Recent Trends in Prevalence of HPV Infection in Nasopharyngeal Carcinoma in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Detection and Typing of HPV
2.2.1. DNA Extraction
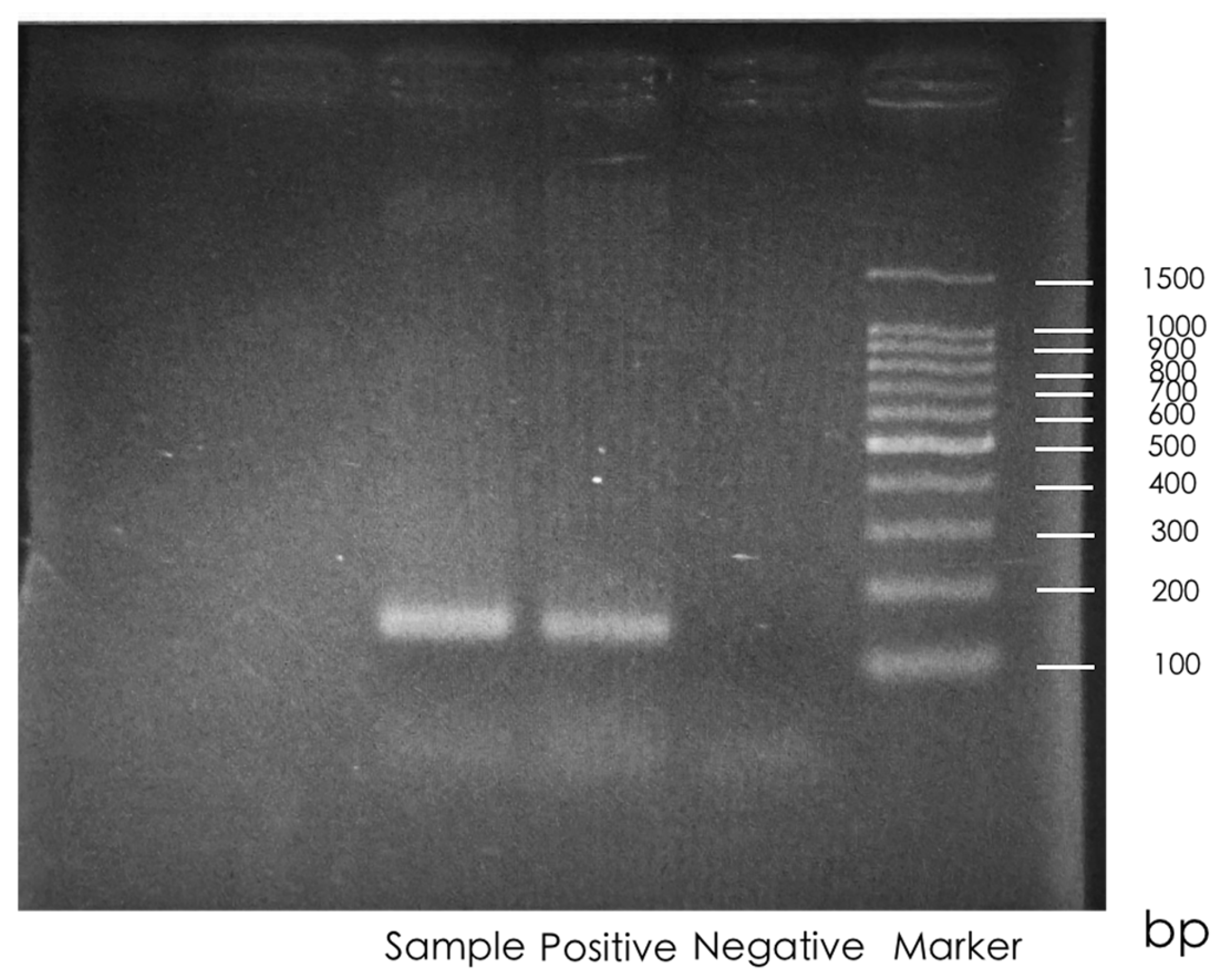
2.2.2. HPV Polymerase Chain Reaction (PCR)
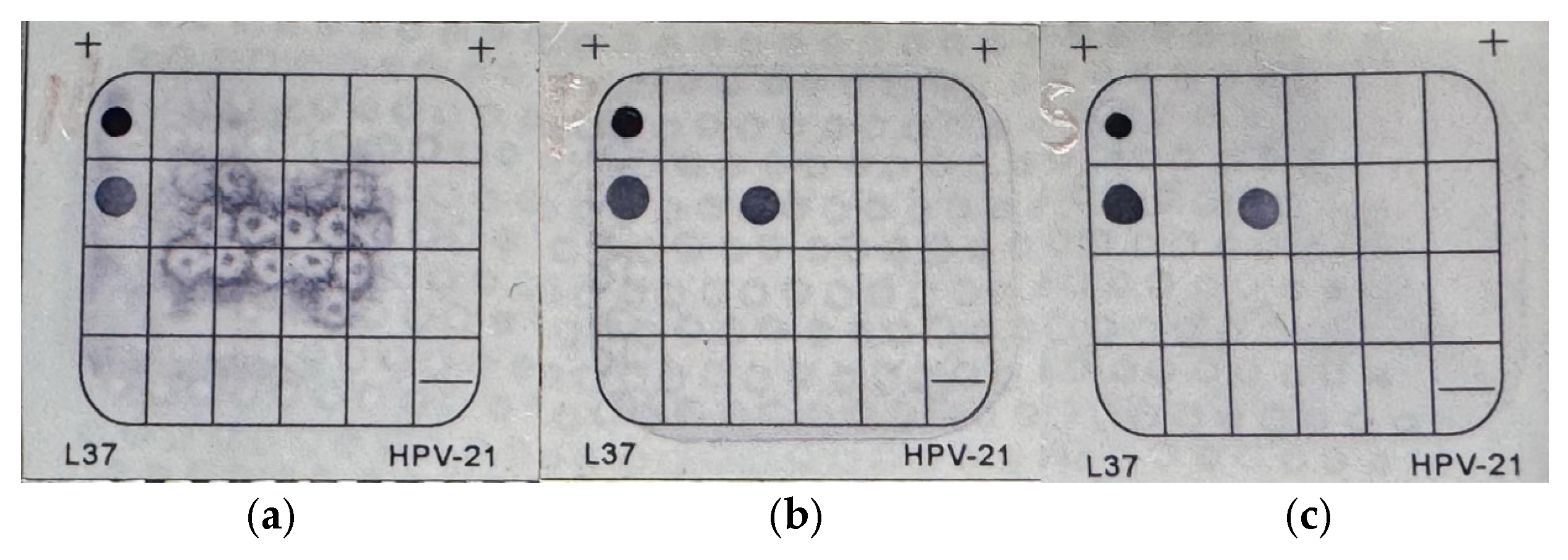
2.2.3. HPV Detection and Genotyping
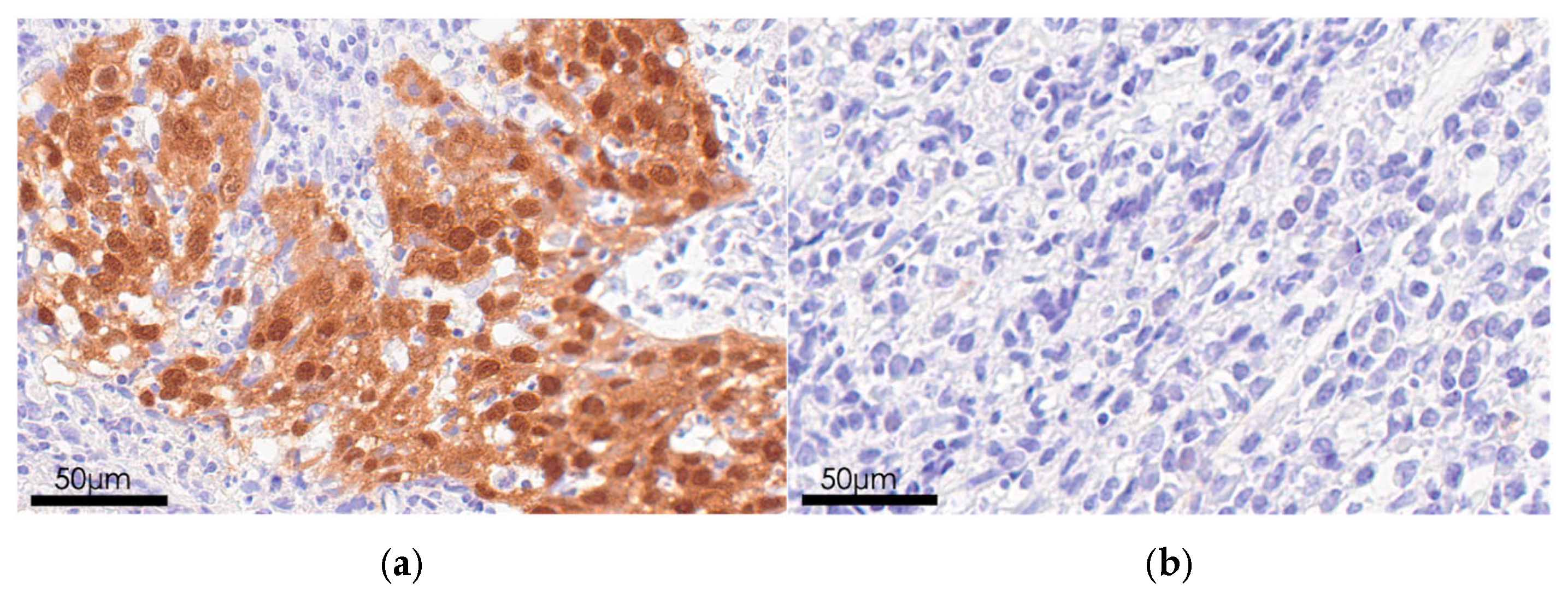
2.3. Immunohistochemical Analysis
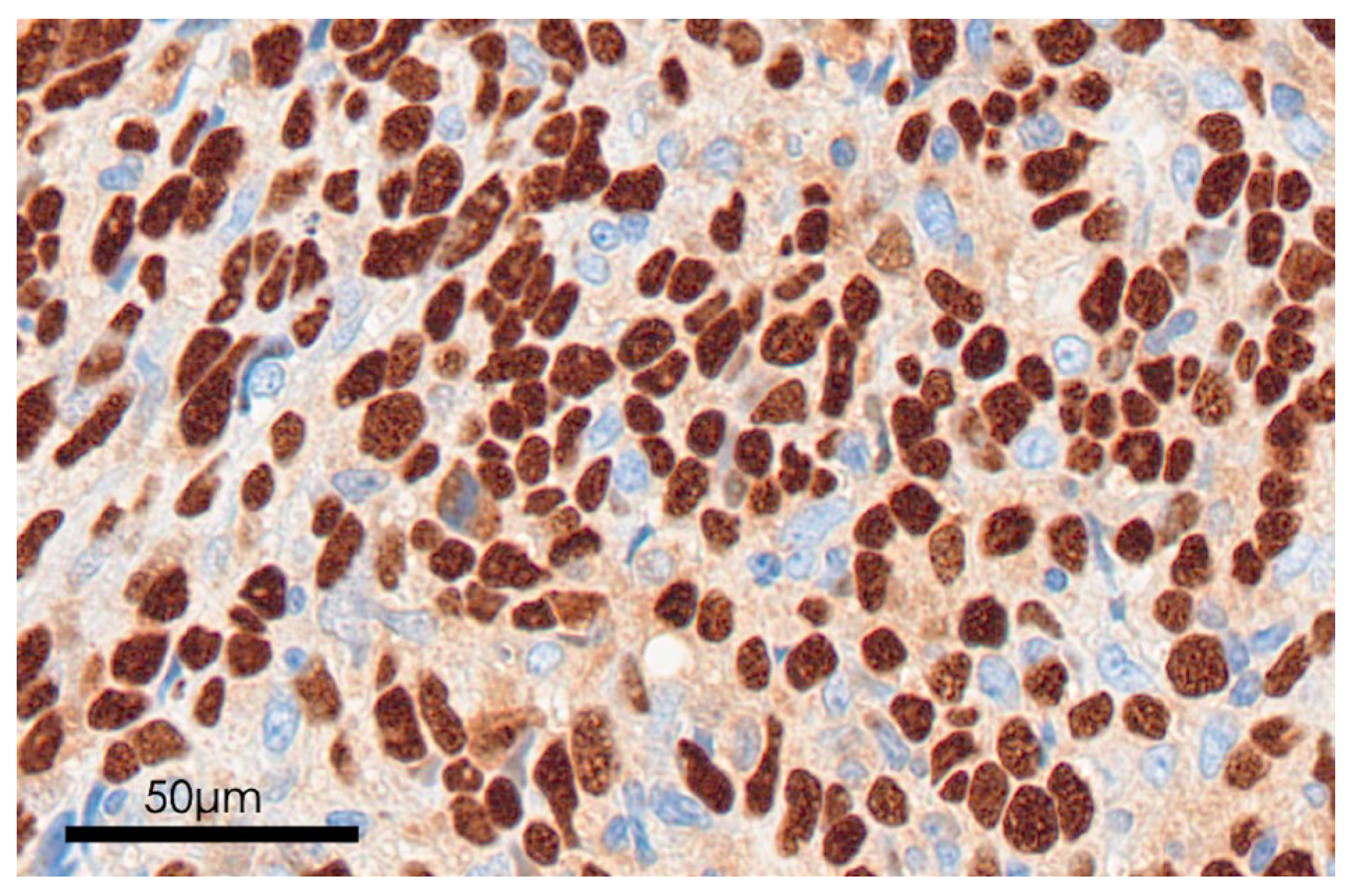
2.4. EBV Detection
3. Results
3.1. HPV Positivity
3.2. HPV Status
3.3. p16 Status
3.4. EBV Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPC | Nasopharyngeal carcinoma |
| EBV | Epstein–Barr virus |
| HPV | Human papilloma virus |
| WHO | World Health Organization |
| AJCC | American Joint Committee on Cancer |
| RT | Radiotherapy |
| Alternating-CRT | Alternating cisplatin and 5-FU chemo-radiotherapy |
| PCE-CCRT | Induction chemotherapy with Paclitaxel, Carboplatin and Cetuximab |
| CCRT | Concurrent chemoradiotherapy |
| PCR | Polymerase Chain Reaction |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| ISH | In situ hybridization |
| EBER | Epstein–Barr virus-encoded RNA |
| IC | Internal control |
| OPC | Oropharyngeal carcinoma |
Appendix A
| Study | Period | Total Cases | Region | Institution | Methods | HPV-Positive Cases (%) | HPV Genotypes | EBV */HPV Co-Infection |
|---|---|---|---|---|---|---|---|---|
