Microbiological Investigation and Clinical Efficacy of Professional Topical Fluoride Application on Streptococcus mutans and Selemonas sputigena in Orthodontic Patients: A Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
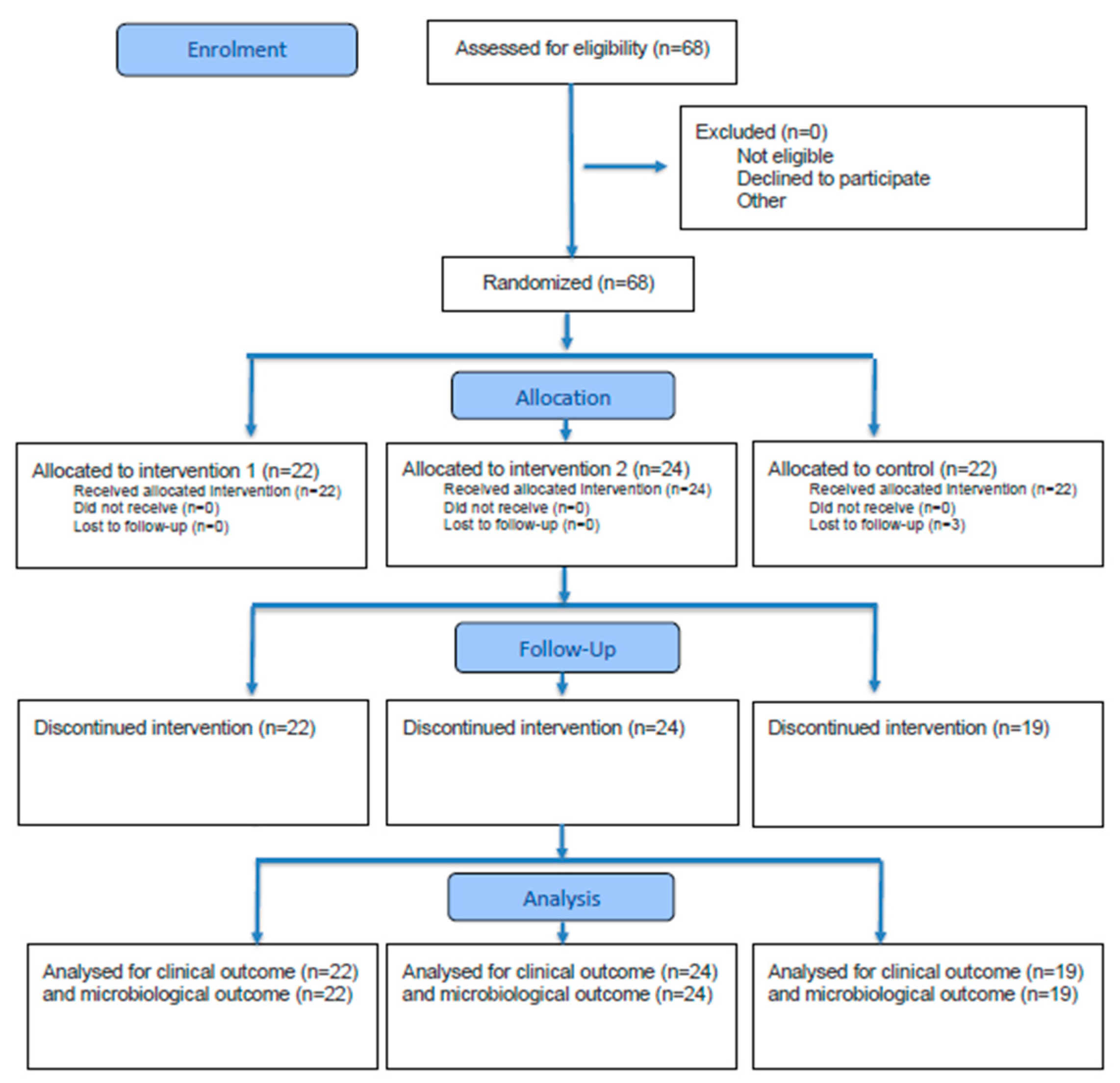
2.1. Trial Design
2.2. Trial Setting
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Recruitment and Consent of Participants
2.5. Intervention and Comparator
2.6. Microbiological Analysis
2.7. Outcomes
- −
- The end-point PCR analysis of plaque samples: the detection and negativization of Streptococcus mutans and Selenomonas sputigena, the key cariogenic and emerging bacterial species.
