholar] [CrossRef]
- Arnold, S.L.M.; Choi, R.; Hulverson, M.A.; Whitman, G.R.; Mccloskey, M.C.; Dorr, C.S.; Vidadala, R.S.R.; Khatod, M.; Morada, M.; Barrett, L.K.; et al. P-Glycoprotein-Mediated Efflux Reduces the in Vivo Efficacy of a Therapeutic Targeting the Gastrointestinal Parasite Cryptosporidium. J. Infect. Dis. 2019, 220, 1188–1198. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Li, Y.; Tian, S.; Sun, H.; Hou, T. ADMET Evaluation in Drug Discovery. 13. Development of in Silico Prediction Models for P-Glycoprotein Substrates. Mol. Pharm. 2014, 11, 716–726. [Google Scholar] [CrossRef]
- Desai, P.V.; Sawada, G.A.; Watson, I.A.; Raub, T.J. Integration of in Silico and in Vitro Tools for Scaffold Optimization during Drug Discovery: Predicting P-Glycoprotein Efflux. Mol. Pharm. 2013, 10, 1249–1261. [Google Scholar] [CrossRef]
- Hanessian, S.; Boyer, N.; Reddy, G.J.; Deschênes-Simard, B. Total Synthesis of Oidiodendrolides and Related Norditerpene Dilactones from a Common Precursor: Metabolites CJ-14,445, LL-Z1271gamma, Oidiolactones A, B, C, and D, and Nagilactone F. Org. Lett. 2009, 11, 4640–4643. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Xu, T. Total Synthesis of Bioactive Tetracyclic Norditerpene Dilactones. Org. Biomol. Chem. 2021, 19, 9138–9147. [Google Scholar] [CrossRef]
- Barrero, A.F.; Arseniyadis, S.; Quílez del Moral, J.F.; Herrador, M.M.; Valdivia, M.; Jiménez, D. First Synthesis of the Antifungal Oidiolactone C from Trans-Communic Acid: Cytotoxic and Antimicrobial Activity in Podolactone-Related Compounds. J. Org. Chem. 2002, 67, 2501–2508. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, L.; Lv, C.; Sun, H.; Wei, S.; Wang, B.; Huang, C.; Jiao, B. Wentilactone B Induces G2/M Phase Arrest and Apoptosis via the Ras/Raf/MAPK Signaling Pathway in Human Hepatoma SMMC-7721 Cells. Cell Death Dis. 2013, 4, e657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Jiang, X.-M.; Huang, M.-Y.; Feng, Z.-L.; Chen, X.; Wang, Y.; Li, H.; Li, A.; Lin, L.-G.; Lu, J.-J. Nagilactone E Suppresses TGF-Β1-Induced Epithelial-Mesenchymal Transition, Migration and Invasion in Non-Small Cell Lung Cancer Cells. Phytomedicine 2019, 52, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Benatrehina, P.A.; Chen, W.-L.; Czarnecki, A.A.; Kurina, S.; Chai, H.-B.; Lantvit, D.D.; Ninh, T.N.; Zhang, X.; Soejarto, D.D.; Burdette, J.E.; et al. Bioactivity-Guided Isolation of Totarane-Derived Diterpenes from Podocarpus neriifolius and Structure Revision of 3-Deoxy-2α-Hydroxynagilactone E. Nat. Products Bioprospect. 2019, 9, 157–163. [Google Scholar] [CrossRef]
- Ichikawa, K.; Hirai, H.; Ishiguro, M.; Kambara, T.; Kato, Y.; Kim, Y.J.; Kojima, Y.; Matsunaga, Y.; Nishida, H.; Shiomi, Y.; et al. Cytokine Production Inhibitors Produced by a Fungus, Oidiodendron griseum. J. Antibiot. 2001, 54, 697–702. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Guo, J.; Jiang, X.-M.; Chen, X.-P.; Wang, Y.-T.; Li, A.; Lin, L.-G.; Li, H.; Lu, J.-J. Identification of Nagilactone E as a Protein Synthesis Inhibitor with Anticancer Activity. Acta Pharmacol. Sin. 2020, 41, 698–705. [Google Scholar] [CrossRef]
- Devkota, K.P.; Ratnayake, R.; Colburn, N.H.; Wilson, J.A.; Henrich, C.J.; McMahon, J.B.; Beutler, J.A. Inhibitors of the Oncogenic Transcription Factor AP-1 from Podocarpus latifolius. J. Nat. Prod. 2011, 74, 374–377. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer Activities and Mechanism of Action of Nagilactones, a Group of Terpenoid Lactones Isolated from Podocarpus Species. Nat. Products Bioprospect. 2020, 10, 367–375. [Google Scholar] [CrossRef] [PubMed]





| Mouse Plasma Stability t½ (min) | Human Plasma Stability t½ (min) | Mouse Microsomal Stability a t½ (min) | Human Microsomal Stability t½ (min) | |
|---|---|---|---|---|
| Oidiolactone A (1) | 105.8 ± 1.7 | 17.5 ± 0.1 | 110.4 ± 4.3 b | 317.7 ± 16.7 |
| Verapamil | 2.8 ± 0.0 | 11.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolas, A.; Rusman, Y.; Maia, A.C.R.G.; Williams, J.M.; Xie, J.; Katekar, R.; Fumuso, F.G.; Cotto-Rosario, A.; Onoh, C.N.; Baggar, H.; et al. Norditerpene Natural Products from Subterranean Fungi with Anti-Parasitic Activity. Microorganisms 2025, 13, 2527. https://doi.org/10.3390/microorganisms13112527
Kolas A, Rusman Y, Maia ACRG, Williams JM, Xie J, Katekar R, Fumuso FG, Cotto-Rosario A, Onoh CN, Baggar H, et al. Norditerpene Natural Products from Subterranean Fungi with Anti-Parasitic Activity. Microorganisms. 2025; 13(11):2527. https://doi.org/10.3390/microorganisms13112527
Chicago/Turabian StyleKolas, Alexandra, Yudi Rusman, Ana C. R. G. Maia, Jessica M. Williams, Jiashu Xie, Roshan Katekar, Fernanda G. Fumuso, Alexis Cotto-Rosario, Chidiebere N. Onoh, Hanen Baggar, and et al. 2025. "Norditerpene Natural Products from Subterranean Fungi with Anti-Parasitic Activity" Microorganisms 13, no. 11: 2527. https://doi.org/10.3390/microorganisms13112527
APA StyleKolas, A., Rusman, Y., Maia, A. C. R. G., Williams, J. M., Xie, J., Katekar, R., Fumuso, F. G., Cotto-Rosario, A., Onoh, C. N., Baggar, H., Piaskowski, M. L., Baigorria, C., Paes, R., Chakrabarti, D., Weible, L. J., Ojo, K. K., O’Connor, R. M., & Salomon, C. E. (2025). Norditerpene Natural Products from Subterranean Fungi with Anti-Parasitic Activity. Microorganisms, 13(11), 2527. https://doi.org/10.3390/microorganisms13112527








