Nanobodies Targeting the GP4 Protein Inhibit PRRSV Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Expression and Purification of PRRSV GP4 Recombinant Protein
2.3. Alpaca Immunization and Library Construction
2.4. Screening of Specific Nanobodies
2.5. Nanobody Prokaryotic Expression
2.6. Nanoantibody Affinity Testing
2.7. Cytotoxicity Assay
2.8. Quantitative Real-Time PCR
2.9. Virus Titration
2.10. Western Blotting Method
2.11. Neutralizing Effect of Nanobodies on Viruses
2.12. Inhibitory Effect of Nanobodies on Viruses
2.13. Determination of the Virus Life Cycle
2.14. Molecular Docking
2.15. Statistical Analysis
3. Results
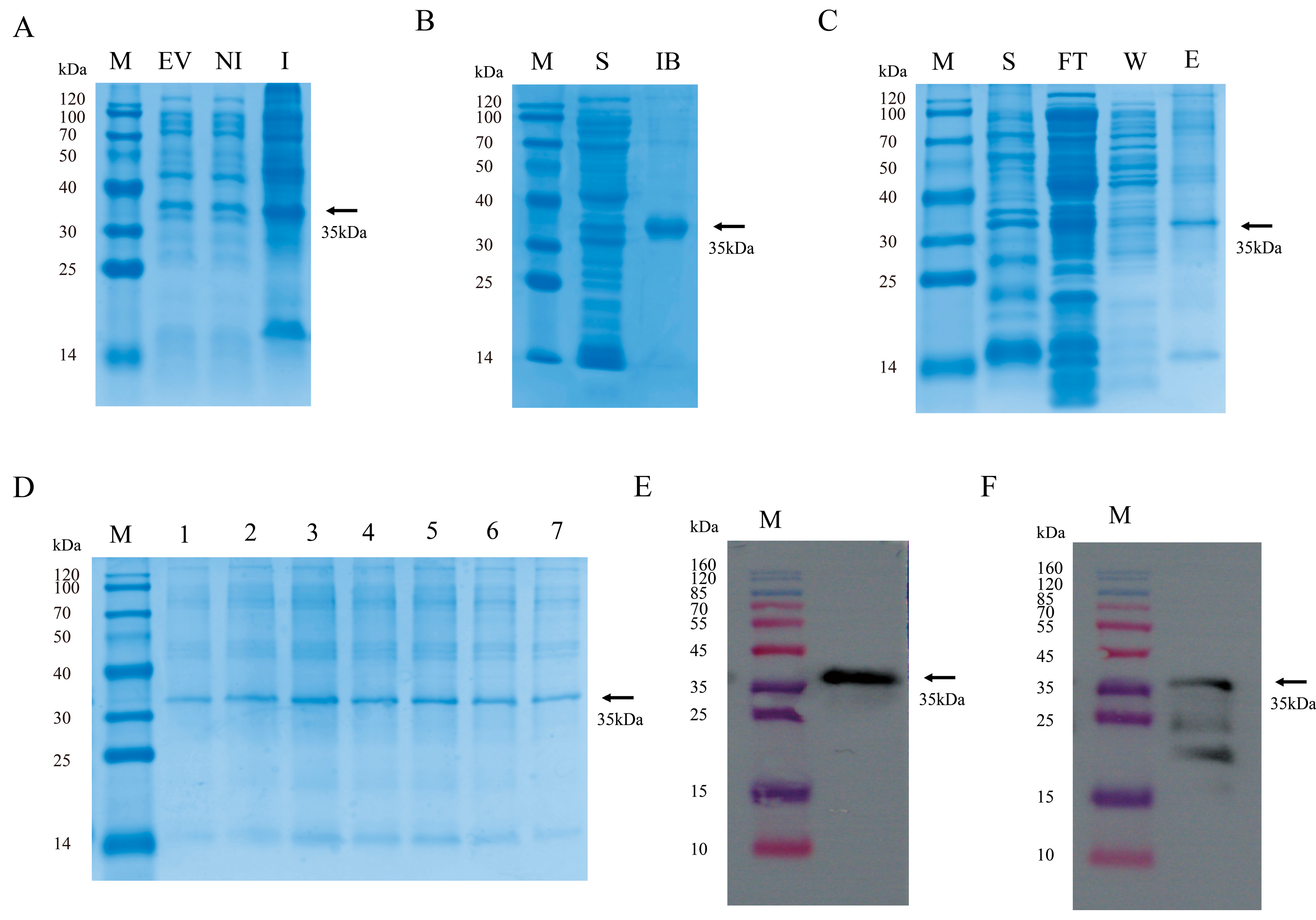
3.1. Preparation of GP4 Antigen Protein and WB Validation
3.2. VHH Library Construction
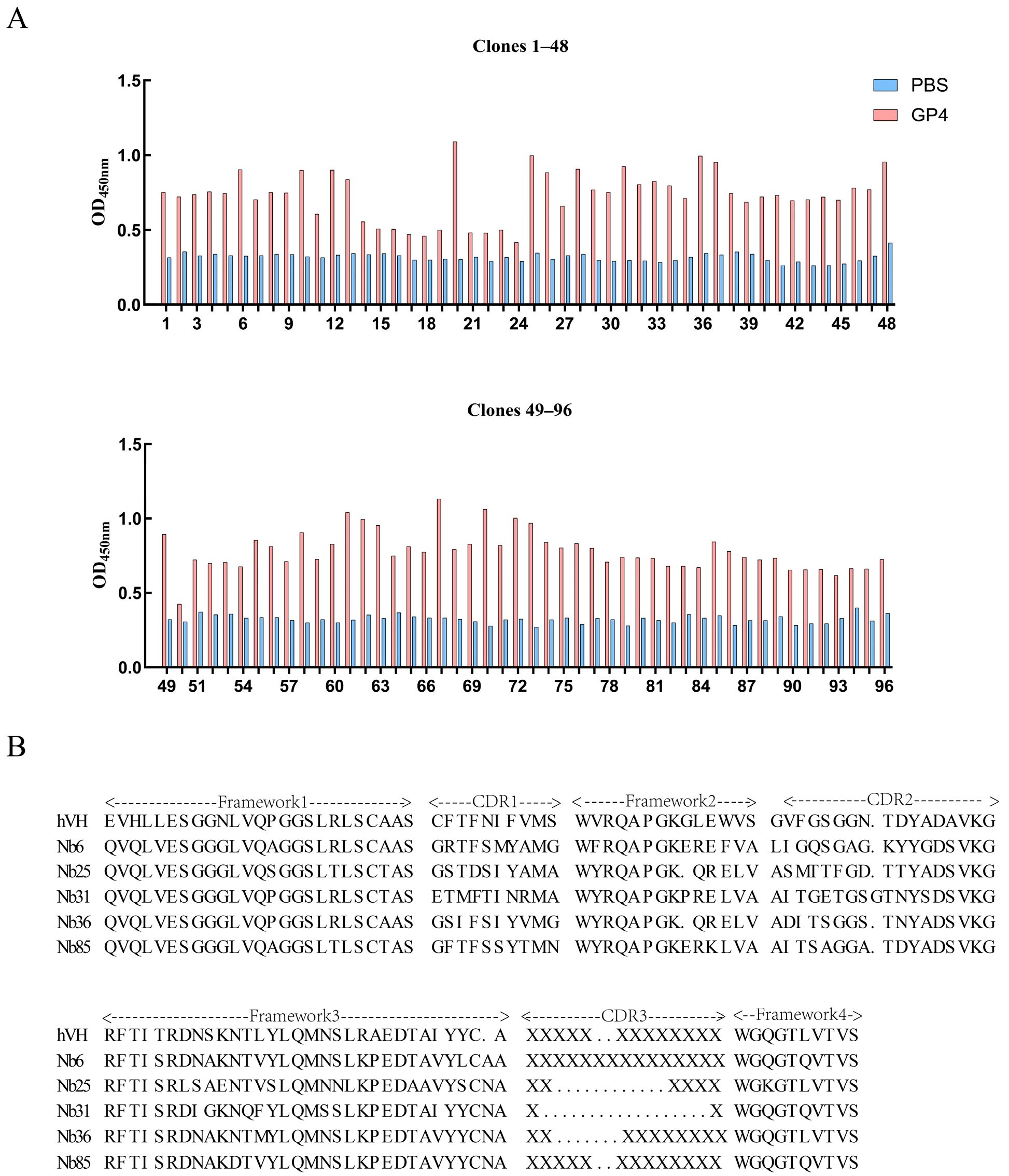
3.3. Separation and Identification of Nanobodies
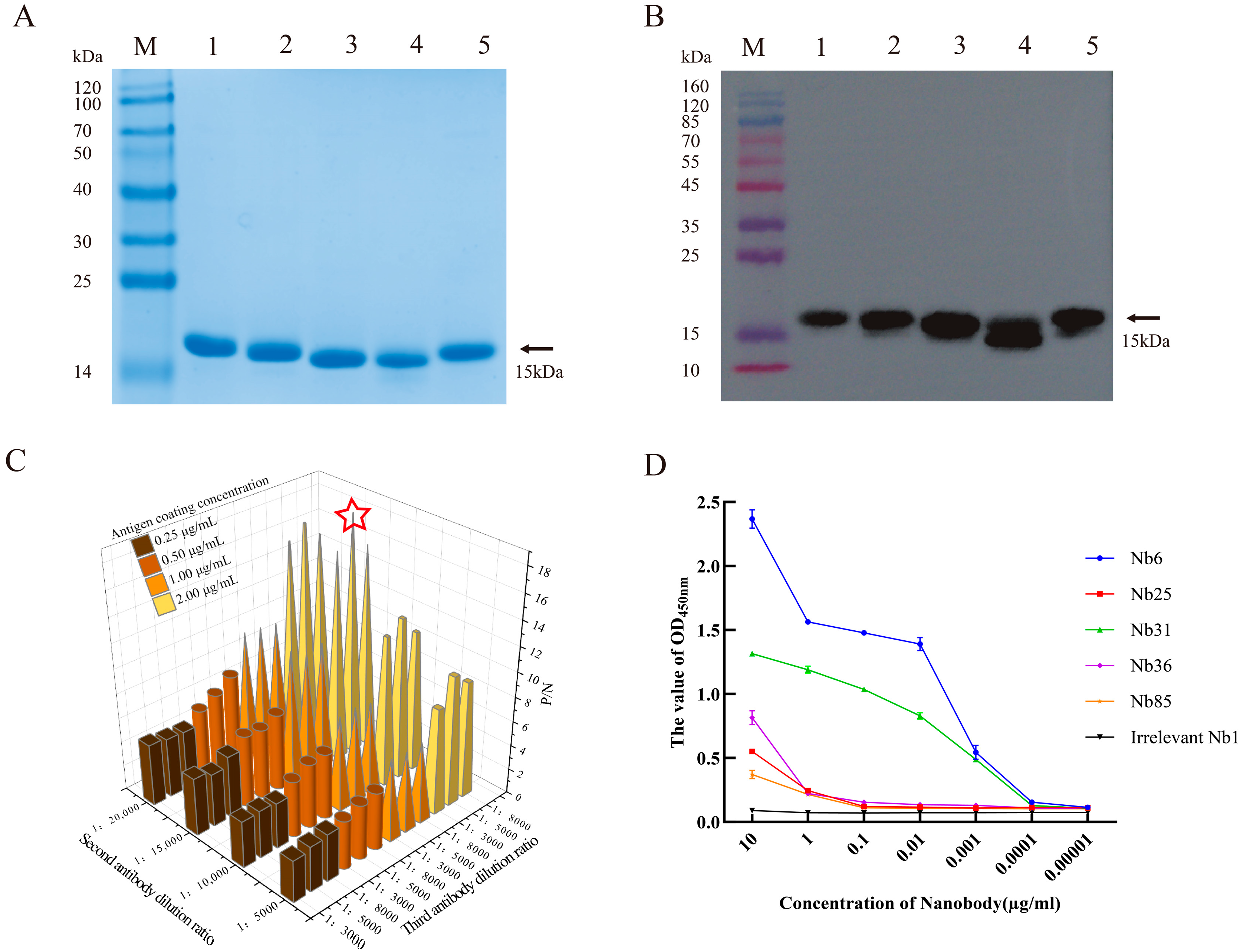
3.4. Preparation of Soluble Nanobodies and Affinity Validation
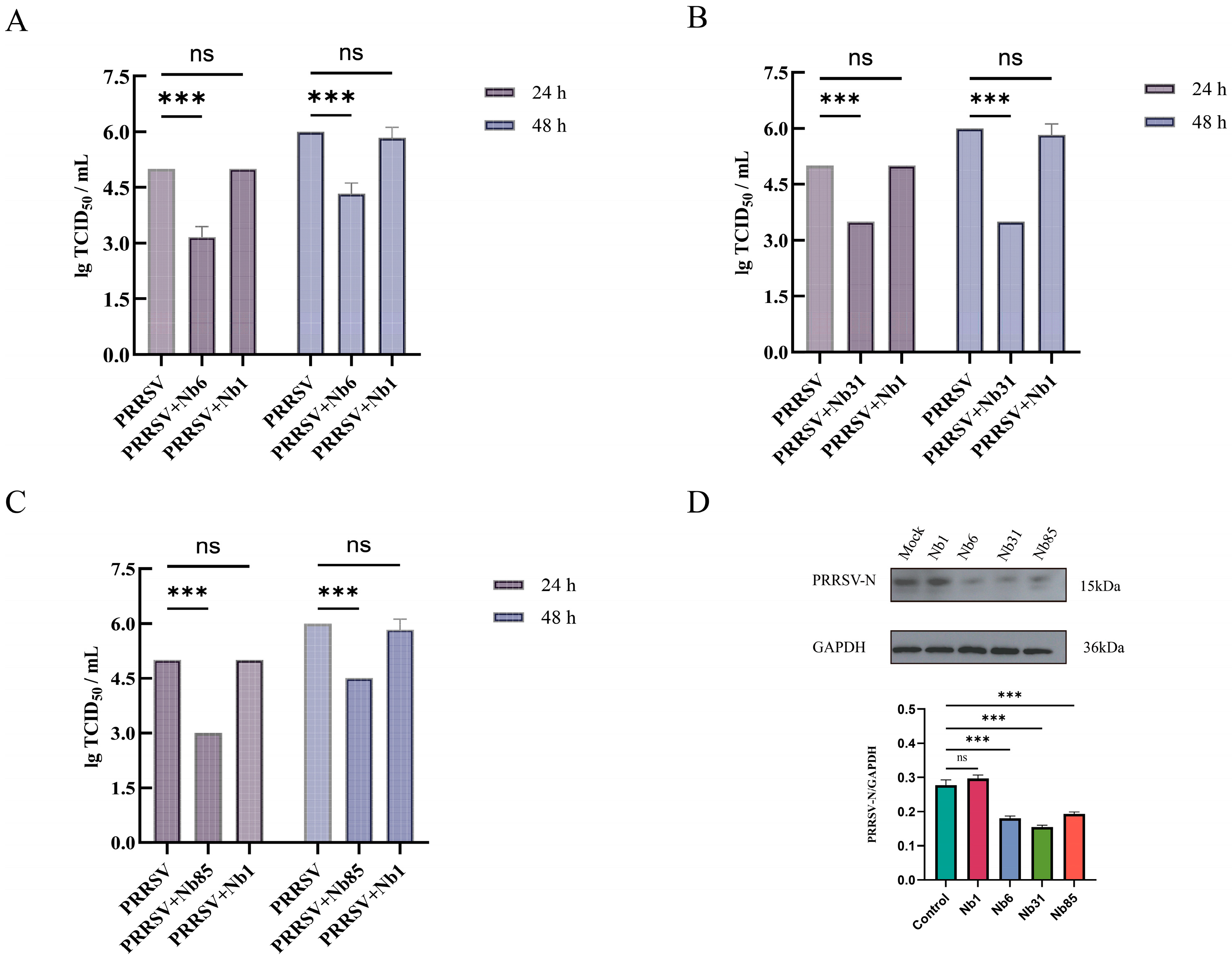
3.5. Cytotoxicity Testing and Neutralization Effect of Nanobodies on Viruses
3.6. Detection of Viral Titer in Offspring
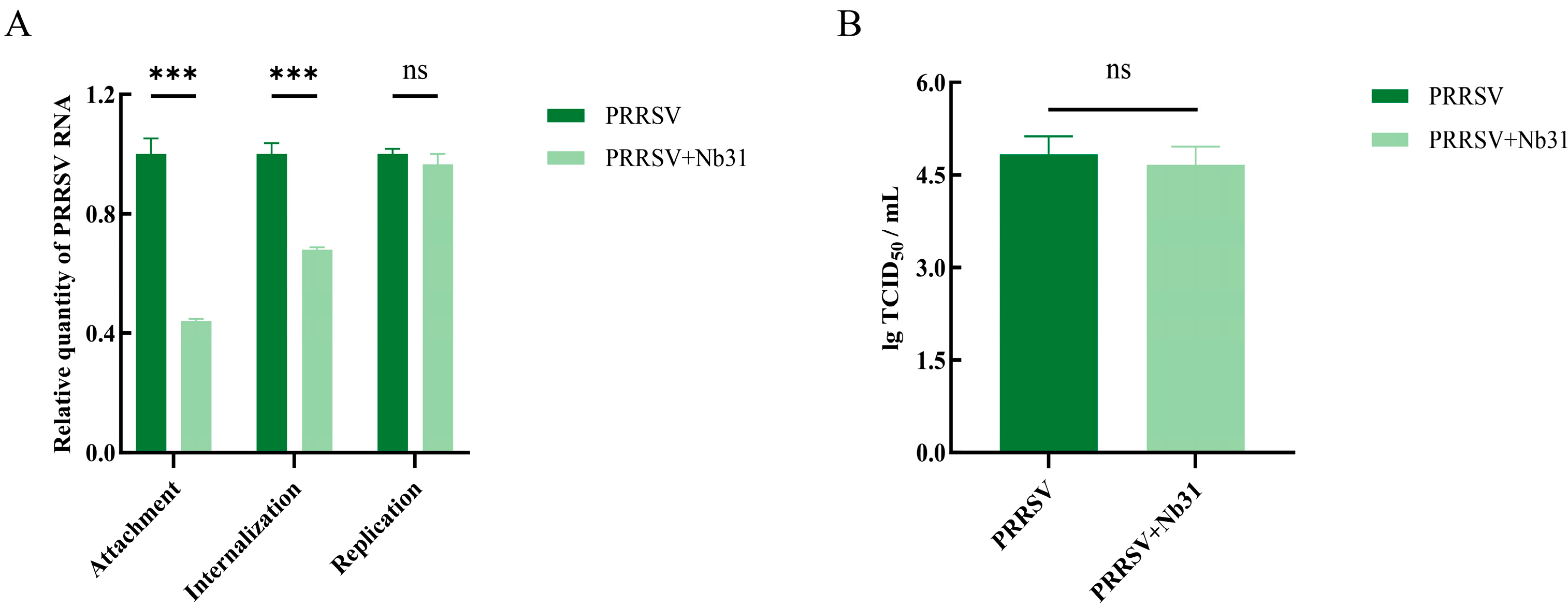
3.7. The Effect of GP4-Nb31 on the Viral Life Cycle
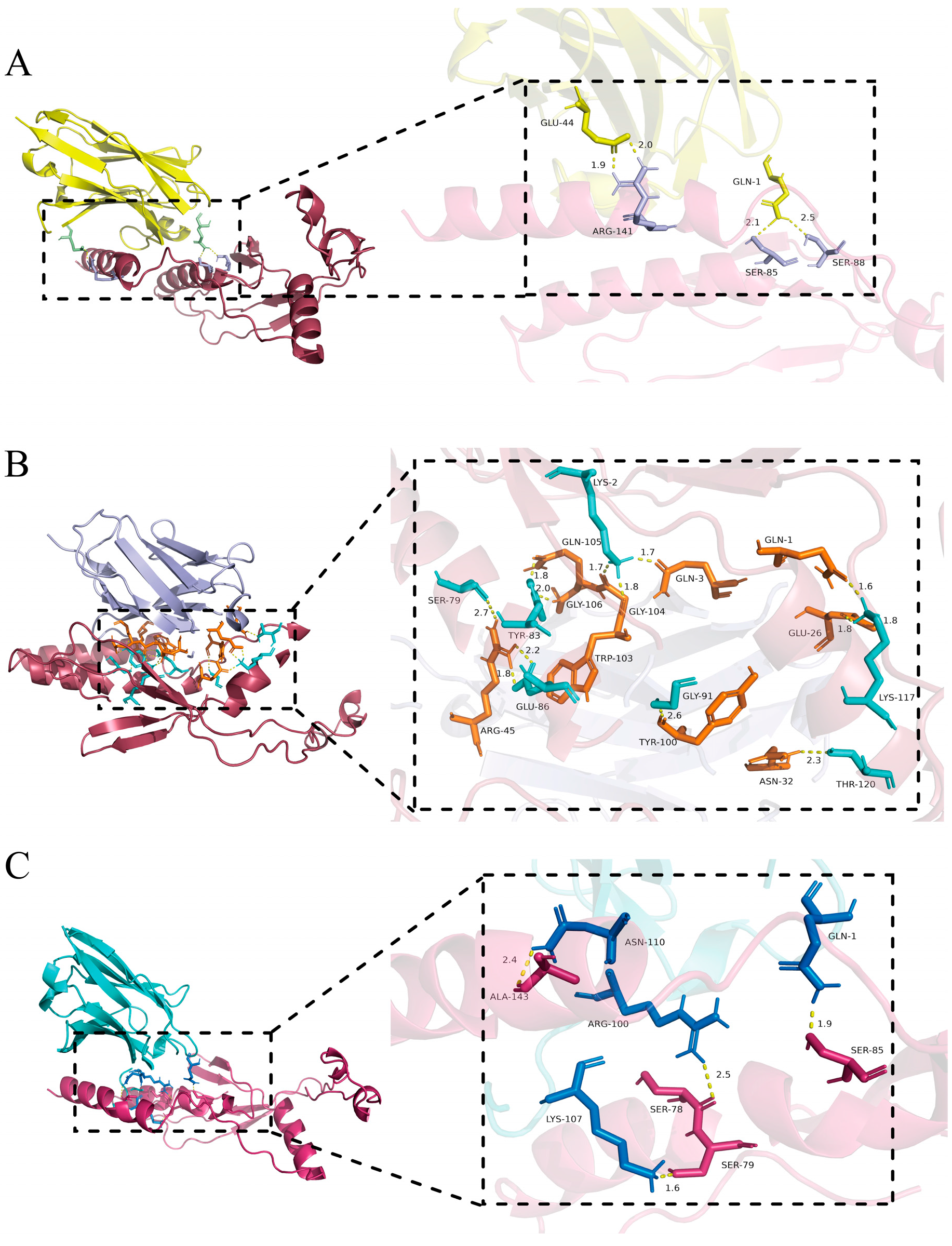
3.8. Molecular Docking of VHH and GP4 Recombinant Proteins
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| Nbs | Nanobodies |
| VHH | The heavy-chain variable domain |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| FCA | Freund’s Complete Adjuvant |
| FIA | Freund’s Incomplete Adjuvant |
| CPEs | cytopathic effects |
| TCID50 | The 50% tissue culture infective dose |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| PBMCs | peripheral blood mononuclear cells |
| iELISA | indirect enzyme-linked immunosorbent assay |
| CDR | Complementarity-Determining Region |
References
