1. Introduction
The transmission of zoonotic pathogens—such as avian influenza virus (AIV) and dengue virus (DENV)—from animals to humans has caused several devastating outbreaks in recent years [
1]. In rural areas, public health authorities often fail to detect zoonotic infections at an early stage, particularly in remote regions with limited healthcare access and diagnostic capacity [
2]. Consequently, these potentially epidemic- or pandemic-prone diseases often remain unreported, especially among populations with limited access to healthcare facilities. Farmed and wild animals in these regions are recognized reservoirs of these zoonotic pathogens [
3].
Bangladesh and Yunnan Province in China, both situated in tropical to subtropical regions, face substantial threats from zoonotic diseases. Since 2007, Bangladesh has experienced multiple outbreaks of highly pathogenic AIV in poultry, with more than 550 outbreaks recorded and eight human cases of H5N1 reported, including one fatality [
4]. In 2025, two human cases of H5N1 were reported in Khulna and Jessore districts of Bangladesh [
5]. Concurrently, dengue fever has shown geographical expansion from core regions (Dhaka and Chattogram) to other areas [
6]. This co-circulation of multiple pathogens underscores the significant challenges in Bangladesh and Yunnan province [
7]. Yunnan Province is also confronted with imported cases of dengue fever from Myanmar and other regions [
8]. Moreover, seasonal migration of wild birds further increases the risk of AIV introduction into Yunnan Province [
9].
Wastewater-based surveillance (WBS) has recently emerged as a robust and essential tool for monitoring public health-relevant analytes by capturing pathogens shed by both symptomatic and asymptomatic individuals, irrespective of their socioeconomic status [
10]. The U.S. Centers for Disease Control and Prevention (CDC) established a WBS covering 45% of the population to track the dynamics of AIV transmission. The system provides an early warning signal that both zoonotic and anthropogenic sources contribute to community infections [
11]. During 2024 in the United States, wastewater monitoring across 48 states during an outbreak of highly pathogenic AIV (H5N1) consistently detected the AIV H5 subtype, confirming that wastewater monitoring serves as a One Health indicator for assessing influenza prevalence in communities [
12]. Complementary research demonstrated the effectiveness of WBS by identifying H5 gene fragments coinciding with elevated prevalence of AIV in municipal wastewater solids in the spring of 2024 [
13]. In addition, metagenomic sequencing of nine municipal wastewater samples was used to successfully identify H5N1 AIVs [
14]. WBS has also demonstrated utility for early detection and assessment of the risk of dengue virus transmission in various countries [
15,
16,
17]. However, rural areas frequently lack centralized sewer networks, posing challenges for implementing wastewater-based surveillance in these settings. Monitoring surface water in rural communities and wildlife habitats may offer a practical alternative [
18]. Nevertheless, whether this approach can provide both early detection and comprehensive tracking of zoonotic outbreaks remains unclear.
To address this issue, in this study, we implemented surface water-based surveillance (SWBS) in a rural area of Bangladesh and China to detect AIV and dengue virus. Together with the sampling of wild animals or digital epidemiology, these findings not only validate the potential role of SWBS for early viral warning systems but also reveal the application value of a multifaceted approach for distinguishing the source of the virus.
2. Materials and Methods
2.1. Study Area and Surface Water Sampling
In this study, we implemented a Sewage and Water-Based Surveillance (SWBS) project in two locations: Dhaka, Bangladesh, and Ruili, China. Both locations face challenges due to limited resources for addressing zoonotic diseases and are situated within an intense monsoon climate regime, characterized by a distinct concentration of high-temperature rainy seasons [
19,
20]. This climatic setting provides favorable environmental conditions for the proliferation of vector mosquitoes and virus transmission. During the rainy season, inadequate drainage systems in Dhaka result in extensive surface water stagnation, forming persistent mosquito breeding habitats in public areas [
21]. In contrast, Ruili’s mosquito proliferation primarily depends primarily on scattered water-holding containers at the household and community levels. These distinct hydrological conditions collectively create numerous microenvironments conducive to mosquito breeding. Furthermore, high-density urbanization and overburdened infrastructure not only increase human-vector contact frequency but also foster a high-risk environment for the cross-species transmission of pathogens such as avian influenza. Ruili’s position on the China-Myanmar border facilitates the continuous introduction of foreign pathogens through frequent cross-border human mobility and trade activities, including poultry trade [
8,
22]. Under suitable local climatic and hydrological conditions, these introduced pathogens can readily initiate local transmission cycles and potentially lead to exported cases.
