Rare emm6.10 Streptococcus pyogenes Causing an Unusual Invasive Infection in a Child: Clinical and Genomic Insights
Abstract
1. Introduction
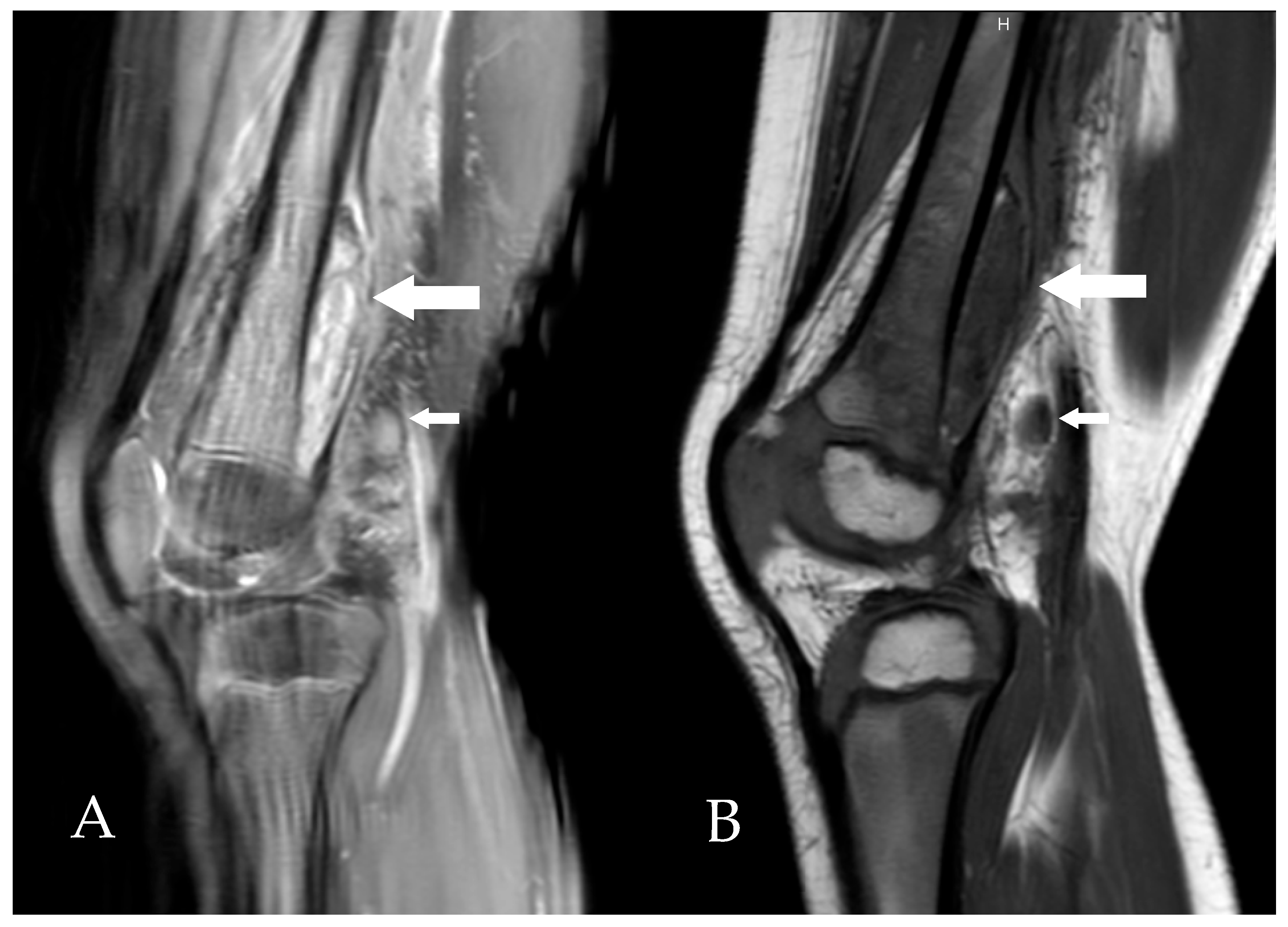
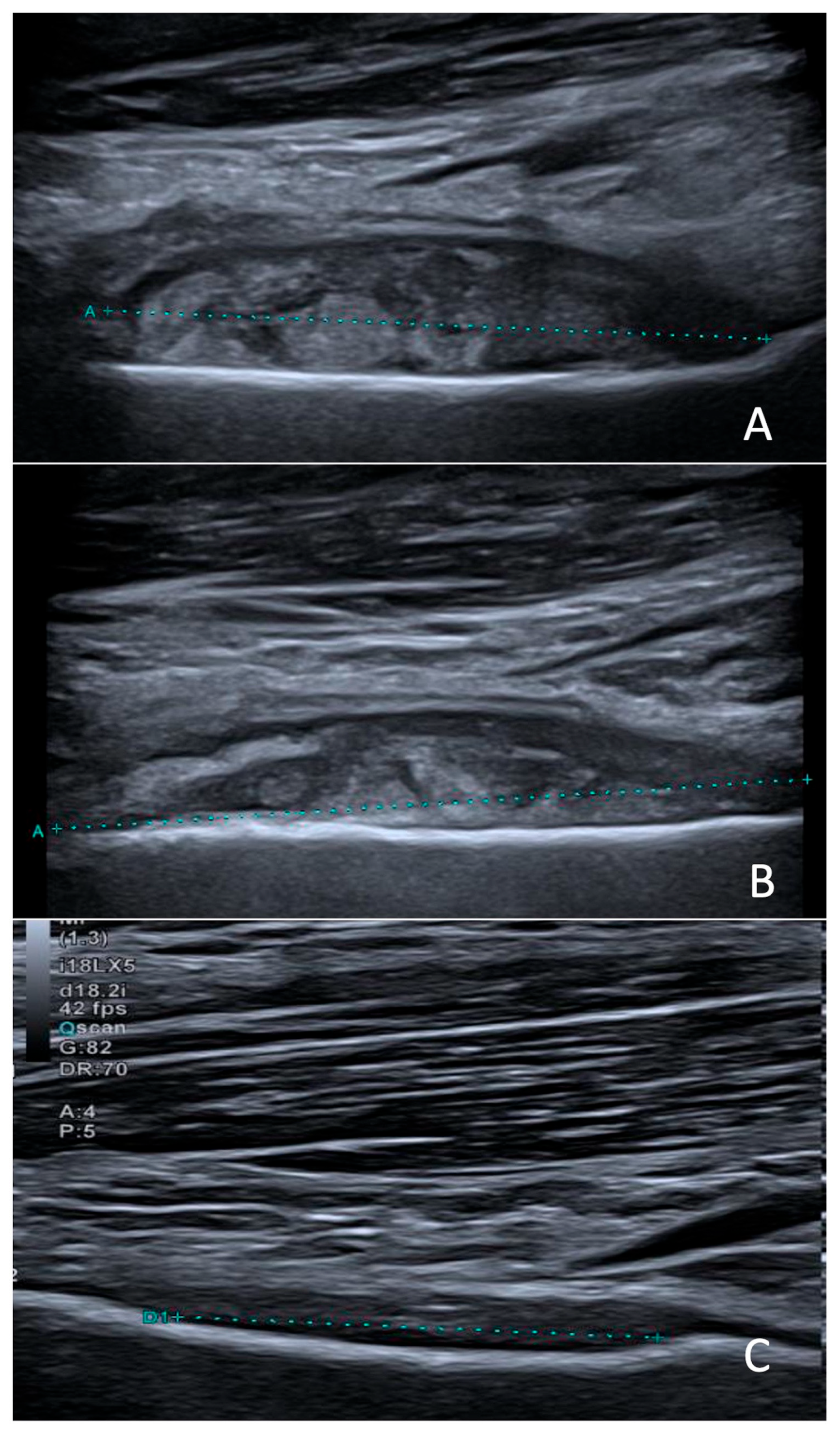
2. Clinical Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flamant, A.; Demirjian, A.; Lamagni, T.; Toubiana, J.; Smeesters, P.R.; Cohen, J.F. Invasive group A streptococcal infections: Lessons learned from the 2022–2023 upsurge. Lancet Infect. Dis. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Sierra Colomina, M.; Flamant, A.; Le Balle, G.; Cohen, J.F.; Berthomieu, L.; Leteurtre, S.; Skov, R.L.; Chalker, V.; Lynskey, N.N.; Ladhani, S.; et al. Severe group A Streptococcus infection among children, France, 2022–2024. Emerg Infect. Dis. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Regional Office for Europe. Increase in Invasive Group A Streptococcal Infections Among Children in Europe, Including Fatalities. 12 December 2022. Available online: https://www.who.int/europe/news/item/12-12-2022-increase-in-invasive-group-a-streptococcal-infections-among-children-in-europe--including-fatalities (accessed on 15 May 2025).