- Hanada, K.; Suzuki, Y.; Nakane, T.; Hirose, O.; Gojobori, T. The origin and evolution of porcine reproductive and respiratory syndrome viruses. Mol. Biol. Evol. 2005, 22, 1024–1031. [Google Scholar] [CrossRef]
- Li, N.; Du, T.; Yan, Y.; Zhang, A.; Gao, J.; Hou, G.; Xiao, S.; Zhou, E.M. MicroRNA let-7f-5p Inhibits Porcine Reproductive and Respiratory Syndrome Virus by Targeting MYH9. Sci. Rep. 2016, 6, 34332. [Google Scholar] [CrossRef]
- Du, Y.; Du, T.; Shi, Y.; Zhang, A.; Zhang, C.; Diao, Y.; Jin, G.; Zhou, E.M. Synthetic Toll-like receptor 7 ligand inhibits porcine reproductive and respiratory syndrome virus infection in primary porcine alveolar macrophages. Antivir. Res. 2016, 131, 9–18. [Google Scholar] [CrossRef]
- Serão, N.V.; Kemp, R.A.; Mote, B.E.; Willson, P.; Harding, J.C.; Bishop, S.C.; Plastow, G.S.; Dekkers, J.C. Genetic and genomic basis of antibody response to porcine reproductive and respiratory syndrome (PRRS) in gilts and sows. Genet. Sel. Evol. GSE 2016, 48, 51. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Genzow, M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine 2011, 29, 8192–8204. [Google Scholar] [CrossRef]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.M. Antiviral Strategies against PRRSV Infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [CrossRef]
- Brar, M.S.; Shi, M.; Hui, R.K.; Leung, F.C. Genomic evolution of porcine reproductive and respiratory syndrome virus (PRRSV) isolates revealed by deep sequencing. PLoS ONE 2014, 9, e88807. [Google Scholar] [CrossRef]
- Bao, D.; Wang, R.; Qiao, S.; Wan, B.; Wang, Y.; Liu, M.; Shi, X.; Guo, J.; Zhang, G. Antibody-dependent enhancement of PRRSV infection down-modulates TNF-α and IFN-β transcription in macrophages. Vet. Immunol. Immunopathol. 2013, 156, 128–134. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Xu, X.; Sun, Z.; Xiao, Y.; Ji, G.; Li, Y.; Tan, F.; Li, X.; Tian, K. Commercial vaccines provide limited protection to NADC30-like PRRSV infection. Vaccine 2016, 34, 5540–5545. [Google Scholar] [CrossRef]