In Bangladesh, we selected a remote suburban area of Dhaka (23.4813° N, 90.2040° E), which includes two sewage discharge outlets (
Figure S1). The catchment area covers approximately 15 km
2, with an average flow velocity of 0.5 m/s during the sampling period. The average monthly rainfall in Dhaka during sampling was 250 mm. From July to November 2023, surface water samples were collected weekly from 0.5 m below the surface at the designated sampling points. In China, river water was collected twice per week from the Ruili River in Ruili City (24.0125° N, 97.5149° E) (
Figure S2). The catchment area is approximately 20 km
2, with a relatively stable flow velocity of about 1.0 m/s. The average monthly rainfall in Ruili during the sampling period was 180 mm. To ensure consistency and reliability, the sampling frequency at both sites was standardized to twice per week. Surface water samples were collected using a Bag-Mediated Filtration System (BMFS), following the recommendations of a previous study [
23]. A total of 3 L of water was filtered through a 0.22 μm pore-size polyethersulfone (PES) membrane (Millipore, Burlington, MA, USA). The filters were immediately stored at −20 °C prior to nucleic acid extraction using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany).
2.2. Quantification and Subtyping of AIV in Surface Water
In this study, to monitor the presence of AIV in surface water, the M gene was first detected using RT-qPCR. A plasmid containing the AIV M gene (Sangon Biotech Co., Ltd., Shanghai, China) was used to generate a standard curve by performing a 10-fold serial dilution (101–107 copies/μL).
Subsequently, a multiplex one-step RT-qPCR assay was employed to identify five AIV subtypes (H1, H5, H7, H9, and H10). Primers and probes were designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA, USA), and their sequences are listed in
Table S1. Samples positive for the M gene but negative for all five HA subtypes were classified as AIV HA untyped.
To assess potential loss of viral genetic material during sample processing, pepper mild mottle virus (PMMoV) was used as a process control. PMMoV was propagated in
Escherichia coli (ATCC 15597) cultures, harvested, and spiked into 35 mL of raw surface water at a concentration of 2.3 × 10
10 PFU/mL. Recovery rates of PMMoV were determined by two methods: first, the modified PEG/precipitation method, followed by quantification of the active PMMoV using the double agar layer assay (in PFU/mL) as described by Cormier and Janes [
24]; second, the PMMoV genome copies were quantified using RT-qPCR, following the method of Miranda and Steward [
25]. Calibration curves were generated using dilutions of 1 × 10
0, 1 × 10
1, 1 × 10
2, 1 × 10
3, and 1 × 10
9 PFU/mL (analyzed in duplicate). The recovery rate was calculated as the percentage of total inoculated viral particles recovered post-sample processing.
Quantitative detection was performed on the Applied Biosystems QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Shanghai, China). Each 20 μL reaction contained 10.0 μL Premix Ex Taq™ (Probe qPCR; TaKaRa, Shiga, Japan), 0.2 μm of each subtype-specific primer, 0.1 μm of the corresponding probe, 1.0 μL RNA template, and nuclease-free water to a final volume of 20 μL. The RT-qPCR program was as follows: reverse transcription at 50 °C for 15 min, initial denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 10 s and 58 °C for 30 s (annealing and extension). Each run included positive controls (standard plasmid) and negative controls (no template control). All tests were performed in triplicate.
