Prospects of Phage DJ6712 and FW6709 in Biocontrol of Aeromonas veronii in Fish Aquaculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Aeromonas Bacteria
2.2. Phage Isolation
2.3. Phage Testing Against the Aeromonas Isolates Obtained from Diseased Fish
2.4. Characterization of the Selected Phages
2.4.1. Host Range
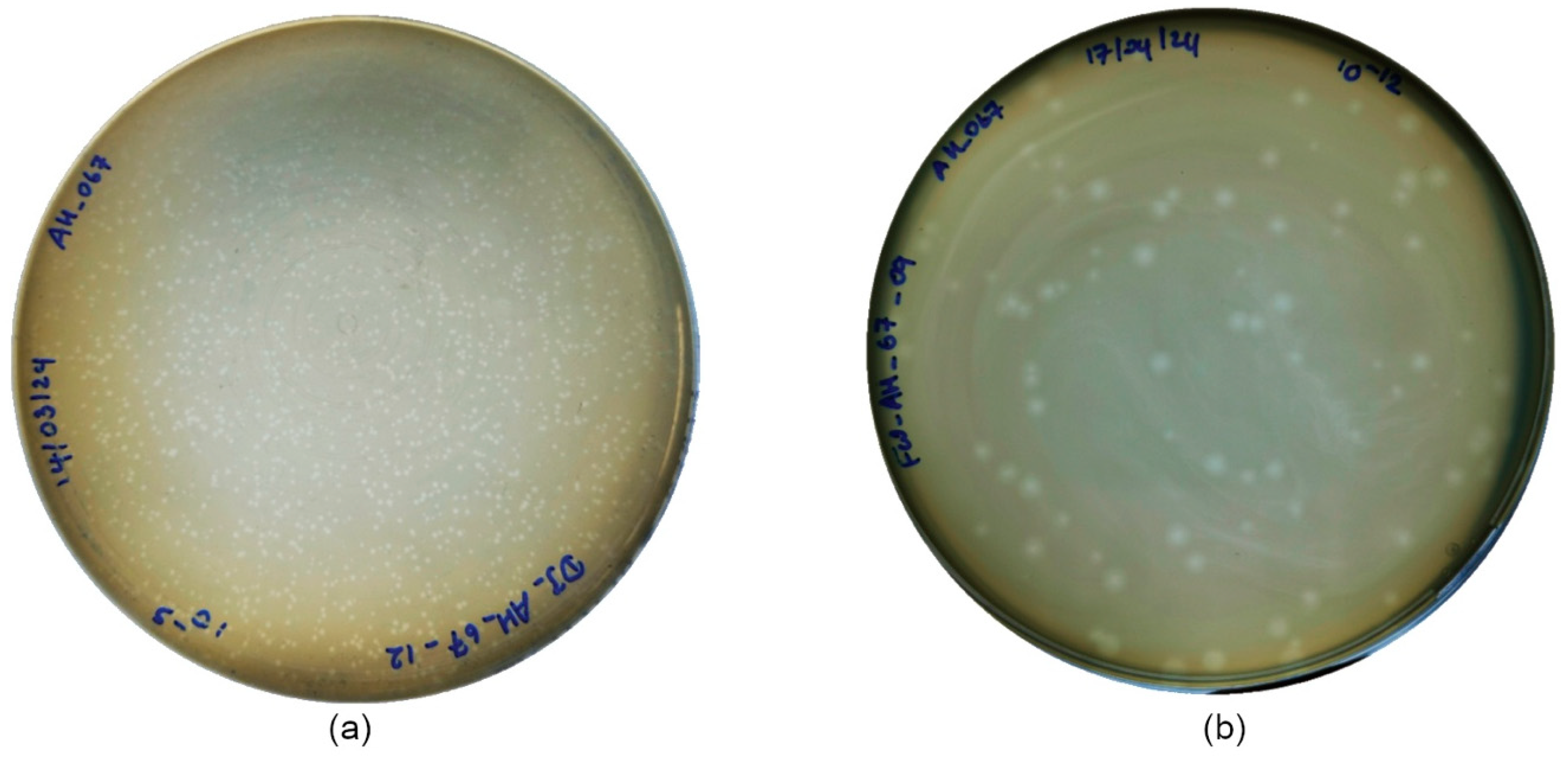
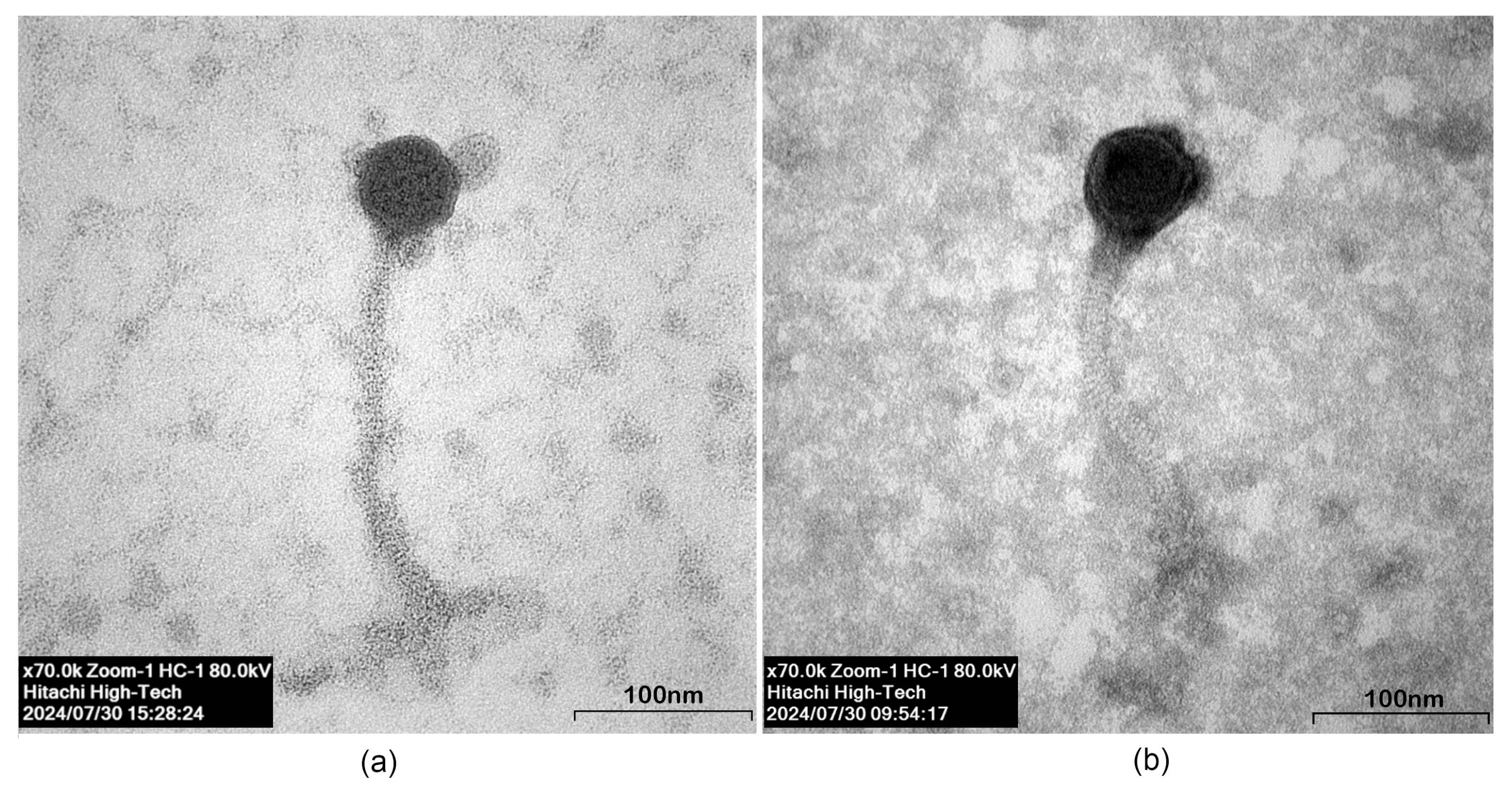
2.4.2. Plaque Formation and Phage Morphology
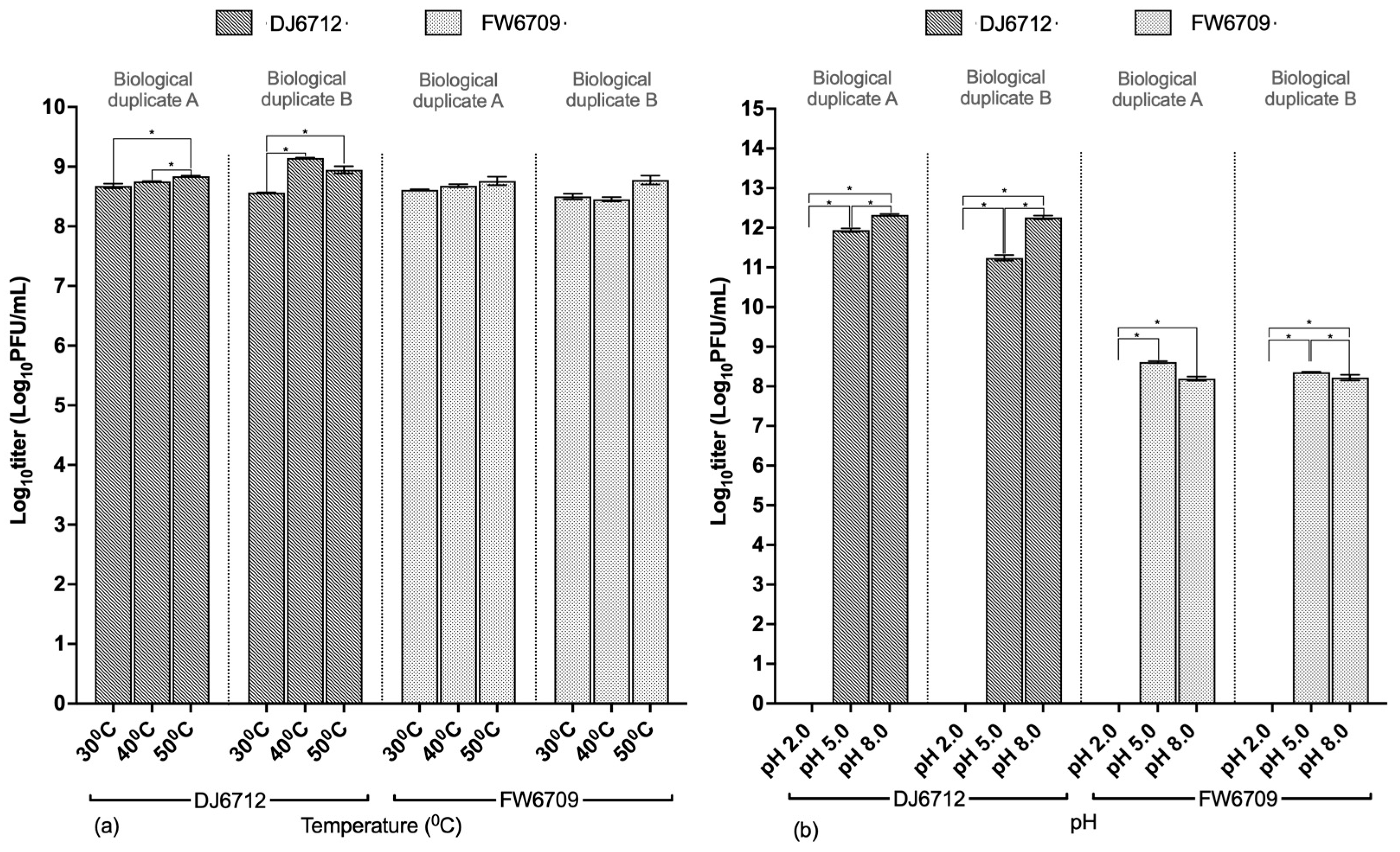
2.4.3. Temperature and pH Stability
2.4.4. Optimal Multiplicity of Infection (MOI)
2.4.5. One-Step Growth Curves
3. Results
3.1. Aeromonas veronii Host Bacterial Confirmation
3.2. Bacteriophage Performance Screening Against Aeromonas Hosts
3.3. Characterization of Phages DJ6712 and FW6709
3.3.1. Host Range
3.3.2. Plaque Formation and Phage Morphology
3.3.3. Temperature and pH Stability
3.3.4. MOI
3.3.5. One-Step Growth Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAS | Motile Aeromonas septicemia |
| WGS | Whole genome sequencing |
| SM | Saline Magnesium |
| PVDF | Polyvinylidene fluoride |
| DAL | Double agar layer |
| OD | Optical density |
| NIH | National institute of health |
| PFU | Plaque forming units |
| AMR | Antimicrobial resistance |
| ARG | Antibiotic resistance genes |
| PCR | Polymerase chain reaction |
| CFSAN | Center for Food Safety and Applied Nutrition |
| FSANZ | Food Standards Australia New Zealand |
| K-FDA | Korean Food and Drug Administration |
| EFSA | The European Food Safety Authority |
References
- Fisheries, F. The State of World Fisheries and Aquaculture 2024 Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Charo-Karisa, H. Tilapia. Encyclopedia of Meat Sciences, 3rd ed.; Dikeman, M., Ed.; Elsevier: Oxford, UK, 2024; pp. 29–39. [Google Scholar]
- Mursalim, M.F.; Budiyansah, H.; Raharjo, H.M.; Debnath, P.P.; Sakulworakan, R.; Chokmangmeepisarn, P.; Yindee, J.; Piasomboon, P.; Elayaraja, S.; Rodkhum, C. Diversity and antimicrobial susceptibility profiles of Aeromonas spp. isolated from diseased freshwater fishes in Thailand. J. Fish Dis. 2022, 45, 1149–1163. [Google Scholar] [CrossRef]
- Chitmanat, C.; Lebel, P.; Whangchai, N.; Promya, J.; Lebel, L. Tilapia diseases and management in river-based cage aquaculture in northern Thailand. J. Appl. Aquac. 2016, 28, 9–16. [Google Scholar] [CrossRef]
- Fauzi, N.; Hamdan, R.H.; Mohamed, M.; Ismail, A.; Mat Zin, A.A.; Mohamad, N.F.A. Prevalence, antibiotic susceptibility, and presence of drug resistance genes in Aeromonas spp. isolated from freshwater fish in Kelantan and Terengganu states, Malaysia. Vet. World 2021, 14, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lv, Z.; Yang, L.; Liu, D.; Ou, Y.; Xu, C.; Liu, W.; Yuan, D.; Hao, Y.; He, J.; et al. Integrated aquaculture contributes to the transfer of mcr-1 between animals and humans via the aquaculture supply chain. Environ. Int. 2019, 130, 104708. [Google Scholar] [CrossRef]