- Smeesters, P.R.; Steer, A.C.; Parks, T.; Henningham, A.; Davies, M.R.; Walker, M.J.; Verhoeven, C.; Botteaux, A.; Steer, A.C. Global Streptococcus pyogenes strain diversity, disease associations, and implications for vaccine development: A systematic review. Lancet Microbe. 2024, 5, e181–e193. [Google Scholar] [CrossRef] [PubMed]
- Steer, A.C.; Carapetis, J.R.; Dale, J.B.; Fraser, J.D.; Good, M.F.; Guilherme, L.; Moreland, N.J.; Mulholland, E.K.; Schodel, F.; Smeesters, P.R.; et al. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 2016, 34, 2953–2958. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Ficari, A.; Romani, L.; De Luca, M.; Tripiciano, C.; Chiurchiù, S.; Carducci, F.I.C.; Cursi, L.; Di Giuseppe, M.; Krzysztofiak, A.; et al. The thousand faces of invasive group A streptococcal infections: Update on epidemiology, symptoms, and therapy. Children 2024, 11, 383. [Google Scholar] [CrossRef]
- Cobo-Vázquez, E.; Aguilera-Alonso, D.; Carrasco-Colom, J.; Calvo, C.; Saavedra-Lozano, J.; Mellado, I.; Grandioso, D.; Rincón, E.; Jové, A.; Cercenado, E.; et al. Increasing incidence and severity of invasive Group A streptococcal disease in Spanish children in 2019–2022. Lancet Reg. Health Eur. 2023, 27, 100597. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, G.M.; Marchisio, P.; Bosi, P.; Castellazzi, M.L.; Lemieux, P. Group A streptococcal infections in pediatric age: Updates about a re-emerging pathogen. Pathogens 2024, 13, 350. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Protocol: Emm Typing Using Conventional PCR and Sequencing. May 2024. Available online: https://www.cdc.gov/strep-lab/media/pdfs/2024/05/emm-Typing-Conventional-PCR-and-Sequencing.pdf (accessed on 17 September 2025).
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Larsen, M.V.; Lund, O.; Villa, L.; Voldby Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Seemann, T. ABRicate: Mass Screening of Contigs for Antimicrobial and Virulence Genes. GitHub. Available online: https://github.com/tseemann/abricate (accessed on 15 September 2025).
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). emm Typing Overview and Guidelines—Group A Streptococcus; CDC: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/strep-lab/php/group-a-strep/emm-typing.html (accessed on 21 October 2025).