- Wei, C.; Dai, A.; Fan, J.; Li, Y.; Chen, A.; Zhou, X.; Luo, M.; Yang, X.; Liu, J. Efficacy of Type 2 PRRSV vaccine against challenge with the Chinese lineage 1 (NADC30-like) PRRSVs in pigs. Sci. Rep. 2019, 9, 10781. [Google Scholar] [CrossRef]
- Kappes, M.A.; Faaberg, K.S. PRRSV structure, replication and recombination: Origin of phenotype and genotype diversity. Virology 2015, 479–480, 475–486. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Bao, C.; Xiang, H.; Gao, Q.; Mao, L. Mechanism and complex roles of HSC70/HSPA8 in viral entry. Virus Res. 2024, 347, 199433. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Huang, B.; Li, K.; Hou, G.; Zhao, Q.; Wu, C.; Nan, Y.; Du, T.; Mu, Y.; et al. A Nanobody Targeting Viral Nonstructural Protein 9 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication. J. Virol. 2019, 93, e01888-18. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, L.; Zhao, S.; Li, W.; Li, J.; Chen, J.; Zhang, Y.; Xia, P. The Host E3-Ubiquitin Ligase TRIM28 Impedes Viral Protein GP4 Ubiquitination and Promotes PRRSV Replication. Int. J. Mol. Sci. 2023, 24, 10965. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Q.; Tang, J.; Feng, W.H. Lipid rafts both in cellular membrane and viral envelope are critical for PRRSV efficient infection. Virology 2015, 484, 170–180. [Google Scholar] [CrossRef]
- Du, Y.; Pattnaik, A.K.; Song, C.; Yoo, D.; Li, G. Glycosyl-phosphatidylinositol (GPI)-anchored membrane association of the porcine reproductive and respiratory syndrome virus GP4 glycoprotein and its co-localization with CD163 in lipid rafts. Virology 2012, 424, 18–32. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Wang, W.; Qiao, Y.; Xu, M.; Liu, Z.; Gu, X.; Wu, A.; Ma, Z.; Chen, C.; et al. PRRSV GP4 subunit vaccine combined with adenovirus heterologous prime-boost immunization strategy induced a significant immune response in mice. BMC Vet. Res. 2025, 21, 379. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Li, C.; Tang, Z.; Hu, Z.; Wang, Y.; Yang, X.; Mo, F.; Lu, X. Natural Single-Domain Antibody-Nanobody: A Novel Concept in the Antibody Field. J. Biomed. Nanotechnol. 2018, 14, 1–19. [Google Scholar] [CrossRef]