- Tuševljak, N.; Dutil, L.; Rajić, A.; Uhland, F.C.; McClure, C.; St-Hilaire, S.; Reid-Smith, R.J.; McEwen, S.A. Antimicrobial Use and Resistance in Aquaculture: Findings of a Globally Administered Survey of Aquaculture-Allied Professionals. Zoonoses Public Health 2013, 60, 426–436. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; Van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef]
- Suyamud, B.; Chen, Y.; Dong, Z.; Zhao, C.; Hu, J. Antimicrobial resistance in aquaculture: Occurrence and strategies in Southeast Asia. Sci. Total Environ. 2023, 907, 167942. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.V.; dos Santos, A.G.V.; de Souza, A.B.; Bueno Junior, G.; de Souza, G.F.; de Souza, E.M.; de Carvalho Nunes, L.; Viana, K.F. Bivalent Vaccine Against Streptococcus agalactiae and Aeromonas hydrophila in Nile Tilapia (Oreochromis niloticus): A Laboratory-Phase and Large-Scale Study. Animals 2023, 13, 3338. [Google Scholar] [CrossRef]
- Plant, K.P.; Lapatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Muliya Sankappa, N.; Shivani Kallappa, G.; Kallihosuru Boregowda, K.; Mandrira Ramakrishna, N.; Kattapuni Suresh, P.; Shriraje Balakrishna, D.; Ballamoole, K.K.; Thangavel, S.; Sahoo, L.; Lange, M.D.; et al. Novel lytic bacteriophage AhFM11 as an effective therapy against hypervirulent Aeromonas hydrophila. Sci. Rep. 2024, 14, 16882. [Google Scholar] [CrossRef]
- Gordola, K.M.C.; Boctuanon, F.A.U.; Diolata, R.A.A.; Pedro, M.B.D.; Gutierrez, T.A.D.; Papa, R.D.S.; Papa, D.M.D. Evaluation of Phage Delivery Systems on Induced Motile Aeromonas Septicemia in Oreochromis niloticus. Phage 2020, 1, 189–197. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Namonyo, S.; Weynberg, K.D.; Guo, J.; Carvalho, G. The effectiveness and role of phages in the disruption and inactivation of clinical P. aeruginosa biofilms. Environ. Res. 2023, 234, 116586. [Google Scholar] [CrossRef] [PubMed]
- Mulia, D.S.; Pratiwi, R.; Asmara, W.; Azzam-Sayuti, M.; Yasin, I.S.M.; Isnansetyo, A. Isolation, genetic characterization, and virulence profiling of different Aeromonas species recovered from moribund hybrid catfish (Clarias spp.). Vet. World 2023, 16, 1974–1984. [Google Scholar] [CrossRef]
- Nawaz, M.; Sung, K.; Khan, S.A.; Khan, A.A.; Steele, R. Biochemical and molecular characterization of tetracycline-resistant Aeromonas veronii isolates from catfish. Appl. Environ. Microbiol. 2006, 72, 6461–6466. [Google Scholar] [CrossRef]
