Genetic and Pathogenic Characteristics of Variant Avian Reovirus Strains Isolated from Diseased Chickens in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Animals, and Reagents
2.2. Clinical Samples and Virus Isolation
2.3. Genomic Sequencing of the Isolated ARV Strains
2.4. Pathogenicity of the ARV Isolates in SPF Chicken
2.5. Statistical Analysis
3. Results
3.1. Virus Isolation and Identification
3.2. Sequencing and Analysis of Complete Genomes of the Three ARVs
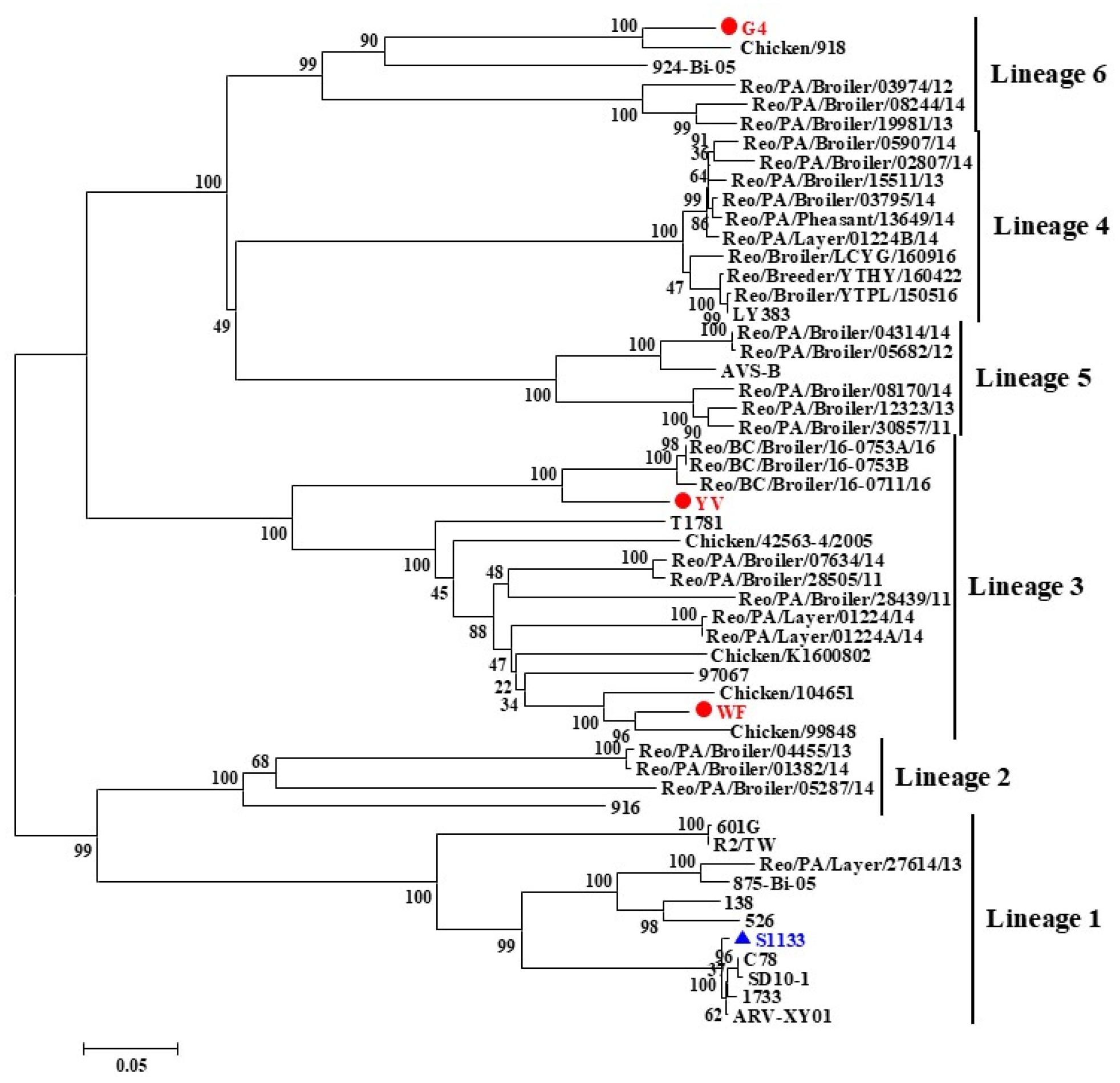
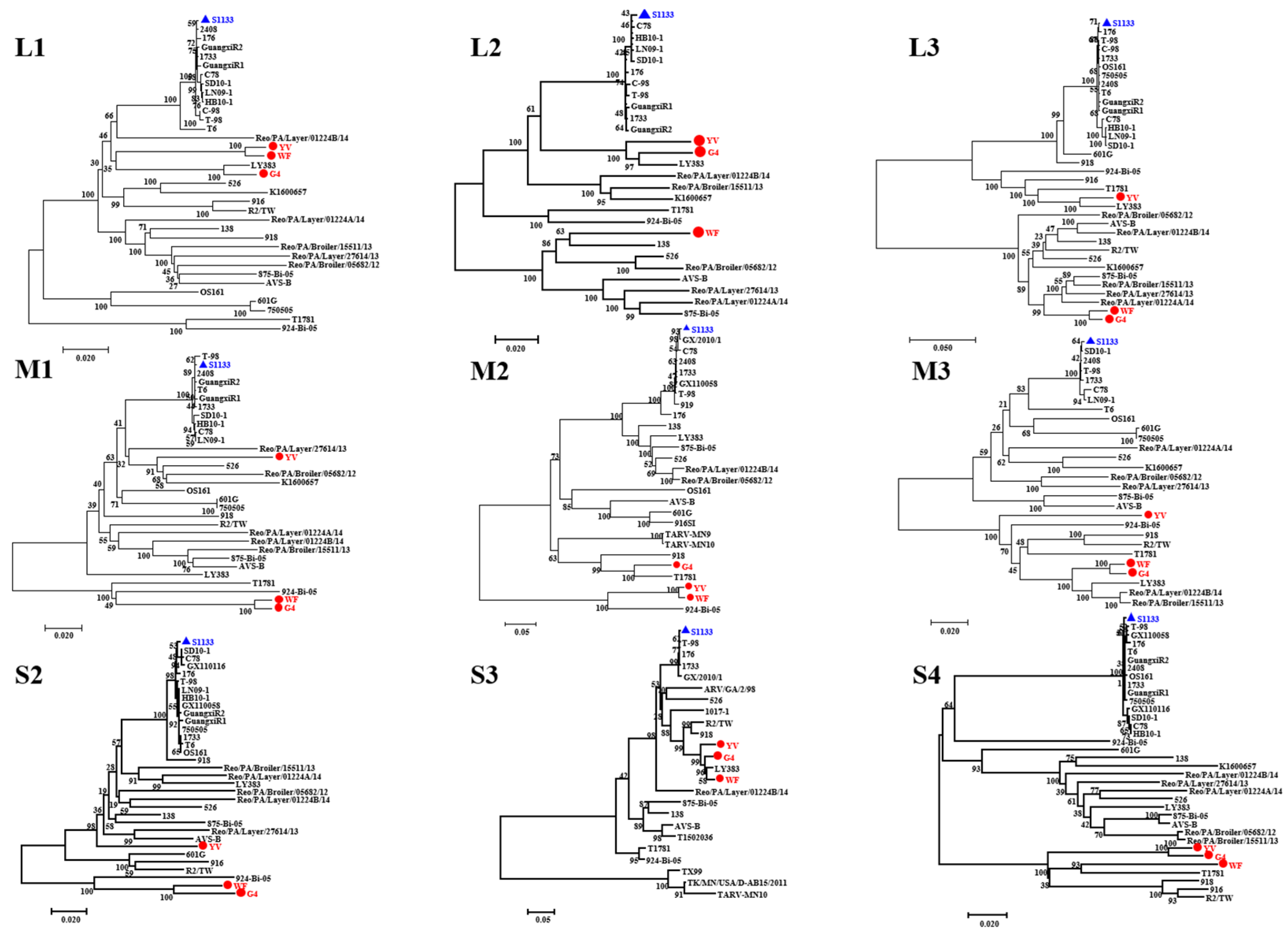