| Kano et al., 2016 [8] | 1996–2015 | 59 | Hokuriku Region | Kanazawa University Hospital; Toyama Prefectural Central Hospital | HPV DNA Isolation; HPV PCR *; p16 IHC *; HPV Detection and Genotyping (13 HPV genotypes) | 2 (3%) | HPV18 (n = 1); HPV16/18 co-infection (n = 1) | 2 |
| Liu et al., 2025 | 2015–2022 | 26 | Hokuriku Region | Kanazawa University Hospital | HPV DNA Isolation; HPV PCR; p16 IHC; HPV Detection and Genotyping (21 HPV genotypes) | 1 (4%) | HPV 18 (n = 1) | 0 |
References
- Cantu, G. Nasopharyngeal carcinoma. A “different” head and neck tumour. Part A: From histology to staging. Acta Otorhinolaryngol. Ital. 2023, 43, 85–98. [Google Scholar] [CrossRef]
- Sinha, S.; Winters, R.; Gajra, A. Nasopharyngeal Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Huang, H.; Yao, Y.; Deng, X.; Huang, Z.; Chen, Y.; Wang, Z.; Hong, H.; Huang, H.; Lin, T. Immunotherapy for nasopharyngeal carcinoma: Current status and prospects. Int. J. Oncol. 2023, 63, 97. [Google Scholar] [CrossRef]
- Ooft, M.L.; van Ipenburg, J.; Braunius, W.W.; Stegeman, I.; Wegner, I.; de Bree, R.; Overbeek, L.I.; Koljenovic, S.; Willems, S.M. A nation-wide epidemiological study on the risk of developing second malignancies in patients with different histological subtypes of nasopharyngeal carcinoma. Oral. Oncol. 2016, 56, 40–46. [Google Scholar] [CrossRef]