2.3. Detection of Dengue Virus in Surface Water
For dengue virus (DENV), the capsid–premembrane (CprM) region of DENV was targeted for detection using RT-qPCR. A standard curve was established using tenfold serial dilutions (3 × 100 – 3 × 105 copies/μL) of CprM gene fragments.
Recovery efficiency for DENV was evaluated by spiking surface water samples with a known amount of DENV RNA and calculating the recovery percentage as (measured copies/spiked copies) × 100%. PMMoV was added as an internal amplification control in each reaction to monitor potential inhibition.
DENV detection was performed using the GoTaq
® 1-Step RT-qPCR System (Promega, Madison, WI, USA) following the method described by Johnson et al. [
26]. Primers targeting the CprM region are shown in
Table S1. Quantitative detection was performed on the Applied Biosystems QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Shanghai, China) in 20 μL reaction volumes. The thermal cycling conditions were: reverse transcription at 45 °C for 15 min, enzyme activation at 95 °C for 2 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s. Each run included a positive control (standard plasmid) and a negative control (no-template control). All experiments were performed in triplicate.
2.4. Determination of LOD for AIV and DENV RT-qPCR Assays
The limits of detection (LOD) for the AIV and DENV RT-qPCR assays were determined using serial dilutions of pseudoviruses. For AIV, pseudoviruses with five different HA genes (Diff-BioTech, Shanghai, China), representing the M, H1, H5, H7, H9, and H10 genes, were used in serial dilution. For DENV, serial dilutions of DENV pseudoviruses (Zhongke Kaipu Biotechnology Co., Ltd., Wuhan, China) were employed.
Spiking experiments were performed by adding pseudoviruses at different concentrations (1 to 20 copies/μL) to AIV-negative or DENV-negative surface water samples, with 20 replicates for each concentration. The LOD was defined as the lowest concentration at which ≥95% of replicates tested positive. The 95% LOD was determined using probit analysis. Probit values were calculated using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and plotted against the log10-transformed concentration data. The 95% LOD was then calculated from the regression equation corresponding to a probability of 0.95 (equivalent to a probit value of 1.64).
2.5. Collection of Fresh Bird Feces and RNA Extraction
Fresh fecal samples (
n = 40) were collected weekly within a 3 km radius of positive sampling site (
Figure S1). The feces originated from five wild bird species (Eurasian Hobby (
Falco subbuteo), Oriental Magpie-Robin (
Copsychus saularis), mallard duck (
Anas platyrhynchos), Ruddy Shelduck (
Tadorna ferruginea), Jungle crow (
Corvus levaillantii)) and domestic chicken. Fresh feces from migratory birds were sampled from grass blades. The samples were collected early in the morning to minimize UV degradation. Fresh droppings were collected only when a single species was present, as confirmed by field surveys. For each sample, droppings from the same species were pooled to provide sufficient material, resulting in a final sample size of 1 g. The fecal samples were collected using a spoon and placed in a 15 mL sterile Falcon tube containing 2 mL of isotonic solution consisting of composed saline (PBS) with 50% glycerol, penicillin (10,000 U/mL), gentamicin (250 mg/mL), and nystatin (2500 U/mL). All samples were placed in sterile containers and transported to the laboratory in Chattogram Veterinary and Animal Sciences University on ice within 24 h of collection. Viral RNA was extracted from bird feces using QIAamp
® Viral RNA (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The presence and subtyping of the AIV was conducted by RT-qPCR as detailed in above section.
2.6. Sequencing of Eight Fragments for AIV
Amplification of the eight segments of AIV was carried out by standard conventional RT-qPCR, as described previously. Among the RT-qPCR positives, the samples with Ct value ≤33 were considered for sequencing. Library preparation was performed using the MGISP-100RS automated sample preparation system (CAT No. 900-000070-00). Sequencing was conducted on the DNBSEQ-E25 sequencer using the DNBSEQ-E25RS high-throughput sequencing reagent kit (FCL PE150) (MGI Tech Co., Ltd., Shenzhen, China). The sequencing data were aligned to the AIV H5N6 reference genome (A/Whooper swan/Mongolia/25/2020) using the MGI FluTrack software (version v1.0).