3.3. Phylogenetic Analysis of ARV Genes
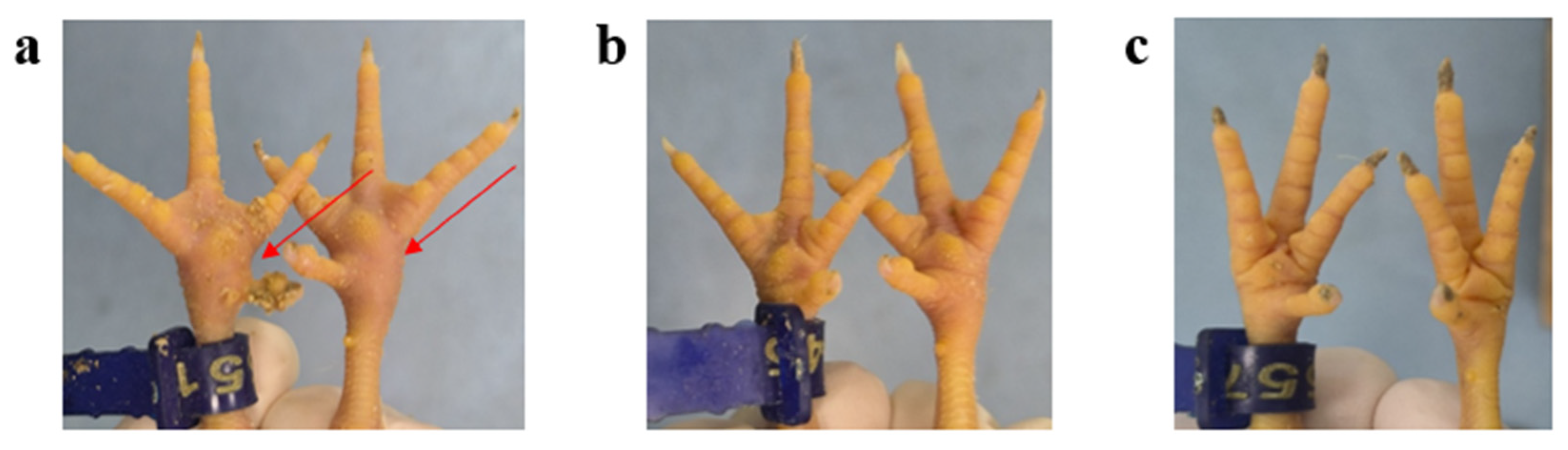
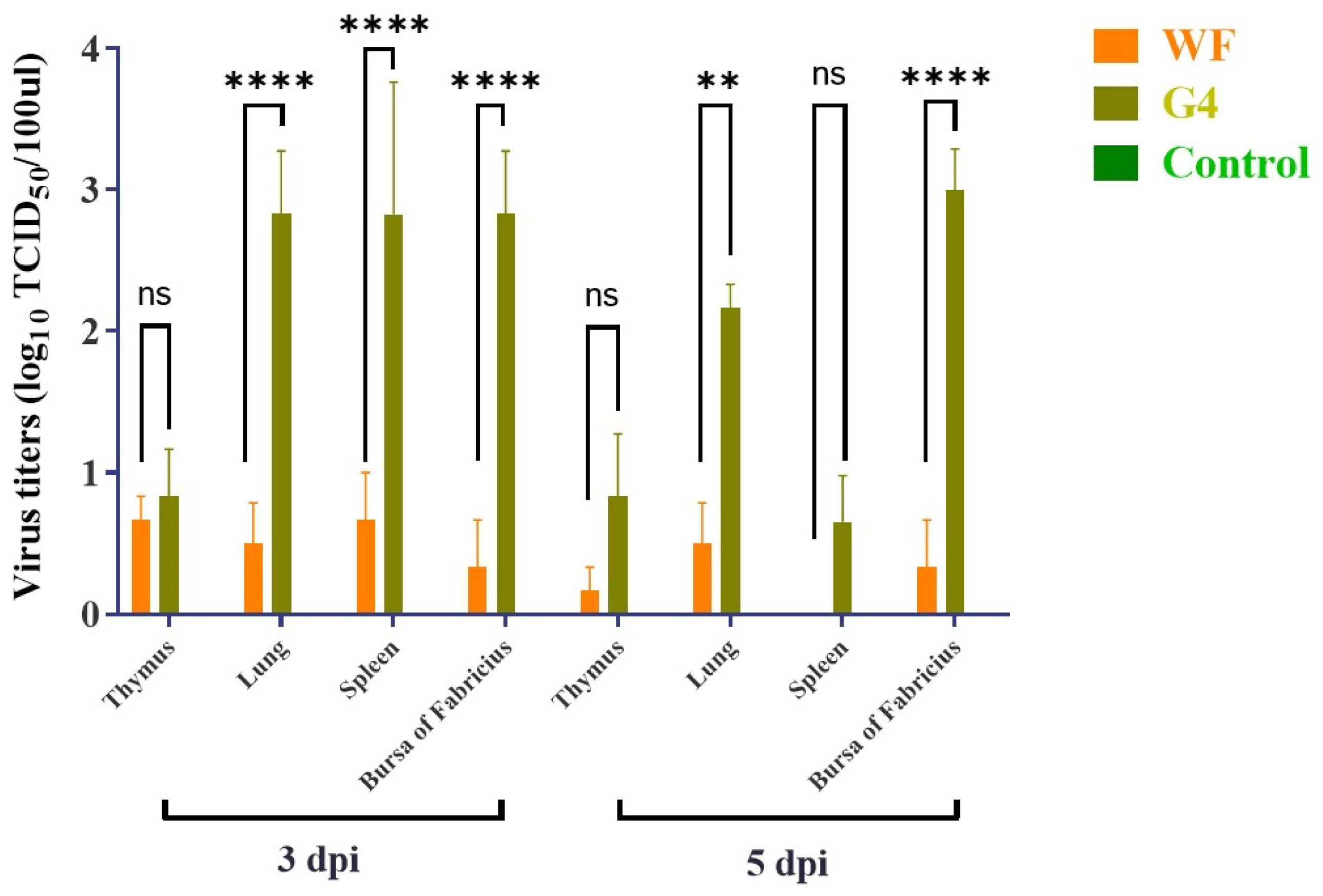
3.4. Pathogenicity of the ARV Isolates in SPF Chickens
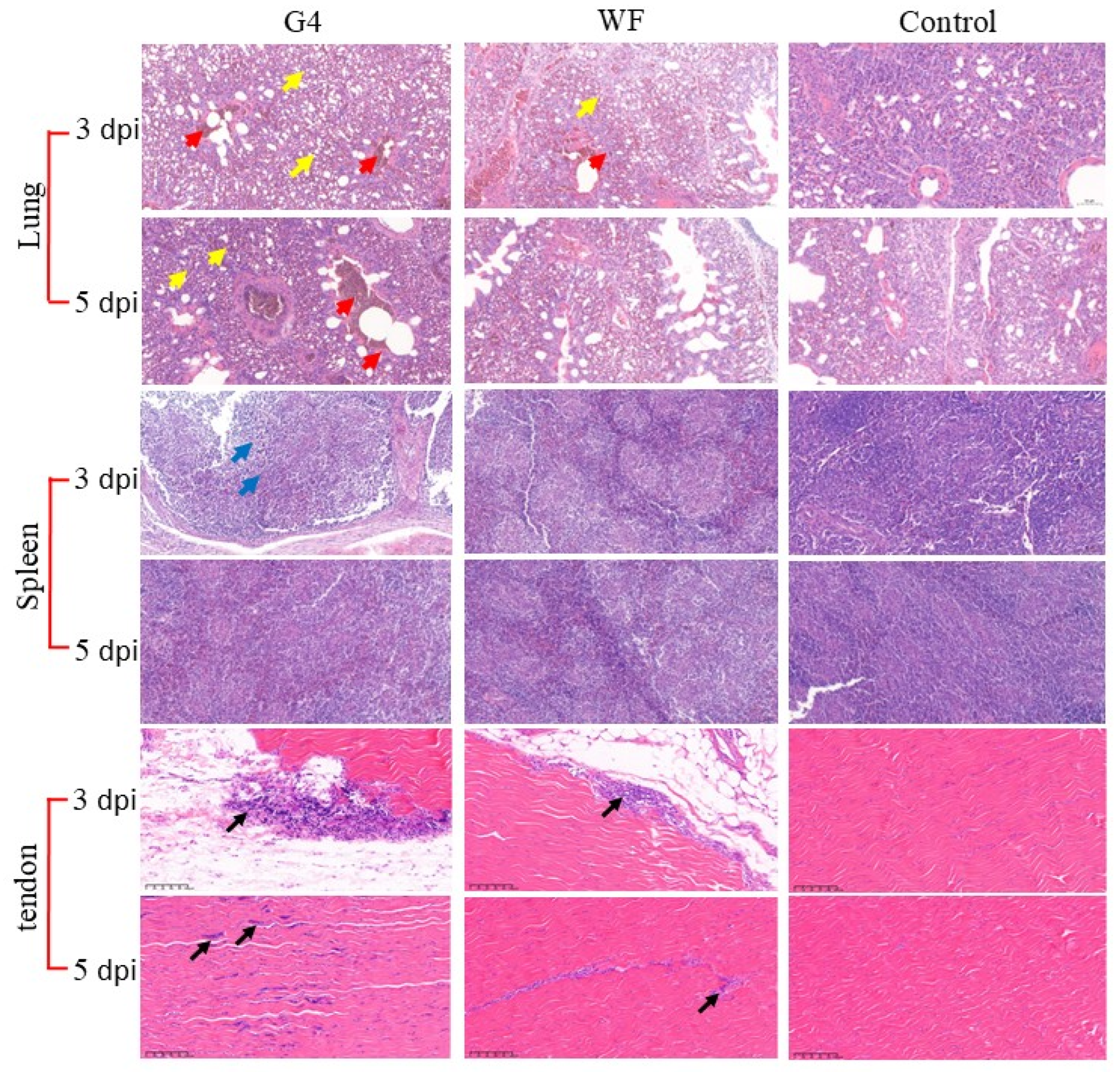
3.5. Histopathological Examination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahey, J.E.; Crawley, J.F. Studies on Chronic Respiratory Disease of Chickens II. Isolation of a Virus. Can. J. Comp. Med. Vet. Sci. 1954, 18, 13–21. [Google Scholar]