- −
- The culture-based analysis of saliva samples: the quantification of total oral Streptococci and Lactobacilli, as well as total bacterial load, to assess shifts in microbial composition.
- −
- Secondary outcomes focused on clinical changes, including the following:
- −
- DMFT index (Decayed, Missing, and Filled Teeth): to monitor changes in caries status over the study period.
- −
- Salivary pH: measured with a calibrated pH meter to evaluate acid–base changes in the oral environment.
- −
- Plaque Control Record (PCR%): calculated to quantify plaque accumulation on tooth surfaces.
2.8. Harms
2.9. Sample Size
2.10. Randomization
- −
- Group 1 (experimental): professional oral hygiene session (standard of care) plus topical fluoride gel application.
- −
- Group 2 (experimental): professional oral hygiene session (standard of care) plus topical fluoride varnish application.
- −
- Group 3 (control): professional oral hygiene session (standard of care) only.
2.11. Blinding
2.12. Statistical Methods
3. Results
3.1. Microbiological Results
3.1.1. Results of the Molecular Biology Analysis of Plaque Samples by PCR
- Group 1 (22 patients): S. mutans: 7 patients were positive at T0 and remained positive at T1; 9 patients were negative at T0 and remained negative at T1; 5 patients were positive at T0 and became negative at T1; 1 patient was negative at T0 and became positive at T1; S. sputigena: all patients, except n. 51 and 61, were positive at T0. Among these, 4 patients became negative at T1, while 1 patient became positive at T1. Among the 4 patients who became negative for S. sputigena, patient n. 2 also became negative for S. mutans.
- Group 2 (24 patients): S. mutans: 9 patients were positive at T0 and remained positive at T1; 9 patients were negative at T0 and remained negative at T1; 3 patients were positive at T0 and became negative at T1; 2 patients were negative at T0 and became positive at T1; S. sputigena: All patients were positive at T0 except patient n. 56. Among these, 5 patients became negative at T1.
- Group 3 (22 patients): S. mutans: 5 patients (n. 24, 32, 42, 43, 45) were positive at T0 and remained positive at T1; 2 patients (n. 35, 60) were positive at T0 and became negative at T1; 1 patient (n. 68) became positive at T1; S. sputigena: All patients were positive at both T0 and T1.
3.1.2. Results of the Culture-Based Microbiological Analysis on Plaque Samples
3.1.3. Results of the Culture-Based Microbiological Analysis on Saliva Samples
3.2. Clinical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCT | Randomized Controlled Trial |
| DMFT | Decayed, Missing, Filled Teeth index |
| PCR% | Plaque Control Record |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| end-point PCR | end-point Polymerase Chain Reaction |
| CONSORT | Consolidated Standards of Reporting Trials |
| WHO | World Health Organization |
| CI | Confidence Interval |
References
- Patano, A.; Malcangi, G.; Sardano, R.; Mastrodonato, A.; Garofoli, G.; Mancini, A.; Inchingolo, A.D.; Di Venere, D.; Inchingolo, F.; Dipalma, G.; et al. White Spots: Prevention in Orthodontics-Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2023, 20, 5608. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A. Oral Microbiota Changes during Orthodontic Treatment. Front. Biosci. (Elite Ed.) 2022, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.S.; Alves, L.S.; Maltz, M.; Zenkner, J.E.D.A. Association between fixed orthodontic treatment and dental caries: A 1-year longitudinal study. Braz. Oral Res. 2020, 35, e002. [Google Scholar] [CrossRef]
- Salerno, C.; Grazia Cagetti, M.; Cirio, S.; Esteves-Oliveira, M.; Wierichs, R.J.; Kloukos, D.; Campus, G. Distribution of initial caries lesions in relation to fixed orthodontic therapy. A systematic review and meta-analysis. Eur. J. Orthod. 2024, 46, cjae008. [Google Scholar] [CrossRef]
- Thanetchaloempong, W.; Koontongkaew, S.; Utispan, K. Fixed Orthodontic Treatment Increases Cariogenicity and Virulence Gene Expression in Dental Biofilm. J. Clin. Med. 2022, 11, 5860. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.K.; Jungbauer, G.; Jungbauer, R.; Wolf, M.; Deschner, J. Biofilm and Orthodontic Therapy. Monogr. Oral Sci. 2021, 29, 201–213. [Google Scholar]
- Cumerlato CBda, F.; Santos CSDos Rotta, R.N.; Cademartori, M.G.; Corrêa, M.B. Is professionally applied topical fluoride effective in treating incipient caries? A systematic review. Braz. Oral Res. 2022, 36, e083. [Google Scholar] [CrossRef]
- Marinho, V.C.C.; Higgins, J.P.T.; Logan, S.; Sheiham, A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002782. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Oral Health. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 9 February 2025).