- Kiska, D.L.; Thiede, B.; Caracciolo, J.; Jordan, M.; Johnson, D.; Kaplan, E.L.; Gruninger, R.P.; Lohr, J.A.; Gilligan, P.H.; Denny, J.F.W. Invasive group A streptococcal infections in North Carolina: Epidemiology, clinical features, and genetic and serotype analysis of causative organisms. J. Infect. Dis. 1997, 176, 992–1000. [Google Scholar] [CrossRef]
- Villalón, P.; Sáez-Nieto, J.A.; Rubio-López, V.; Medina-Pascual, M.J.; Garrido, N.; Carrasco, G.; Pino-Rosa, S.; Valdezate, S. Invasive Streptococcus pyogenes disease in Spain: A microbiological and epidemiological study covering the period 2007–2019. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Southon, S.B.; Beres, S.B.; Kachroo, P.; Ojeda Saavedra, M.; Erlendsdóttir, H.; Haraldsson, G.; Yerramilli, P.; Pruitt, L.; Zhu, L.; Musser, J.M.; et al. Population genomic molecular epidemiological study of macrolide-resistant Streptococcus pyogenes in Iceland, 1995 to 2016: Identification of a large clonal population with a pbp2x mutation conferring reduced in vitro β-lactam susceptibility. J. Clin. Microbiol. 2020, 58, e00638-20. [Google Scholar] [CrossRef]
- de Crombrugghe, G.; Schiavolin, L.; Osowicki, J.; Steer, A.C.; Botteaux, A.; Smeesters, P.R. M1 and done? Global assessment of the invasive potential of group A streptococcal strains. Lancet Microbe. 2025, 6, 101123. [Google Scholar] [CrossRef]
- Hammond-Collins, K.; Strauss, B.; Barnes, K.; Demczuk, W.; Domingo, M.C.; Lamontagne, M.C.; Lu, D.; Martin, I.; Tepper, M. Group A Streptococcus outbreak in a Canadian Armed Forces training facility. Mil. Med. 2019, 184, e197–e204. [Google Scholar] [CrossRef]
- Friães, A.; Melo-Cristino, J.; Ramirez, M. Changes in emm types and superantigen gene content of Streptococcus pyogenes causing invasive infections in Portugal. Sci. Rep. 2019, 9, 18051. [Google Scholar] [CrossRef]
- Rivera, A.; Rebollo, M.; Sánchez, F.; Navarro, F.; Miró, E.; Mirelis, B.; Coll, P. Characterization of fluoroquinolone-resistant clinical isolates of Streptococcus pyogenes in Barcelona, Spain. Clin. Microbiol. Infect. 2005, 11, 759–761. [Google Scholar] [CrossRef][Green Version]
- Malhotra-Kumar, S.; Van Heirstraeten, L.; Lammens, C.; Chapelle, S.; Goossens, H. Emergence of high-level fluoroquinolone resistance in emm6 Streptococcus pyogenes and in vitro resistance selection with ciprofloxacin, levofloxacin and moxifloxacin. J. Antimicrob. Chemother. 2009, 63, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Barnett, T.; Indraratna, A.; Sanderson-Smith, M. Chapter 13, Secreted Virulence Factors of Streptococcus Pyogenes. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations, 2nd ed.; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 8 October 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK587095/ (accessed on 17 September 2025).
- Sciensano. Report 2012–2024 Streptococci; Sciensano: Brussels, Belgium. Available online: https://www.sciensano.be/sites/default/files/report_2012-2024_streptococci_v1.0.pdf (accessed on 17 September 2025).