- U-Taynapun, K.; Nganwisuthiphan, T.; Chirapongsatonkul, N. Species diversity and existence of virulence genes in clinical Aeromonas spp. causing motile Aeromonas septicemia (MAS) isolated from cultured Nile tilapia (Oreochromis niloticus). Int. J. Agric. Technol. 2020, 16, 749–760. [Google Scholar]
- Hu, H.; Cai, L.; Shao, L.; Xu, X.; Wang, H.; Zhou, G. Characterization and genome analysis of two Aeromonas phages isolated from various sources related to chilled chicken. LWT 2024, 212, 117028. [Google Scholar] [CrossRef]
- Pan, L.; Li, D.; Lin, W.; Liu, W.; Qu, C.; Qian, M.; Cai, R.; Zhou, Q.; Wang, F.; Tong, Y. Novel Aeromonas Phage Ahy-Yong1 and Its Protective Effects against Aeromonas hydrophila in Brocade Carp (Cyprinus aka Koi). Viruses 2022, 14, 2498. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, S.; Han, S.; Wang, D.; Zhao, J.; Xu, L.; Liu, H.; Lu, T. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 2020, 523, 735193. [Google Scholar] [CrossRef]
- Akmal, M.; Rahimi-Midani, A.; Hafeez-ur-Rehman, M.; Hussain, A.; Choi, T.-J. Isolation, Characterization, and Application of a Bacteriophage Infecting the Fish Pathogen Aeromonas hydrophila. Pathogens 2020, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Paquet, V.E.; Bernatchez, A.; Tremblay, D.M.; Moineau, S.; Charette, S.J. Characterization and diversity of phages infecting Aeromonas salmonicida subsp. salmonicida. Sci. Rep. 2017, 7, 7054. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, P.; Zhou, X.; Zhang, Y.; Wang, Q.; Liu, X.; Shao, S.; Liu, Q. Isolation of a Virulent Aeromonas salmonicida subsp. masoucida Bacteriophage and Its Application in Phage Therapy in Turbot (Scophthalmus maximus). Appl. Environ. Microbiol. 2021, 87, e01468-21. [Google Scholar] [CrossRef]
- Golomidova, A.K.; Kulikov, E.E.; Kuznetsov, A.S.; Pechenov, P.Y.; Belalov, I.S.; Letarov, A.V.; Galyov, E.E. Isolation and complete genome sequence of Aeromonas bacteriophage Gekk3-15. F1000Research 2024, 13, 380. [Google Scholar] [CrossRef]
- Ye, Y.; Tong, G.; Chen, G.; Huang, L.; Huang, L.; Jiang, X.; Wei, X.; Lin, M. The characterization and genome analysis of a novel phage phiA034 targeting multiple species of Aeromonas. Virus Res. 2023, 336, 199193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, J.; Jiang, G.; Zhou, D.; Hua, J.; Li, Q. Characterization and genomic analysis of the novel Aeromonas veronii phage pAEv1812. Arch. Virol. 2022, 167, 1865–1870. [Google Scholar] [CrossRef]