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Castilho ARFde Mialhe, F.L.; Barbosa Tde, S.; Puppin-Rontani, R.M. Influence of family environment on children’s oral health: A systematic review. J. Pediatr. (Rio. J.) 2013, 89, 116–123. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry reference manual 2011–2012. Pediatr. Dent. 2011, 33, 1–349.
- Young, D.A.; Featherstone, J.D.; Roth, J.R.; Anderson, M.; Autio-Gold, J.; Christensen, G.J.; Fontana, M.; Kutsch, V.K.; Peters, M.C.; Simonsen, R.J.; et al. Caries management by risk assessment: Implementation guidelines. J. Calif. Dent. Assoc. 2007, 35, 799–805. [Google Scholar] [CrossRef]
- Yost, J.; Li, Y. Promoting oral health from birth through childhood: Prevention of early childhood caries. MCN Am. J. Matern. Child. Nurs. 2008, 33, 17–23, quiz 24–5. [Google Scholar] [CrossRef]
- Cho, H.; Ren, Z.; Divaris, K.; Roach, J.; Lin, B.M.; Liu, C.; Azcarate-Peril, M.A.; Simancas-Pallares, M.A.; Shrestha, P.; Orlenko, A.; et al. Selenomonas sputigena acts as a pathobiont mediating spatial structure and biofilm virulence in early childhood caries. Nat. Commun. 2023, 14, 2919. [Google Scholar] [CrossRef]
- Richter, A.E.; Arruda, A.O.; Peters, M.C.; Sohn, W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 657–664. [Google Scholar] [CrossRef]
- Ministero della Salute. Linee Guida Nazionali per la Promozione Della Salute Orale e la Prevenzione Delle Patologie Orali in età Evolutiva. 2013. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_867_allegato.pdf (accessed on 4 June 2025).