- Mir, M.A.; Mehraj, U.; Sheikh, B.A.; Hamdani, S.S. Nanobodies: The “Magic Bullets” in therapeutics, drug delivery and diagnostics. Hum. Antibodies 2020, 28, 29–51. [Google Scholar] [CrossRef]
- Arezumand, R.; Alibakhshi, A.; Ranjbari, J.; Ramazani, A.; Muyldermans, S. Nanobodies As Novel Agents for Targeting Angiogenesis in Solid Cancers. Front. Immunol. 2017, 8, 1746. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Yan, Q.; Budachetri, K.; Hou, L.; Sahni, A.; Liu, H.; Han, N.C.; Lakritz, J.; Pei, D.; et al. An intracellular nanobody targeting T4SS effector inhibits Ehrlichia infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2024102118. [Google Scholar] [CrossRef]
- De Genst, E.; Silence, K.; Decanniere, K.; Conrath, K.; Loris, R.; Kinne, J.; Muyldermans, S.; Wyns, L. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl. Acad. Sci. USA 2006, 103, 4586–4591. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, X.; Pan, S.; Yu, Y.; Shi, Y.; Lin, T.; Duan, H.; Liu, X.; Chen, W.; Yang, X.; et al. An ultrapotent RBD-targeted biparatopic nanobody neutralizes broad SARS-CoV-2 variants. Signal Transduct. Target. Ther. 2022, 7, 44. [Google Scholar] [CrossRef]
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 6–13. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, B.; Wang, X.; Ni, F.; Ao, M.; Wang, R.; Zheng, B.; Chen, C.; Xue, J.; Zhu, L.; et al. Highly potent and broadly neutralizing anti-CD4 trimeric nanobodies inhibit HIV-1 infection by inducing CD4 conformational alteration. Nat. Commun. 2024, 15, 6961. [Google Scholar] [CrossRef]