- Commons, R.; Rogers, S.; Gooding, T.; Danchin, M.; Carapetis, J.; Robins-Browne, R.; Curtis, N. Superantigen genes in group A streptococcal isolates and their relationship with emm types. J. Med. Microbiol. 2008, 57 Pt 10, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef]
- Lynskey, N.N.; Jauneikaite, E.; Li, H.K.; Zhi, X.; Turner, C.E.; Mosavie, M.; Pearson, M.; Asai, M.; Lobkowicz, L.; Chow, J.Y.; et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: A population-based molecular epidemiological study. Lancet Infect. Dis. 2019, 19, 1209–1218. [Google Scholar] [CrossRef]
- Davies, M.R.; Holden, M.T.G.; Coupland, P.; Chen, J.H.K.; Venturini, C.; Barnett, T.C.; Ben Zakour, N.L.; Tse, H.; Dougan, G.; Yuen, K.-Y.; et al. Emergence of scarlet fever Streptococcus pyogenes emm12 clones in Hong Kong is associated with toxin acquisition and multidrug resistance. Nat. Genet. 2015, 47, 84–87. [Google Scholar] [CrossRef]
- Zangarini, L.; Martiny, D.; Miendje Deyi, V.Y.; Hites, M.; Maillart, E.; Hainaut, M.; Delforge, M.; Botteaux, A.; Matheeussen, V.; Goossens, H.; et al. Incidence and clinical and microbiological features of invasive and probable invasive streptococcal group A infections in children and adults in the Brussels-Capital Region, 2005–2020. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, J.P.; Lin, Q.; Lammens, C.; Smeesters, P.R.; van Kleef-van Koeveringe, S.; Matheeussen, V.; Malhotra-Kumar, S. Increase in bloodstream infections caused by emm1 group A Streptococcus correlates with emergence of toxigenic M1UK, Belgium, May 2022 to August 2023. Euro Surveill. 2023, 28, 2300422. [Google Scholar] [CrossRef] [PubMed]
- Peetermans, M.; Matheeussen, V.; Moerman, C.; De Rydt, F.; Thieren, S.; Pollet, E.; Casaer, M.; De Backer, B.; De Paep, R.; Debaveye, Y.; et al. Clinical and molecular epidemiological features of critically ill patients with invasive group A Streptococcus infections: A Belgian multicenter case-series. Ann. Intensive Care 2024, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, T.; Chujo, T.; Tsuge, M.; Kondo, Y. Paediatric case of group A streptococcal pharyngitis, arthritis and osteomyelitis associated with dental neglect. BMJ Case Rep. 2021, 14, e239196. [Google Scholar] [CrossRef]
- Di Pietro, G.M.; Borzani, I.M.; Aleo, S.; Bosis, S.; Marchisio, P.; Tagliabue, C. Pediatric Septic Arthritis of the Knee Due to a Multi-Sensitive Streptococcus pyogenes Strain Responsive to Clindamycin—A Case Report. Children 2021, 8, 189. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Nadeem, M.; Bandi, S. A rare case of osteomyelitis of the clavicle in a child due to group A streptococcal infection. BMJ Case Rep. 2019, 12, e227090. [Google Scholar] [CrossRef]
- Naik-Mathuria, B.; Ng, G.; Olutoye, O.O. Lytic rib lesion in a 1-year-old child: Group A beta-streptococcal osteomyelitis mimicking tumor. Pediatr Surg Int. 2006, 22, 837–839. [Google Scholar] [CrossRef]
- Trobisch, A.; Schweintzger, N.A.; Kohlfürst, D.S.; Sagmeister, M.G.; Sperl, M.; Grisold, A.J.; Feierl, G.; Herberg, J.A.; Carrol, E.D.; Paulus, S.C.; et al. Osteoarticular infections in pediatric hospitals in Europe: A prospective cohort study from the EUCLIDS consortium. Front. Pediatr. 2022, 10, 744182. [Google Scholar] [CrossRef]
- Walkinshaw, D.R.; Wright, M.E.E.; Mullin, A.E.; Excler, J.L.; Kim, J.H.; Steer, A.C. The Streptococcus pyogenes vaccine landscape. Npj Vaccines 2023, 8, 16. [Google Scholar] [CrossRef] [PubMed]