- Walsh, L.J.; Healey, D.L. Prevention and caries risk management in teenage and orthodontic patients. Aust. Dent. J. 2019, 64 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef]
- Al Tuma, R.R.; Yassir, Y.A. Effect of calcium fluoride nanoparticles in prevention of demineralization during orthodontic fixed appliance treatment: A randomized clinical trial. Eur. J. Orthod. 2023, 45, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Tubert-Jeannin, S.; Auclair, C.; Amsallem, E.; Tramini, P.; Gerbaud, L.; Ruffieux, C.; Schulte, A.G.; Koch, M.J.; Rège-Walther, M.; Ismail, A. Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing dental caries in children. Cochrane Database Syst. Rev. 2011, 2011, CD007592. [Google Scholar] [CrossRef]
- Benson, P.E.; Parkin, N.; Dyer, F.; Millett, D.T.; Furness, S.; Germain, P. Fluorides for the prevention of early tooth decay (demineralised white lesions) during fixed brace treatment. Cochrane Database Syst. Rev. 2019, 2019, CD003809. [Google Scholar] [CrossRef] [PubMed]
- Cortesi Addizzone, V.; Pejrone, C. Fluoro. In Igienista Orale; Cortesi Ardizzone, V., Abbinante, A., Eds.; Edra: Milano, Italy, 2013; pp. 149–158. [Google Scholar]
- Sharma, A.; Schwartz, S.M.; Méndez, E. Hospital volume is associated with survival but not multimodality therapy in Medicare patients with advanced head and neck cancer. Cancer 2013, 119, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.V.; Ulrich, J.; Costa, L.; Ribeiro, L.A. Multiple Primary Malignancies in Head and Neck Cancer: A University Hospital Experience Over a Five-Year Period. Cureus 2021, 13, e17349. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Espelid, I. Caries preventive effect of fluoride in milk, salt and tablets: A literature review. Eur. Arch. Paediatr. Dent. 2009, 10, 149–156. [Google Scholar] [CrossRef]
- Medikeri, R.S. Quantification of Selenomonas sputigena in Chronic Periodontitis in Smokers Using 16S rDNA Based PCR Analysis. J. Clin. Diagn. Res. 2015, 9, ZC13–ZC17. [Google Scholar] [CrossRef]
- Miyatani, F.; Kuriyama, N.; Watanabe, I.; Nomura, R.; Nakano, K.; Matsui, D.; Ozaki, E.; Koyama, T.; Nishigaki, M.; Yamamoto, T.; et al. Relationship between Cnm-positive Streptococcus mutans and cerebral microbleeds in humans. Oral Dis. 2015, 21, 886–893. [Google Scholar] [CrossRef]
- Lombardo, G.; Signoriello, A.; Corrocher, G.; Signoretto, C.; Burlacchini, G.; Pardo, A.; Nocini, P.F. A Topical Desiccant Agent in Association with Manual Debridement in the Initial Treatment of Peri-Implant Mucositis: A Clinical and Microbiological Pilot Study. Antibiotics 2019, 8, 82. [Google Scholar] [CrossRef]
- Quaranta, A.; Marchisio, O.; D’Isidoro, O.; Genovesi, A.M.; Covani, U. Single-blind randomized clinical trial on the efficacy of an interdental cleaning device in orthodontic patients. Minerva Dent. Oral Sci. 2018, 67, 141–147. [Google Scholar] [CrossRef]