- Kawakita, D.; Oze, I.; Iwasaki, S.; Matsuda, T.; Matsuo, K.; Ito, H. Trends in the incidence of head and neck cancer by subsite between 1993 and 2015 in Japan. Cancer Med. 2022, 11, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Nibu, K.I.; Oridate, N.; Saito, Y.; Roset, M.; Fores Maresma, M.; Cuadras, D.; Morais, E.; Roberts, C.; Chen, Y.T.; Spitzer, J.; et al. Human papillomavirus-driven head and neck cancers in Japan during 2008–2009 and 2018–2019: The BROADEN study. Cancer Sci. 2024, 115, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Simone, C.B., 2nd; Lin, C. Human papillomavirus and nasopharyngeal cancer. Head Neck 2018, 40, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Kondo, S.; Wakisaka, N.; Moriyama-Kita, M.; Nakanishi, Y.; Endo, K.; Murono, S.; Nakamura, H.; Yoshizaki, T. The influence of human papillomavirus on nasopharyngeal carcinoma in Japan. Auris Nasus Larynx 2017, 44, 327–332. [Google Scholar] [CrossRef]
- Carioli, G.; Negri, E.; Kawakita, D.; Garavello, W.; La Vecchia, C.; Malvezzi, M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: Focus on low-risk areas. Int. J. Cancer 2017, 140, 2256–2264. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Gronhoj Larsen, C.; Gyldenlove, M.; Jensen, D.H.; Therkildsen, M.H.; Kiss, K.; Norrild, B.; Konge, L.; von Buchwald, C. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: A systematic review. Br. J. Cancer 2014, 110, 1587–1594. [Google Scholar] [CrossRef]