- Li, Z.; Hua, J.; Zhou, D.; Feng, J.; Zhou, K.; Li, Q. Genomic analysis of a novel Aeromonas veronii phage pAEv1810, belonging to the genus Petsuvirus. Arch. Microbiol. 2022, 204, 304. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhou, D.; Li, Q.; Jiang, Z.; Zhang, J.; Qiao, G. Evaluation on the effects of phage cocktail in preventing Aeromonas veronii infection in Gibel carps (Carassius auratus gibelio). Aquaculture 2023, 563, 738998. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Hou, Y.; Chen, Y.; Wu, Z.; Zhang, Y.-A.; Zhou, Y. Bacteriophage adhering to mucus provide protection against Aeromonas veronii infection in scaleless fish. Aquaculture 2024, 586, 740804. [Google Scholar] [CrossRef]
- Aly, S.M.; Abou-El-Atta, M.E.; El-Mahallawy, H.S.; Elaswad, A.; ElAbyad, F.A.; ElBanna, N.I. Aeromonas veronii and ulcerative syndrome in cultured Nile tilapia (Oreochromis niloticus) and their associated factors. Aquac. Int. 2023, 31, 2867–2881. [Google Scholar] [CrossRef]
- Pulpipat, T.; Heckman, T.I.; Boonyawiwat, V.; Kerddee, P.; Phatthanakunanan, S.; Soto, E.; Surachetpong, W. Concurrent infections of Streptococcus iniae and Aeromonas veronii in farmed Giant snakehead (Channa micropeltes). J. Fish Dis. 2023, 46, 629–641. [Google Scholar] [CrossRef]
- Persson, S.; Al-Shuweli, S.; Yapici, S.; Jensen, J.N.; Olsen, K.E. Identification of clinical Aeromonas species by rpoB and gyrB sequencing and development of a multiplex PCR method for detection of Aeromonas hydrophila, A. caviae, A. veronii, and A. media. J. Clin. Microbiol. 2015, 53, 653–656. [Google Scholar] [CrossRef]
- Van Twest, R.; Kropinski, A.M. Bacteriophage enrichment from water and soil. Methods Mol. Biol. 2009, 501, 15–21. [Google Scholar] [CrossRef]
- Peters, D.L.; Harris, G.; Davis, C.M.; Dennis, J.J.; Chen, W. Bacteriophage isolation, purification, and characterization techniques against ubiquitous opportunistic pathogens. Curr. Protoc. 2022, 2, e594. [Google Scholar] [CrossRef]
- Khan Mirzaei, M.; Nilsson, A.S. Isolation of Phages for Phage Therapy: A Comparison of Spot Tests and Efficiency of Plating Analyses for Determination of Host Range and Efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Birk, T.; Sørensen, M.C.H.; Brøndsted, L. Methods for Isolation, Purification, and Propagation of Bacteriophages of Campylobacter jejuni. In Campylobacter jejuni: Methods and Protocols; Butcher, J., Stintzi, A., Eds.; Springer: New York, NY, USA, 2017; pp. 19–28. [Google Scholar]
- Booncharoen, N.; Mongkolsuk, S.; Sirikanchana, K. Comparative persistence of human sewage-specific Enterococcal bacteriophages in freshwater and seawater. Appl. Microbiol. Biotechnol. 2018, 102, 6235–6246. [Google Scholar] [CrossRef]