- Memarpour, M.; Fakhraei, E.; Dadaein, S.; Vossoughi, M. Efficacy of Fluoride Varnish and Casein Phosphopeptide-Amorphous Calcium Phosphate for Remineralization of Primary Teeth: A Randomized Clinical Trial. Med. Princ. Pract. 2015, 24, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Baccini, F.; De Manzoni, R.; Viviani, M.; Pancheri, L.; Zangani, A.; Faccioni, P.; Luciano, U.; Zuffellato, N.; Signoriello, A.; et al. Removal of bacterial biofilm in patients undergoing fixed orthodontic treatment: A literature review. J. Appl. Cosmetol. 2023, 41, 1. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Mikłasz, P.; Zawiślak, A.; Sobolewska, E.; Janiszewska-Olszowska, J. Fluoride varnish, ozone and octenidine reduce the incidence of white spot lesions and caries during orthodontic treatment: Randomized controlled trial. Sci. Rep. 2022, 12, 13985. [Google Scholar] [CrossRef] [PubMed]
- Sonesson, M.; Twetman, S. Prevention of white spot lesions with fluoride varnish during orthodontic treatment with fixed appliances: A systematic review. Eur. J. Orthod. 2023, 45, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Piwat, S.; Sophatha, B.; Teanpaisan, R. An assessment of adhesion, aggregation and surface charges of Lactobacillus strains derived from the human oral cavity. Lett. Appl. Microbiol. 2015, 61, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aleem, M.A.E.A.; Ezzat, M.A.; Hafez, A.I. Effect of xylitol and calcium combination to fluoride gel versus conventional fluoride gel on plaque bacterial count and salivary ph in high caries risk adult patients over three months follow up: A randomized clinical trial. Egypt. Dent. J. 2024, 70, 3859–3868. [Google Scholar] [CrossRef]
- Baik, A.; Alamoudi, N.; El-Housseiny, A.; Altuwirqi, A. Fluoride Varnishes for Preventing Occlusal Dental Caries: A Review. Dent. J. 2021, 9, 64. [Google Scholar] [CrossRef]
- ten Cate, J. Contemporary perspective on the use of fluoride products in caries prevention. Br. Dent. J. 2013, 214, 161–167. [Google Scholar] [CrossRef]
- Koopman, J.E.; van der Kaaij, N.C.W.; Buijs, M.J.; Elyassi, Y.; van der Veen, M.H.; Crielaard, W.; Cate, J.M.T.; Zaura, E. The Effect of Fixed Orthodontic Appliances and Fluoride Mouthwash on the Oral Microbiome of Adolescents—A Randomized Controlled Clinical Trial. PLoS ONE 2015, 10, e0137318. [Google Scholar] [CrossRef]
- Pardo, A.; Signoriello, A.; Zangani, A.; Messina, E.; Gheza, S.; Faccioni, P.; Albanese, M.; Lombardo, G. Home Biofilm Management in Orthodontic Aligners: A Systematic Review. Dent. J. 2024, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Miragliotta, G.; Mosca, A. Il Biofilm Batterico Nel Cavo Orale. In Igienista Orale; Cortesi Ardizzone, V., Abbinante, A., Eds.; Edra: Milano, Italy, 2013; pp. 122–126. [Google Scholar]
- Mamani-Cori, V.; Calcina-Asillo, T.P.; Chino-Mamani, M.; Mendoza-Quispe, Y.R.; Yucra-Sardón, S.O.; Arbildo-Vega, H.I.; Padilla-Cáceres, T.C.; Quispe-Quispe, B.; Coronel-Zubiate, F.T. Topical fluoride and regulation of salivary pH in Peruvian Altiplano schoolchildren: A comparative longitudinal study. Front. Oral Health 2025, 6, 1620432. [Google Scholar] [CrossRef]
| PLAQUE | Streptococci T0 (Mean ± SD) | Streptococci T1 (Mean ± SD) | Δ Streptococci T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 1.45 × 107 ± 3.40 × 107 | 1.45 × 107 ± 3.40 × 107 | 1.45 × 107 ± 3.40 × 107 | 0.03 * |
| Group 1 (gel, n = 22) | 2.54 × 107 ± 4.28 × 107 | 2.54 × 107 ± 4.28 × 107 | 2.54 × 107 ± 4.28 × 107 | 0.13 |