- Hammarstedt, L.; Holzhauser, S.; Zupancic, M.; Kapoulitsa, F.; Ursu, R.G.; Ramqvist, T.; Haeggblom, L.; Nasman, A.; Dalianis, T.; Marklund, L. The value of p16 and HPV DNA in non-tonsillar, non-base of tongue oropharyngeal cancer. Acta Otolaryngol. 2021, 141, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.T.; Jin, Y.T.; Mann, R.B.; Ambinder, R.F. Epstein-Barr virus detection in nasopharyngeal tissues of patients with suspected nasopharyngeal carcinoma. Cancer 1998, 82, 1449–1453. [Google Scholar] [CrossRef]
- Xu, M.; Yao, Y.; Chen, H.; Zhang, S.; Cao, S.M.; Zhang, Z.; Luo, B.; Liu, Z.; Li, Z.; Xiang, T.; et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 2019, 51, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Y.; Hirayama, S.; Goss, D.; Zhao, Y.; Faden, D.L. Human papillomavirus-associated nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral. Oncol. 2024, 159, 107057. [Google Scholar] [CrossRef]
- Udager, A.M.; McHugh, J.B. Human Papillomavirus-Associated Neoplasms of the Head and Neck. Surg. Pathol. Clin. 2017, 10, 35–55. [Google Scholar] [CrossRef]
- Lo, E.J.; Bell, D.; Woo, J.S.; Li, G.; Hanna, E.Y.; El-Naggar, A.K.; Sturgis, E.M. Human papillomavirus and WHO type I nasopharyngeal carcinoma. Laryngoscope 2010, 120, 1990–1997. [Google Scholar] [CrossRef]
- Richardo, T.; Prattapong, P.; Ngernsombat, C.; Wisetyaningsih, N.; Iizasa, H.; Yoshiyama, H.; Janvilisri, T. Epstein-Barr Virus Mediated Signaling in Nasopharyngeal Carcinoma Carcinogenesis. Cancers 2020, 12, 2441. [Google Scholar] [CrossRef]