2.7. Phylogenetic Analysis of AIV Fragments
The obtained sequences were subjected to Clustal W multiple sequence alignment and residue analyses using the BioEdit 7.1.5 program. HA subtypes and nucleotide identity were confirmed using basic local alignment (BLASTn) searches (
https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 26 October 2025). Clade information was identified using the H5 clade classification tool incorporated within the Influenza Research Database. We downloaded (2 June 2024) all HA sequences of H5 viruses (
n = 564) deposited in the Global Initiative for Sharing All Influenza Data (GISAID;
https://gisaid.org/) [
27] since 2010. Sequences shorter than 1500 bp or lacking complete metadata were excluded. We only kept sequences from chicken, duck, and wild birds, resulting in a total of 429 sequences.
Sequences were aligned using the MAFFT [
28]. The best fit substitution model was identified as the lowest BIC using ModelFinder within IQ-TREE [
29], and we inferred maximum likelihood phylogenetic trees, using IQ-TREE version 1.68, with 1000 bootstraps. In addition to these sequences from Bangladesh, we used the BLASTn search tool to identify closely related sequences belonging to clade 2.3.4.4 in both GenBank and the GISAID Epiflu database. Similarly, NA, M, NS, and NP sequences from Bangladesh and China were also downloaded from GISAID. Phylogenetic relationships of above genes were also analyzed as described above.
2.8. Sequencing of Dengue Virus from Surface Water
The CprM gene in the viral polyprotein gene was amplified by standard conventional RT-qPCR, which was performed as previously described by Gomes et al. [
30]. Of the RT-qPCR-positive samples, those with Ct values ≤ 33 were selected for sequencing. Phylogenetic relationships of CprM gene were analyzed using the same approach employed for AIV.
2.9. Collection of Clinical Data
We collected dengue fever case data from Bangladesh, provided by the World Health Organization [
31]. All reported dengue cases from Ruili City in 2023 and Guangzhou or Shenzhen in 2024 were obtained through the local Disease Control and Prevention or literature [
32]. Statistical analyses were performed using the R package v4.3.2, Pearson’s correlation analysis was employed to evaluate the association between the number of infected patients and abundance of dengue virus in surface water.
To quantitatively assess the temporal lead-lag relationship between environmental surveillance data and clinical case reports, a cross-correlation function (CCF) analysis was conducted. This method computes correlation coefficients across a range of time lags, identifying the specific lag at which the two time series exhibit the strongest association. Prior to CCF analysis, a prewhitening procedure was applied to both time series to remove internal autocorrelation, thus preventing spurious correlations. All analyses were performed using the ‘forecast’ package in R software (version 4.3.2). For a time series of length n, the 95% confidence interval for the cross-correlation coefficients was defined as
. A correlation peak exceeding this threshold was considered statistically significant.
2.10. Derivation of Web Search Sentiment Indices for Dengue
Web search activity was used as a proxy for public sentiment towards dengue. Indices were extracted from Google Trends (
https://trends.google.gg), the Bing Webmaster Index (
https://www.bing.com/webmasters) and the Baidu Index (
http://index.baidu.com) for 1 February 2022–31 July 2023. Next, we adopted a three-step approach for understanding the role of web search activity for the early warning of dengue.