| Group 2 (varnish, n = 24) | 1.73 × 107 ± 3.78 × 107 | 1.73 × 107 ± 3.78 × 107 | 1.73 × 107 ± 3.78 × 107 | 0.01 * |
| Group 3 (control, n = 22) | 9.90 × 105 ± 1.63 × 106 | 9.90 × 105 ± 1.63 × 106 | 9.90 × 105 ± 1.63 × 106 | 0.25 |
| p value | 0.43 | 0.04 * | 0.07 |
| PLAQUE | Lactobacilli T0 (Mean ± SD) | Lactobacilli T1 (Mean ± SD) | Δ Lactobacilli T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 1.56 × 106 ± 1.21 × 107 | 1.56 × 106 ± 1.21 × 107 | 1.56 × 106 ± 1.21 × 107 | 0.9 |
| Group 1 (gel, n = 22) | 4.56 × 106 ± 2.13 × 107 | 4.56 × 106 ± 2.13 × 107 | 4.56 × 106 ± 2.13 × 107 | 0.34 |
| Group 2 (varnish, n = 24) | 4.97 × 104 ± 2.04 × 105 | 4.97 × 104 ± 2.04 × 105 | 4.97 × 104 ± 2.04 × 105 | 0.56 |
| Group 3 (control, n = 22) | 2.20 × 105 ± 5.08 × 105 | 2.20 × 105 ± 5.08 × 105 | 2.20 × 105 ± 5.08 × 105 | 0.3 |
| p value | 0.45 | 0.12 | 0.6 |
| PLAQUE | Total Count T0 (Mean ± SD) | Total Count T1 (Mean ± SD) | Δ Total Count T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 3.32 × 107 ± 1.31 × 108 | 3.32 × 107 ± 1.31 × 108 | 3.32 × 107 ± 1.31 × 108 | 0.02 * |
| Group 1 (gel, n = 22) | 5.87 × 107 ± 2.07 × 108 | 5.87 × 107 ± 2.07 × 108 | 5.87 × 107 ± 2.07 × 108 | 0.64 |
| Group 2 (varnish, n = 24) | 1.49 × 107 ± 2.67 × 107 | 1.49 × 107 ± 2.67 × 107 | 1.49 × 107 ± 2.67 × 107 | 0.2 |
| Group 3 (control, n = 22) | 2.76 × 107 ± 1.01 × 108 | 2.76 × 107 ± 1.01 × 108 | 2.76 × 107 ± 1.01 × 108 | 0.17 |
| p value | 0.58 | 0.09 | 0.001 * |
| SALIVA | Streptococci T0 (Mean ± SD) | Streptococci T1 (Mean ± SD) | Δ Streptococci T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 1.46 × 107 ± 3.40 × 107 | 1.46 × 107 ± 3.40 × 107 | 1.46 × 107 ± 3.40 × 107 | 0.47 |
| Group 1 (gel, n = 22) | 2.54 × 107 ± 4.28 × 107 | 2.54 × 107 ± 4.28 × 107 | 2.54 × 107 ± 4.28 × 107 | 0.25 |
| Group 2 (varnish, n = 24) | 1.77 × 107 ± 3.77 × 107 | 1.77 × 107 ± 3.77 × 107 | 1.77 × 107 ± 3.77 × 107 | 0.001 * |
| Group 3 (control, n = 22) | 9.67 × 105 ± 2.24 × 106 | 9.67 × 105 ± 2.24 × 106 | 9.67 × 105 ± 2.24 × 106 | 0.5 |
| p value | 0.35 | 0.6 | 0.2 |
| SALIVA | Lactobacilli T0 (Mean ± SD) | Lactobacilli T1 (Mean ± SD) | Δ Lactobacilli T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 1.77 × 105 ± 1.21 × 106 | 1.77 × 105 ± 1.21 × 106 | 1.77 × 105 ± 1.21 × 106 | 0.99 |
| Group 1 (gel, n = 22) | 4.93 × 104 ± 1.12 × 105 | 4.93 × 104 ± 1.12 × 105 | 4.93 × 104 ± 1.12 × 105 | 0.63 |
| Group 2 (varnish, n = 24) | 4.27 × 105 ± 2.04 × 106 | 4.27 × 105 ± 2.04 × 106 | 4.27 × 105 ± 2.04 × 106 | 0.71 |
| Group 3 (control, n = 22) | 3.29 × 104 ± 6.12 × 104 | 3.29 × 104 ± 6.12 × 104 | 3.29 × 104 ± 6.12 × 104 | 0.1 |
| p value | 0.4 | 0.52 | 0.41 |
| SALIVA | Total Count T0 (Mean ± SD) | Total Count T1 (Mean ± SD) | Δ Total Count T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 2.38 × 108 ± 7.43 × 108 | 2.38 × 108 ± 7.43 × 108 | 2.38 × 108 ± 7.43 × 108 | 0.85 |
| Group 1 (gel, n = 22) | 1.55 × 108 ± 5.70 × 108 | 1.55 × 108 ± 5.70 × 108 | 1.55 × 108 ± 5.70 × 108 | 0.37 |
| Group 2 (varnish, n = 24) | 1.56 × 108 ± 2.04 × 108 | 1.56 × 108 ± 2.04 × 108 | 1.56 × 108 ± 2.04 × 108 | 0.7 |