- Shimizu, Y.; Murakami, N.; Mori, T.; Takahashi, K.; Kubo, Y.; Yoshimoto, S.; Honma, Y.; Nakamura, S.; Okamoto, H.; Iijima, K.; et al. Clinical impact of p16 positivity in nasopharyngeal carcinoma. Laryngoscope Investig. Otolaryngol. 2022, 7, 994–1001. [Google Scholar] [CrossRef]
- Robinson, M.; Suh, Y.E.; Paleri, V.; Devlin, D.; Ayaz, B.; Pertl, L.; Thavaraj, S. Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: An observational study of correlation with ethnicity, histological subtype and outcome in a UK population. Infect. Agent. Cancer 2013, 8, 30. [Google Scholar] [CrossRef]
- Worsham, M.J.; Chen, K.M.; Tiwari, N.; Pals, G.; Schouten, J.P.; Sethi, S.; Benninger, M.S. Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head. Neck Surg. 2006, 132, 409–415. [Google Scholar] [CrossRef]
- Stephen, J.K.; Divine, G.; Chen, K.M.; Chitale, D.; Havard, S.; Worsham, M.J. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer Clin. Oncol. 2013, 2, 51–61. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-related oropharyngeal cancer: A review on burden of the disease and opportunities for prevention and early detection. Hum. Vaccines Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 26) |
|---|---|
| Age | 58.46 ± 12.79 |
| Sex (Male/Female) | 20/6 |
| WHO type 1 (I/II/III) | 1/20/5 |
| Tumor classification (T1–T4) | 8/10/5/3 |
| Nodal classification (N0–N3) | 2/11/11/2 |
| Metastasis classification (M0/M1) | 25/1 |
| AJCC stage (I–IVB) | 1/8/11/6/5/1 |
| Treatment (RT 2/Alternating CRT 3/PCE-CCRT 4) | 1/24/1 |