- Duan, H.; Chen, X.; Zhang, Z.; Zhang, Z.; Li, Z.; Wang, X.; Zhao, J.; Nan, Y.; Liu, B.; Zhang, A.; et al. A nanobody inhibiting porcine reproductive and respiratory syndrome virus replication via blocking self-interaction of viral nucleocapsid protein. J. Virol. 2024, 98, e0131923. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Cao, S.; Lv, H.; Huang, J.; Zhang, G.; Tabynov, K.; Zhao, Q.; Zhou, E.M. Nanobody Nb6 fused with porcine IgG Fc as the delivering tag to inhibit porcine reproductive and respiratory syndrome virus replication in porcine alveolar macrophages. Vet. Res. 2021, 52, 25. [Google Scholar] [CrossRef]
- Duan, H.; Chen, X.; Zhao, J.; Zhu, J.; Zhang, G.; Fan, M.; Zhang, B.; Wang, X.; Sun, Y.; Liu, B.; et al. Development of a Nanobody-Based Competitive Enzyme-Linked Immunosorbent Assay for Efficiently and Specifically Detecting Antibodies against Genotype 2 Porcine Reproductive and Respiratory Syndrome Viruses. J. Clin. Microbiol. 2021, 59, e0158021. [Google Scholar] [CrossRef]
- Yang, H.; Sun, M.; Qiu, H.; Xu, H.; Deng, Z.; Gu, H.; Wang, N.; Du, L.; Shi, F.; Zhou, J.; et al. Nanobody peptide conjugate: A novel CD163 based broad neutralizing strategy against porcine reproductive and respiratory syndrome virus. J. Nanobiotechnology 2024, 22, 388. [Google Scholar] [CrossRef]
- Vincke, C.; Gutiérrez, C.; Wernery, U.; Devoogdt, N.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S. Generation of single domain antibody fragments derived from camelids and generation of manifold constructs. In Antibody Engineering; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 907, pp. 145–176. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, A.; Zhang, C.; Ni, H.; Gao, J.; Wang, C.; Zhao, Q.; Wang, X.; Wang, X.; Ma, C.; et al. Heme oxygenase-1 acts as an antiviral factor for porcine reproductive and respiratory syndrome virus infection and over-expression inhibits virus replication in vitro. Antivir. Res. 2014, 110, 60–69. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Wang, X.F.; Zhang, J.L.; Wu, Y.; Wang, J.; Wang, J.F. Melatonin inhibits bovine viral diarrhea virus replication by ER stress-mediated NF-κB signal pathway and autophagy in MDBK cells. Front. Cell. Infect. Microbiol. 2024, 14, 1431836. [Google Scholar] [CrossRef]