| Virulence Factor Category | Virulence Genes | Virulence Factor |
|---|---|---|
| Adherence | fbp54, lmb, cpa, sclA, sclB, plr/gapA, scpA/scpB, eno | Fibronectin, laminin, collagen-binding proteins, C5a peptidase |
| Exoenzyme | sda, mf/spd, mf2, mf3, speB, ska, cppA, htrA/degP, slaA, hylA, hylP | Streptodornase, mitogenic factors, Streptokinase, proteases, hyaluronidase |
| Exotoxin | speA, speC, speG, speK, smeZ, speH, slo, sagA, spyA | Streptococcal exotoxins (Spe superantigens), hemolysins (Streptolysin O and Streptolysin S) |
| Immune modulation | ideS/mac, endoS, hasA, hasB, hasC, galU, rfbB | Immune evasion enzymes (IgG protease), hyaluronic acid capsule |
| Nutritional/Metabolic factor | psaA, isdE, shr | PsaA, NEAT-type surface protein direct heme uptake system |
| Stress survival | tig/ropA | Trigger factor involved in stress tolerance |
| Year | Sex | Age | Sample Type | Survival | emm Typing | Other Positive GAS Sites | Clinical Presentation and Associated Infections |
|---|---|---|---|---|---|---|---|
| 2015 | F | 17 mo | Blood | No | 12 | – | Herpetic stomatitis; Purpura fulminans |
| 2016 | M | 12 mo | Blood | Yes | 6 | – | – |
| 2016 | F | 3 y | Blood | Yes | 1 | Pleural | Complicated pneumonia; Family viral illness |
| 2017 | F | 8 mo | Blood | No | 1 | – | Cervical abscess |
| 2018 | M | 15 mo | Blood | Yes | 1 | Throat | Pneumonia |
| 2018 | M | 2 y | Blood | Yes | 6 | – | Ethmoiditis |
| 2018 | F | 5 y | Blood | Yes | 4 | – | Septic osteomyelitis and elbow arthritis |
| 2019 | M | 5 y | Blood | Yes | 6 | – | Cervical adenitis; Hip arthritis |
| 2021 | F | 3 y | Blood | Yes | 87 | – | Pharyngitis; Enterovirus infection |
| 2022 | M | 13 mo | Blood | Yes | 75 | Ear | Mastoiditis; Influenza A infection |
| 2022 | F | 4 y | Blood | No | 1 | Pleural | Toxic shock syndrome; Varicella pneumonia |
| 2022 | F | 17 mo | Blood | Yes | N/A | Ear | Bilateral perforated otitis media |
| 2023 | F | 15 mo | Blood | Yes | 1 | – | Bacteremia without focus |
| 2024 | M | 5 y | Blood | Yes | N/A | Ear | Rhinovirus infection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blairon, L.; Tré-Hardy, M.; Matheeussen, V.; Koster, S.D.; Cassart, M.; Heenen, S.; Nebbioso, A.; Vitali, N. Rare emm6.10 Streptococcus pyogenes Causing an Unusual Invasive Infection in a Child: Clinical and Genomic Insights. Microorganisms 2025, 13, 2475. https://doi.org/10.3390/microorganisms13112475
Blairon L, Tré-Hardy M, Matheeussen V, Koster SD, Cassart M, Heenen S, Nebbioso A, Vitali N. Rare emm6.10 Streptococcus pyogenes Causing an Unusual Invasive Infection in a Child: Clinical and Genomic Insights. Microorganisms. 2025; 13(11):2475. https://doi.org/10.3390/microorganisms13112475
Chicago/Turabian StyleBlairon, Laurent, Marie Tré-Hardy, Veerle Matheeussen, Sien De Koster, Marie Cassart, Sarah Heenen, Andrea Nebbioso, and Nancy Vitali. 2025. "Rare emm6.10 Streptococcus pyogenes Causing an Unusual Invasive Infection in a Child: Clinical and Genomic Insights" Microorganisms 13, no. 11: 2475. https://doi.org/10.3390/microorganisms13112475
APA StyleBlairon, L., Tré-Hardy, M., Matheeussen, V., Koster, S. D., Cassart, M., Heenen, S., Nebbioso, A., & Vitali, N. (2025). Rare emm6.10 Streptococcus pyogenes Causing an Unusual Invasive Infection in a Child: Clinical and Genomic Insights. Microorganisms, 13(11), 2475. https://doi.org/10.3390/microorganisms13112475