| Group | All Patients (n = 26) |
|---|---|
| EBER*+/p16− | 19 (73%) |
| EBER−/p16+ | 2 (8%) |
| EBER−/p16− | 5 (19%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Hirai, N.; Kondo, S.; Moriyama-Kita, M.; Nakazawa, R.; Komura, S.; Kano, M.; Uno, D.; Inaba, M.; Ueno, T.; et al. Recent Trends in Prevalence of HPV Infection in Nasopharyngeal Carcinoma in Japan. Microorganisms 2025, 13, 2514. https://doi.org/10.3390/microorganisms13112514
Liu L, Hirai N, Kondo S, Moriyama-Kita M, Nakazawa R, Komura S, Kano M, Uno D, Inaba M, Ueno T, et al. Recent Trends in Prevalence of HPV Infection in Nasopharyngeal Carcinoma in Japan. Microorganisms. 2025; 13(11):2514. https://doi.org/10.3390/microorganisms13112514
Chicago/Turabian StyleLiu, Luyao, Nobuyuki Hirai, Satoru Kondo, Makiko Moriyama-Kita, Ryotaro Nakazawa, Shigetaka Komura, Makoto Kano, Daisuke Uno, Manabu Inaba, Takayoshi Ueno, and et al. 2025. "Recent Trends in Prevalence of HPV Infection in Nasopharyngeal Carcinoma in Japan" Microorganisms 13, no. 11: 2514. https://doi.org/10.3390/microorganisms13112514
APA StyleLiu, L., Hirai, N., Kondo, S., Moriyama-Kita, M., Nakazawa, R., Komura, S., Kano, M., Uno, D., Inaba, M., Ueno, T., Nakanishi, Y., Endo, K., Sugimoto, H., & Yoshizaki, T. (2025). Recent Trends in Prevalence of HPV Infection in Nasopharyngeal Carcinoma in Japan. Microorganisms, 13(11), 2514. https://doi.org/10.3390/microorganisms13112514