| Group 3 (control, n = 22) | 4.10 × 108 ± 1.16 × 109 | 4.10 × 108 ± 1.16 × 109 | 4.10 × 108 ± 1.16 × 109 | 0.09 |
| p value | 0.4 | 0.3 | 0.18 |
| DMFT | DMFT T0 Mean ± SD | DMFT T1 Mean ± SD | Δ DMFT T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 3 ± 2.30 | 3.05 ± 2.36 | 0.05 | 0.09 |
| Group 1 (gel, n = 22) | 2.95 ± 2.23 | 2.95 ± 2.23 | 0 | 0.87 |
| Group 2 (varnish, n = 24) | 2.58 ± 2.14 | 2.56 ± 2.19 | −0.02 | 0.12 |
| Group 3 (control, n = 22) | 3.5 ± 2.52 | 3.74 ± 2.64 | 0.24 | 0.23 |
| p value | 0.45 | 0.32 | 0.56 |
| PCR | PCR T0 Mean ± SD | PCR T1 Mean ± SD | Δ PCR T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 96.29 ± 4.99 | 89.33 ± 6.27 | −6.96 | 0.001 * |
| Group 1 (gel, n = 22) | 94.21 ± 6.79 | 88.13 ± 6.28 | −6.08 | 0.001 * |
| Group 2 (varnish, n = 24) | 97.58 ± 3.72 | 87.27 ± 5.27 | −10.31 | 0.001 * |
| Group 3 (control, n = 22) | 96.73 ± 3.48 | 94.43 ± 5.32 | −2.3 | 0.14 |
| p value | 0.35 | 0.02 * | 0.001 * |
| pH | pH T0 Mean ± SD | pH T1 Mean ± SD | Δ pH T1–T0 | p Value |
|---|---|---|---|---|
| Total sample (n = 68) | 6.97 ± 0.61 | 7.40 ± 0.64 | 0.43 | 0.08 |
| Group 1 (gel, n = 22) | 6.86 ± 0.56 | 7.58 ± 0.79 | 0.72 | 0.03 * |
| Group 2 (varnish, n = 24) | 7.05 ± 0.70 | 7.34 ± 0.75 | 0.29 | 0.23 |
| Group 3 (control, n = 22) | 6.97 ± 0.58 | 7.17 ± 0.24 | 0.2 | 0.46 |
| p value | 0.07 | 0.92 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, A.; Marcoccia, S.; Montagnini, C.; Signoriello, A.; Messina, E.; Gaibani, P.; Burlacchini, G.; Salgarelli, C.; Signoretto, C.; Zerman, N. Microbiological Investigation and Clinical Efficacy of Professional Topical Fluoride Application on Streptococcus mutans and Selemonas sputigena in Orthodontic Patients: A Randomized Controlled Clinical Trial. Microorganisms 2025, 13, 2506. https://doi.org/10.3390/microorganisms13112506
Pardo A, Marcoccia S, Montagnini C, Signoriello A, Messina E, Gaibani P, Burlacchini G, Salgarelli C, Signoretto C, Zerman N. Microbiological Investigation and Clinical Efficacy of Professional Topical Fluoride Application on Streptococcus mutans and Selemonas sputigena in Orthodontic Patients: A Randomized Controlled Clinical Trial. Microorganisms. 2025; 13(11):2506. https://doi.org/10.3390/microorganisms13112506
Chicago/Turabian StylePardo, Alessia, Stefano Marcoccia, Camilla Montagnini, Annarita Signoriello, Elena Messina, Paolo Gaibani, Gloria Burlacchini, Camillo Salgarelli, Caterina Signoretto, and Nicoletta Zerman. 2025. "Microbiological Investigation and Clinical Efficacy of Professional Topical Fluoride Application on Streptococcus mutans and Selemonas sputigena in Orthodontic Patients: A Randomized Controlled Clinical Trial" Microorganisms 13, no. 11: 2506. https://doi.org/10.3390/microorganisms13112506
APA StylePardo, A., Marcoccia, S., Montagnini, C., Signoriello, A., Messina, E., Gaibani, P., Burlacchini, G., Salgarelli, C., Signoretto, C., & Zerman, N. (2025). Microbiological Investigation and Clinical Efficacy of Professional Topical Fluoride Application on Streptococcus mutans and Selemonas sputigena in Orthodontic Patients: A Randomized Controlled Clinical Trial. Microorganisms, 13(11), 2506. https://doi.org/10.3390/microorganisms13112506