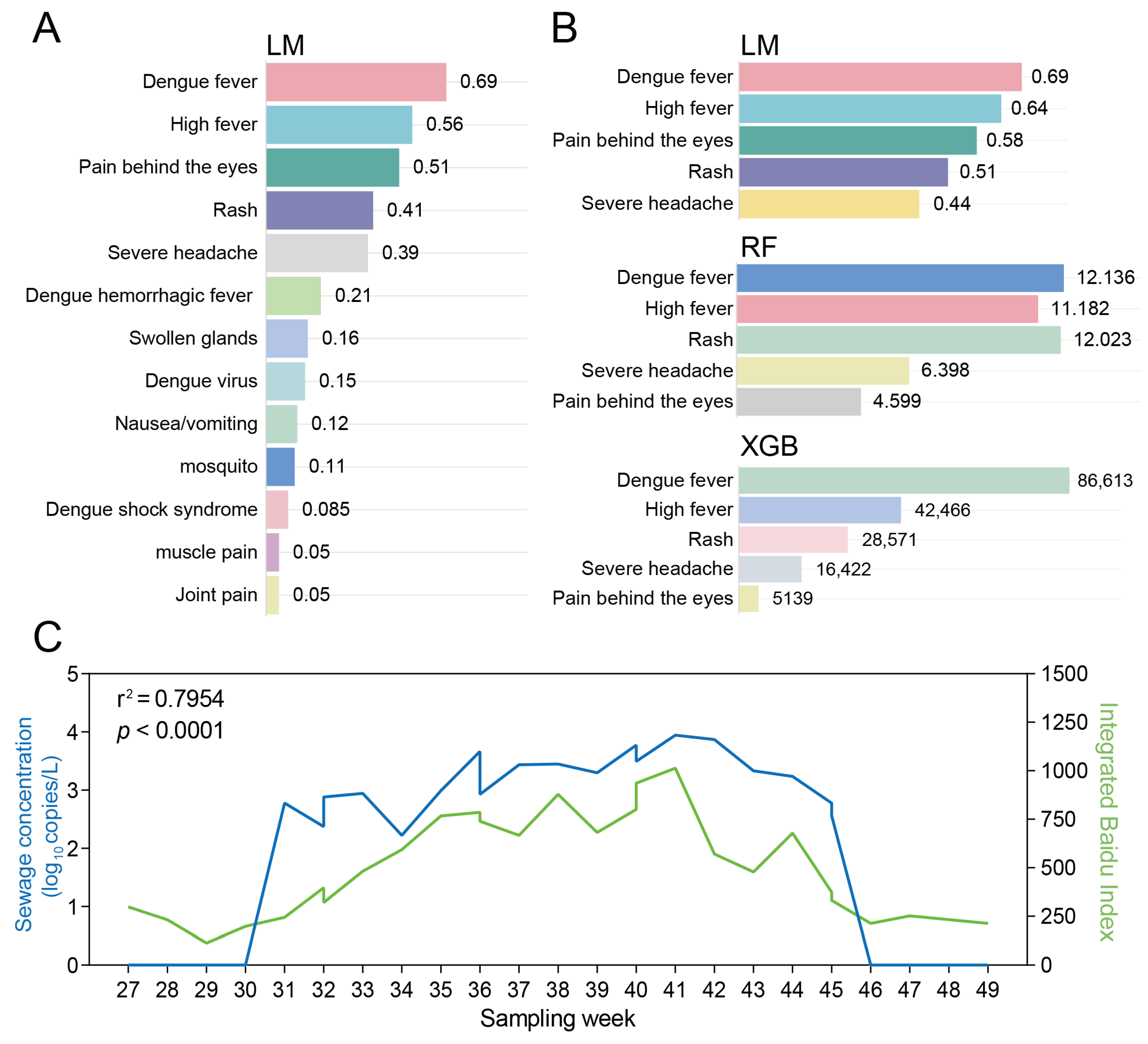
Step 1—Keyword compilation
The term “dengue” was queried weekly on each platform. All 50 unique indices returned were pooled, and their weekly search volumes were averaged across platforms. The 15 indices with the highest mean volume were retained for further analysis.