- Rakocy, J. Oreochromis niloticus. In Cultured Aquatic Species Fact Sheets; Edited and compiled by Valerio; FAO: Rome, Italy, 2011. [Google Scholar]
- Moriarty, D.J.W. The physiology of digestion of blue-green algae in the cichlid fish, Tilapia nilotica. J. Zool. 1973, 171, 25–39. [Google Scholar] [CrossRef]
- Bhujel, R.C. On-Farm Feed Management Practices for Nile tilapia (Oreochromis niloticus) in Thailand; FAO: Rome, Italy, 2014. [Google Scholar]
- Svanberga, K.; Avsejenko, J.; Jansons, J.; Fridmanis, D.; Kazaka, T.; Berzins, A.; Dislers, A.; Kazaks, A.; Zrelovs, N. Isolation and Characterization of a Novel Aeromonas salmonicida-Infecting Studiervirinae Bacteriophage, JELG-KS1. Microorganisms 2024, 12, 542. [Google Scholar] [CrossRef]
- Benala, M.; Vaiyapuri, M.; Visnuvinayagam, S.; George, J.C.; Raveendran, K.; George, I.; Mothadaka, M.P.; Badireddy, M.R. A revisited two-step microtiter plate assay: Optimization of in vitro multiplicity of infection (MOI) for Coliphage and Vibriophage. J. Virol. Methods 2021, 294, 114177. [Google Scholar] [CrossRef] [PubMed]
- Gehring, K.; Charbit, A.; Brissaud, E.; Hofnung, M. Bacteriophage lambda receptor site on the Escherichia coli K-12 LamB protein. J. Bacteriol. 1987, 169, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Washizaki, A.; Yonesaki, T.; Otsuka, Y. Characterization of the interactions between Escherichia coli receptors, LPS and OmpC, and bacteriophage T4 long tail fibers. Microbiologyopen 2016, 5, 1003–1015. [Google Scholar] [CrossRef]
- Mathieu, K.; Javed, W.; Vallet, S.; Lesterlin, C.; Candusso, M.-P.; Ding, F.; Xu, X.N.; Ebel, C.; Jault, J.-M.; Orelle, C. Functionality of membrane proteins overexpressed and purified from E. coli is highly dependent upon the strain. Sci. Rep. 2019, 9, 2654. [Google Scholar] [CrossRef]
- Piel, D.; Bruto, M.; Labreuche, Y.; Blanquart, F.; Goudenège, D.; Barcia-Cruz, R.; Chenivesse, S.; Le Panse, S.; James, A.; Dubert, J.; et al. Phage–host coevolution in natural populations. Nat. Microbiol. 2022, 7, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Thanki, A.M.; Mignard, G.; Atterbury, R.J.; Barrow, P.; Millard, A.D.; Clokie, M.R.J. Prophylactic Delivery of a Bacteriophage Cocktail in Feed Significantly Reduces Salmonella Colonization in Pigs. Microbiol. Spectr. 2022, 10, e00422-22. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Nitin, N. Edible bacteriophage based antimicrobial coating on fish feed for enhanced treatment of bacterial infections in aquaculture industry. Aquaculture 2019, 502, 18–25. [Google Scholar] [CrossRef]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of Bacteriophage Felix O1 into Chitosan-Alginate Microspheres for Oral Delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Aríñez-Soriano, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Sci. Rep. 2017, 7, 41441. [Google Scholar] [CrossRef]