- Kim, D.E.; Chivian, D.; Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004, 32, W526–W531. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Fiers, J.; Cay, A.B.; Maes, D.; Tignon, M. A Comprehensive Review on Porcine Reproductive and Respiratory Syndrome Virus with Emphasis on Immunity. Vaccines 2024, 12, 942. [Google Scholar] [CrossRef]
- Liu, B.; Luo, L.; Shi, Z.; Ju, H.; Yu, L.; Li, G.; Cui, J. Research Progress of Porcine Reproductive and Respiratory Syndrome Virus NSP2 Protein. Viruses 2023, 15, 2310. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, X.; Hu, Y.; Ou, W.; Wan, Y. Streptavidin-biotin-based directional double Nanobody sandwich ELISA for clinical rapid and sensitive detection of influenza H5N1. J. Transl. Med. 2014, 12, 352. [Google Scholar] [CrossRef]
- Kang, N.R.; Im, J.; Biondo, J.R.; Sharpes, C.E.; Rhea, K.A.; Garden, P.M.; Jaramillo Montezco, J.J.; Ringaci, A.; Grinstaff, M.W.; Phillips, D.A.; et al. A Rapid and Modular Nanobody Assay for Plug-and-Play Antigen Detection. ACS Synth. Biol. 2025, 14, 3423–3433. [Google Scholar] [CrossRef]
- Hanke, L.; Das, H.; Sheward, D.J.; Perez Vidakovics, L.; Urgard, E.; Moliner-Morro, A.; Kim, C.; Karl, V.; Pankow, A.; Smith, N.L.; et al. A bispecific monomeric nanobody induces spike trimer dimers and neutralizes SARS-CoV-2 in vivo. Nat. Commun. 2022, 13, 155. [Google Scholar] [CrossRef]
- Yang, J.; Lin, S.; Chen, Z.; Yang, F.; Guo, L.; Wang, L.; Duan, Y.; Zhang, X.; Dai, Y.; Yin, K.; et al. Development of a bispecific nanobody conjugate broadly neutralizes diverse SARS-CoV-2 variants and structural basis for its broad neutralization. PLoS Pathog. 2023, 19, e1011804. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, L.; Fu, M.; Huang, B.; Zhu, L.; Xu, S.; Shi, H.; Zhang, D.; Yuan, H.; Nawaz, W.; et al. A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration. Cell Rep. 2021, 37, 109869. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, Y.; Sivaccumar, J.P.; An, Z.; Xia, N.; Luo, W. Research progress and applications of nanobody in human infectious diseases. Front. Pharmacol. 2022, 13, 963978. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chatterjee, S.; Lee, S.S.; Chakraborty, C. Therapeutic applications of nanobodies against SARS-CoV-2 and other viral infections: Current update. Int. J. Biol. Macromol. 2023, 229, 70–80. [Google Scholar] [CrossRef]
- Hanke, L.; Sheward, D.J.; Pankow, A.; Vidakovics, L.P.; Karl, V.; Kim, C.; Urgard, E.; Smith, N.L.; Astorga-Wells, J.; Ekström, S.; et al. Multivariate mining of an alpaca immune repertoire identifies potent cross-neutralizing SARS-CoV-2 nanobodies. Sci. Adv. 2022, 8, eabm0220. [Google Scholar] [CrossRef]
- Barrass, S.V.; Butcher, S.J. Advances in high-throughput methods for the identification of virus receptors. Med. Microbiol. Immunol. 2020, 209, 309–323. [Google Scholar] [CrossRef]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef]
- Stoian, A.M.M.; Rowland, R.R.R. Challenges for Porcine Reproductive and Respiratory Syndrome (PRRS) Vaccine Design: Reviewing Virus Glycoprotein Interactions with CD163 and Targets of Virus Neutralization. Vet. Sci. 2019, 6, 9. [Google Scholar] [CrossRef]
- Zhu, J.; He, X.; Bernard, D.; Shen, J.; Su, Y.; Wolek, A.; Issacs, B.; Mishra, N.; Tian, X.; Garmendia, A.; et al. Identification of New Compounds against PRRSV Infection by Directly Targeting CD163. J. Virol. 2023, 97, e0005423. [Google Scholar] [CrossRef]


| Primer Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| GP4-F | CGGGATCCTGCAAACCATGTTTCTCCAGCT | pSumo-mut -GP4 |
| GP4-R | AACTGCAGGATAGCCAGCAGAATCGCAAC | |
| CALL001 | GTCCTGGCTGCTCTTCTACAAGG | |