Step 2—Daily search volume acquisition
Weekly indices were collected for Dhaka (Bangladesh) and Ruili (China) concomitantly with wastewater sampling. Analogous weekly data were obtained from two prior European studies (Portugal,
n = 273; Italy,
n = 30) covering both mobile and PC traffic [
16,
33]. Linear regression between the 13 candidate indices and the corresponding wastewater dengue RNA concentrations yielded five indices with R
2 > 0.70 (
p < 0.05). Daily average search volumes for these five indices were extracted for the same cities and periods.
Step 3—Machine learning modelling
Three algorithms—linear regression (LM), random forest (RF) and extreme gradient boosting (XGB)—were trained to predict dengue virus RNA levels in wastewater from the 13 retained search terms (
Table S3). Training data comprised 280 samples: Portugal (
n = 273), Italy (
n = 30), China (
n = 40) and Bangladesh (
n = 40). LM was implemented with the lm function (R 4.3.2, stats package). For RF, the percentage increase in mean squared error (%IncMSE) was calculated over 1000 permutations; the relative importance of each term was expressed as the %IncMSE of the term divided by the total %IncMSE of all terms. For XGB, mean importance (IMP) was computed from the same 1000 permutations. Terms were retained if they met any of the following criteria: R
2 ≥ 0.70 in LM; contribution ≥ 10% in RF; or IMP ≥ 10% in XGB.
Model performance was evaluated on an independent test set of 24 samples collected in Ruili city (1 July–15 September 2024) and validated on another 24 samples from Ruili city in the next two months (16 September–7 December 2024) (
Figure S2). Wastewater sampling, RNA extraction and RT-qPCR were performed as described previously.
4. Discussion
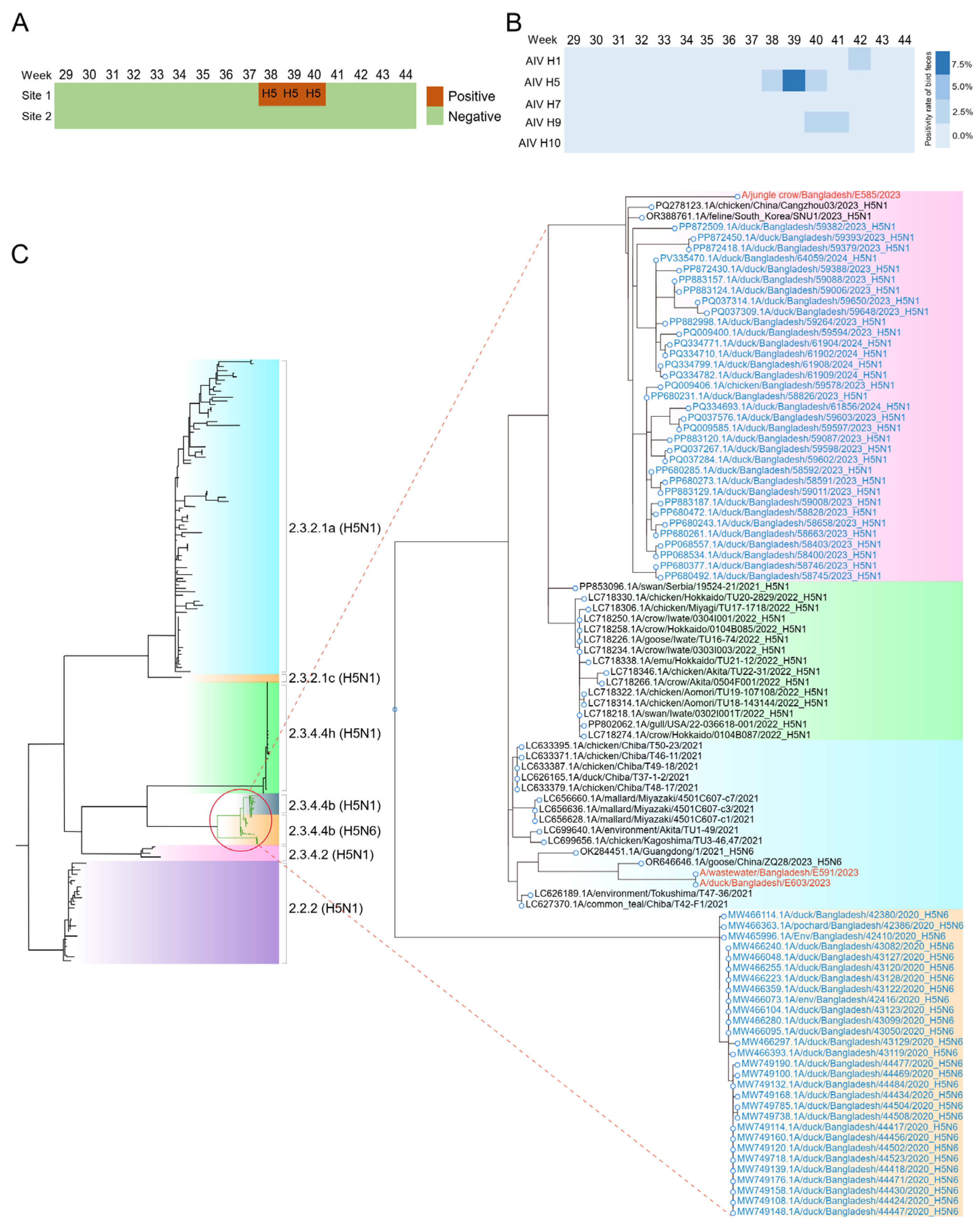
In this study, we conducted SWBS in a rural area near Dhaka city, Bangladesh and Ruili city, China from July to November 2023 to evaluate the early-warning capacity of SWBS for AIV and dengue virus. The results showed that continuous detection of both AIV and dengue viruses in the surface water in a rural area of Dhaka is feasible. After the detection of AIV H5N6 in sewage, we compared our data with those from public health authorities and found no evidence of human cases of avian influenza during this period. A report from the United States also documented a similar phenomenon, with AIV detected in sewage, but no corresponding cases identified in the human population [