- Richards, K.; Malik, D.J. Microencapsulation of Bacteriophages Using Membrane Emulsification in Different pH-Triggered Controlled Release Formulations for Oral Administration. Pharmaceuticals 2021, 14, 424. [Google Scholar] [CrossRef]
- Abedon, S.T.; Hyman, P.; Thomas, C. Experimental Examination of Bacteriophage Latent-Period Evolution as a Response to Bacterial Availability. Appl. Environ. Microbiol. 2003, 69, 7499–7506. [Google Scholar] [CrossRef]
- Ongenae, V.; Azeredo, J.; Kropinski, A.M.; Rozen, D.; Briegel, A.; Claessen, D. Genome sequence and characterization of Streptomyces phage Pablito, representing a new species within the genus Janusvirus. Sci. Rep. 2022, 12, 17785. [Google Scholar] [CrossRef]
- Kannoly, S.; Oken, G.; Shadan, J.; Musheyev, D.; Singh, K.; Singh, A.; Dennehy, J.J. Single-Cell Approach Reveals Intercellular Heterogeneity in Phage-Producing Capacities. Microbiol. Spectr. 2023, 11, e0266321. [Google Scholar] [CrossRef]
- Silveira, C.B.; Luque, A.; Rohwer, F. The landscape of lysogeny across microbial community density, diversity and energetics. Environ. Microbiol. 2021, 23, 4098–4111. [Google Scholar] [CrossRef]
- Naknaen, A.; Samernate, T.; Wannasrichan, W.; Surachat, K.; Nonejuie, P.; Chaikeeratisak, V. Combination of genetically diverse Pseudomonas phages enhances the cocktail efficiency against bacteria. Sci. Rep. 2023, 13, 8921. [Google Scholar] [CrossRef]
- Schulz, P.; Robak, S.; Dastych, J.; Siwicki, A.K. Influence of bacteriophages cocktail on European eel (Anguilla anguilla) immunity and survival after experimental challenge. Fish Shellfish. Immunol. 2019, 84, 28–37. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, R.Y.K.; Britton, W.J.; Morales, S.; Kutter, E.; Chan, H.K. Synergy of nebulized phage PEV20 and ciprofloxacin combination against Pseudomonas aeruginosa. Int. J. Pharm. 2018, 551, 158. [Google Scholar] [CrossRef]
- Manohar, P.; Madurantakam Royam, M.; Loh, B.; Bozdogan, B.; Nachimuthu, R.; Leptihn, S. Synergistic Effects of Phage–Antibiotic Combinations Against Citrobacter amalonaticus. ACS Infect. Dis. 2022, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chatain-Ly, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Tamanna, S.K.; Akter, S.; Mazumder, L.; Akter, S.; Hasan, M.R.; Acharjee, M.; Esti, I.Z.; Islam, M.S.; Shihab, M.M.R.; et al. Role of bacteriophages in shaping gut microbial community. Gut Microbes 2024, 16, 2390720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, G.X.; Sanchez-Quete, F.; Loeb, S.K. Systematic Review and Meta-Analysis on the Inactivation Rate of Viruses and Bacteriophage by Solar Wavelength Radiation. Environ. Sci. Technol. 