| CALL002 | GGTACGTGCTGTTGAACTGTTCC | |
| Cam-For-sfi I | CATGCCATGACTGTGGCCCAGGCGGCCCAGGTGCAGCTCGTGGAGTCTGGRGGAGG | 1 |
| Cam-Rev-sfi I | CATGCCATGACTCGCGGCCGGCCTGGCCGGAGACGGTGACCWGGGT | 1 |
| Pcomb3xss-F | AAGACAGCTATCGCGATTGCA G | Pcomb3xss |
| Pcomb3xss-R | GCCCCCTTATTAGCGTTTGCCATC | |
| ORF7-F | AGATCATCATCGCCCAACAAAAC | RT-qPCR |
| ORF7-R | GACACAATTGCCGCTCACTA | |
| β-actin-F | TCCCTGGAGAAGAGCTACGA | RT-qPCR |
| β-actin-R | AGCACTGTGTTGGCGTACAG |
| Round of Panning | Input Phage (pfu/mL) | P Output (pfu/mL) | N Output (pfu/mL) | Recovery (P/Input) | P/N |
|---|---|---|---|---|---|
| 1st round | 5.00 × 1012 | 1.09 × 106 | 2.00 × 104 | 2.18 × 10−7 | 5.45 × 101 |
| 2nd round | 5.00 × 1012 | 3.13 × 107 | 1.98 × 105 | 6.26 × 10−6 | 1.58 × 102 |
| 3rd round | 2.12 × 1012 | 3.46 × 108 | 2.41 × 106 | 1.63 × 10−4 | 1.43 × 102 |
| 4th round | 5.00 × 1012 | 7.50 × 108 | 1.05 × 106 | 1.50 × 10−4 | 7.14 × 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wu, A.; Li, H.; He, T.; Dong, Q.; Zhang, H.; Chen, J.; Jiang, S.; Sheng, J. Nanobodies Targeting the GP4 Protein Inhibit PRRSV Replication. Microorganisms 2025, 13, 2524. https://doi.org/10.3390/microorganisms13112524
Zhang W, Wu A, Li H, He T, Dong Q, Zhang H, Chen J, Jiang S, Sheng J. Nanobodies Targeting the GP4 Protein Inhibit PRRSV Replication. Microorganisms. 2025; 13(11):2524. https://doi.org/10.3390/microorganisms13112524
Chicago/Turabian StyleZhang, Wenxiang, Aodi Wu, Honghuan Li, Tao He, Qianqian Dong, Hanwen Zhang, Jie Chen, Song Jiang, and Jinliang Sheng. 2025. "Nanobodies Targeting the GP4 Protein Inhibit PRRSV Replication" Microorganisms 13, no. 11: 2524. https://doi.org/10.3390/microorganisms13112524
APA StyleZhang, W., Wu, A., Li, H., He, T., Dong, Q., Zhang, H., Chen, J., Jiang, S., & Sheng, J. (2025). Nanobodies Targeting the GP4 Protein Inhibit PRRSV Replication. Microorganisms, 13(11), 2524. https://doi.org/10.3390/microorganisms13112524