12]. It was speculated that the AIV in sewage might originate from poultry. To investigate this, we collected fecal samples from wild birds and poultry around the sewage sampling sites and tested them using RT-qPCR. We identified multiple avian influenza genotypes, including H5N6, H9N2, and H1N3 in wild birds. Sequencing analysis revealed that the HA and NA genes of H5N6 in sewage were homologous to those in wild birds, suggesting that wild birds may be one source of AIV in sewage. Nevertheless, we did not rule out the possibility of the contribution of poultry. Live poultry markets in Bangladesh are high-risk sites for AIV transmission [
34]. Results herein suggested that SWBS can promptly detect the presence and spread of AIV, thereby implementing effective preventive measures to reduce the risk of virus transmission to humans and wild birds.
In recent years, dengue fever has also been a severe public health issue in Bangladesh and Yunnan Province of China. A previous study suggested that wastewater surveillance at WWTPs or community can act as a powerful tool to provide near real-time monitoring results for the entire population in a cost-effective manner [
35,
36]. However, the detection of dengue fever in surface water has not yet been reported. Recently, Gouthro et al. (2025) demonstrated the potential of surface water surveillance using passive samplers as a scalable strategy for detecting AIVs with successful detection of AIVs and hemagglutinin subtype H5 genes in surface waters across various sites in Nova Scotia, Canada [
37]. However, it is still uncertain whether this method can track the entire epidemic cycle of dengue fever. In this study, SWBS for DENV effectively tracks the epidemic cycle of dengue, with viral concentrations in wastewater preceding clinical case reports by approximately two weeks and correlating significantly with case counts (r = 0.934,
p < 0.001), thus providing a robust early warning signal for dengue outbreaks.