2025, 59, 7421–7439. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.J.; Costa, L.; Pereira, C.; Cunha, Â.; Calado, R.; Gomes, N.C.; Almeida, A. Influence of environmental variables in the efficiency of phage therapy in aquaculture. Microb. Biotechnol. 2014, 7, 401–413. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of Environmental Factors on Phage–Bacteria Interaction and on the Efficacy and Infectivity of Phage P100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef]
- Basri, L.; Nor, R.M.; Salleh, A.; Md Yasin, I.S.; Saad, M.Z.; Abd Rahaman, N.Y.; Barkham, T.; Amal, M.N.A. Co-Infections of Tilapia Lake Virus, Aeromonas hydrophila and Streptococcus agalactiae in Farmed Red Hybrid Tilapia. Animals 2020, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Mon-on, N.; Jaemwimol, P.; Tattiyapong, P.; Surachetpong, W. Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis spp.). Aquaculture 2020, 520, 734746. [Google Scholar] [CrossRef]
- O’Meara, D.M. Examining the Role of Horizontal Gene Transfer on the Evolution of CRISPR-Cas; University of California: Riverside, CA, USA, 2018. [Google Scholar]
- Nolan, T.M.; Sala-Comorera, L.; Reynolds, L.J.; Martin, N.A.; Stephens, J.H.; O’Hare, G.M.P.; O’Sullivan, J.J.; Meijer, W.G. Bacteriophages from faecal contamination are an important reservoir for AMR in aquatic environments. Sci. Total Environ. 2023, 900, 165490. [Google Scholar] [CrossRef] [PubMed]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.-A. Phages rarely encode antibiotic resistance genes: A cautionary tale for virome analyses. ISME J. 2017, 11, 237–247. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Meng, Q.; Hu, Z.; Fu, J.; Dang, C. New perspectives on bacterial chlorine resistance: Phages encoding chlorine resistance genes improve bacterial adaptation. Water Res. 2025, 282, 123607. [Google Scholar] [CrossRef]
- Vos, M.; Birkett, P.J.; Birch, E.; Griffiths, R.I.; Buckling, A. Local Adaptation of Bacteriophages to Their Bacterial Hosts in Soil. Science 2009, 325, 833. [Google Scholar] [CrossRef]
- Wolf, A.; Wiese, J.; Jost, G.; Witzel, K.-P. Wide Geographic Distribution of Bacteriophages That Lyse the Same Indigenous Freshwater Isolate (Sphingomonas sp. Strain B18). Appl. Environ. Microbiol. 2003, 69, 2395–2398. [Google Scholar] [CrossRef]
- Brown, T.L.; Charity, O.J.; Adriaenssens, E.M. Ecological and functional roles of bacteriophages in contrasting environments: Marine, terrestrial and human gut. Curr. Opin. Microbiol. 2022, 70, 102229. [Google Scholar] [CrossRef]
- Niazi, S.K. Bacteriophage Therapy: Discovery, Development, and FDA Approval Pathways. Pharmaceuticals 2025, 18, 1115. [Google Scholar] [CrossRef]


| A. veronii Fish Clinical Isolate | Bacteriophage Infection Ability 1 | ||||
|---|---|---|---|---|---|
| FW6709 | DJ6712 | FP6811 | FW6813 | FW8211 | |
| KLK101 | + | ++ | + | + | − |
| KLK102 | + | ++ | + | + | − |
| KLK103 | + | ++ | + | + | − |
| KLL1 | + | ++ | + | + | − |
| KLK201 | + | + | + | + | − |
| KLK202 | + | ++ | − | − | − |
| KLK203 | + | ++ | − | + | − |
| KLL2 | + | + | − | + | − |
| KLL3 | + | + | − | − | + |
| KLK401 | + | ++ | − | + | − |
| KLK402 | − | − | − | − | − |
| KLK403 | − | + | − | + | + |
| KLL4 | − | ++ | − | − | − |
| KLK502 | + | ++ | − | + | + |
| KLK503 | + | ++ | − | + | + |
| KLL5 | + | ++ | − | + | + |
| KLK701 | − | − | − | − | − |
| KLK702 | − | − | − | − | − |
| KLK703 | − | + | − | − | − |
| UTK3 | + | + | − | − | − |
| UTK4 | − | − | − | − | − |
| UTK6 | ++ | ++ | − | ++ | ++ |
| Host Bacteria | Bacteriophage Infection Ability 1 | ||
|---|---|---|---|
| FW6709 | DJ6712 | ||
| Aeromonas dhakensis | AH62 | − | − |
| A. veronii | AH67 | ++ | ++ |
| AH68 | + | + | |
| AH82 | + | + | |
| Escherichia coli | ATCC15922 | − | − |
| DH5α | − | − | |
| Streptococcus isolates | Streptococcus agalactiae | − | − |
| Streptococcus iniae | − | − | |
| Pseudomonas aeruginosa | − | − | |
| Klebsiella pneumoniae | − | − | |
| Salmonella typhi | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallage, T.P.; Paisantham, P.; Surachetpong, W.; Mongkolsuk, S.; Sirikanchana, K. Prospects of Phage DJ6712 and FW6709 in Biocontrol of Aeromonas veronii in Fish Aquaculture. Microorganisms 2025, 13, 2503. https://doi.org/10.3390/microorganisms13112503
Gallage TP, Paisantham P, Surachetpong W, Mongkolsuk S, Sirikanchana K. Prospects of Phage DJ6712 and FW6709 in Biocontrol of Aeromonas veronii in Fish Aquaculture. Microorganisms. 2025; 13(11):2503. https://doi.org/10.3390/microorganisms13112503
Chicago/Turabian StyleGallage, Tharindu Pollwatta, Phongsawat Paisantham, Win Surachetpong, Skorn Mongkolsuk, and Kwanrawee Sirikanchana. 2025. "Prospects of Phage DJ6712 and FW6709 in Biocontrol of Aeromonas veronii in Fish Aquaculture" Microorganisms 13, no. 11: 2503. https://doi.org/10.3390/microorganisms13112503
APA StyleGallage, T. P., Paisantham, P., Surachetpong, W., Mongkolsuk, S., & Sirikanchana, K. (2025). Prospects of Phage DJ6712 and FW6709 in Biocontrol of Aeromonas veronii in Fish Aquaculture. Microorganisms, 13(11), 2503. https://doi.org/10.3390/microorganisms13112503