In the surveillance of dengue fever, given the scarcity of public health personnel in rural areas, which makes long-term and sustained mosquito density monitoring difficult, we established an early warning framework for dengue fever in rural regions by integrating SWBS with web searches related to dengue fever symptoms. While recent studies have also employed web search engine data to predict dengue fever outbreaks, the current search terms are often limited to the word “dengue” itself [
38]. In this study, we further expanded the search scope to include all symptoms related to dengue fever and ranked these search keywords according to their relevance using machine learning algorithms. This approach is expected to significantly enhance the efficiency of detecting early dengue fever cases in rural areas and would effectively compensate for the delay in dengue fever early warning caused by the lag in clinical monitoring in rural areas. The five symptom-related queries captured >80% of the predictive power, suggesting that syndromic keywords alone are sufficient for operational surveillance. Nevertheless, search data cannot discriminate dengue from other arboviral infections that provoke similar symptoms. Thus, we suggested that wastewater surveillance together with real-time web search data can complement each other’s strengths and weaknesses and create a two-tier early-warning system. By initiating enhanced wastewater sampling only when web search indices for dengue symptoms exceed a predefined threshold, the frequency of sampling can be dynamically adjusted. This approach not only maintains the efficiency of detecting viral circulation but also minimizes unnecessary sampling efforts, thereby lowering the overall cost of surveillance while ensuring timely public health responses. This workflow was successfully piloted in Ruili during 2024; no additional field resources were required beyond routine sewage collection. The integration of digital epidemiology with environmental surveillance is particularly suited to resource-limited rural settings where traditional syndromic reporting is sparse. By using freely accessible search-engine data to modulate wastewater sampling intensity, public-health authorities can allocate testing kits, vector-control teams and clinical supplies more efficiently and at lower cost.
This study is also subject to a few limitations. First of all, environmental conditions, particularly rainfall and water flow, can significantly influence viral detection and persistence in surface water. Heavy rainfall may dilute viral concentrations, reducing the likelihood of detection, while reduced water flow can concentrate viruses, enhancing detection rates. In our study, we collected samples during the period from July to November 2023, which corresponds to the rainy season in Bangladesh and China. This period may have influenced our detection rates. Future studies should consider longer-term sampling to better account for seasonal variations and their impact on viral detection. Secondly, the relatively short surveillance window (July–November 2023) limits the ability to generalize temporal patterns or assess inter-annual variability. While our study demonstrates the feasibility of WBS for detecting zoonotic viruses in rural settings, longer-term studies are necessary to fully understand the dynamics of these viruses. Inter-annual studies would be particularly valuable to capture variability in viral prevalence and to better inform public health strategies.
Overall, we present an integrated surveillance framework that combines SWBS, wild bird monitoring, and web-based search data to enhance early detection and response to AIV and dengue virus in rural settings. Our findings demonstrate that SWBS can effectively capture the temporal dynamics of two virus cycles, significantly improving the timeliness and coverage of surveillance compared to traditional methods. By detecting AIV in surface water, we can strategically guide wild bird sampling efforts based on viral concentration and geographic distribution of positive samples. This targeted approach reduces sampling effort and cost while enhancing surveillance efficiency. Furthermore, integrating SWBS with web search data shows promise for improving early detection of dengue fever cases in rural areas, where clinical reporting is often delayed. This combination helps mitigate the lag in traditional surveillance systems and enables more timely public health responses.
In summary, this study effectively addressed its primary objectives. First, we established the feasibility of SWBS by successfully tracking the transmission dynamics of both avian influenza (AIV) and dengue viruses in the resource-limited rural settings of Bangladesh and China. Second, we validated its correlation with clinical data, demonstrating a significant lead time of approximately two weeks for dengue and confirming the identity of water-derived and clinical viruses through genomic sequencing. Finally, by integrating SWBS with wild bird sampling and web search indices, we developed a cost-effective, scalable, digital-environmental integrated surveillance framework.