- Attoui, H.; Billoir, F.; Biagini, P.; de Micco, P.; de Lamballerie, X. Complete sequence determination and genetic analysis of Banna virus and Kadipiro virus: Proposal for assignment to a new genus (Seadornavirus) within the family Reoviridae. J. Gen. Virol. 2000, 81 Pt 6, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; Friedman, M.H.; Olson, N.O. Electron microscopic study of an avian reovirus that causes arthritis. J. Ultrastruct. Res. 1972, 41, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.D.; Villegas, P.; Kleven, S.H. Role of route of exposure, age, sex, and type of chicken on the pathogenicity of avian reovirus strain 81-176. Avian Dis. 1986, 30, 460–467. [Google Scholar] [CrossRef]
- Van Der Heide, L. Viral arthritis/tenosynovitis: A review. Avian Pathol. 1977, 6, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Pomeroy, B.S. Isolation and characterization of an enterovirus from baby chicks having an enteric infection II. Physical and chemical characteristics and ultrastructure. Avian Dis. 1967, 11, 9–14. [Google Scholar] [CrossRef]
- van Loon, A.A.; Koopman, H.C.; Kosman, W.; Mumczur, J.; Szeleszczuk, O.; Karpinska, E.; Kosowska, G.; Lütticken, D. Isolation of a new serotype of avian reovirus associated with malabsorption syndrome in chickens. Vet. Q. 2001, 23, 129–133. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Davis, J.F.; McNulty, M.S.; Brown, J.; Player, E.C. Enteritis (so-called runting stunting syndrome) in Georgia broiler chicks. Avian Dis. 1993, 37, 451–458. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, S.J. The Roles of MicroRNAs (miRNAs) in Avian Response to Viral Infection and Pathogenesis of Avian Immunosuppressive Diseases. Int. J. Mol. Sci. 2019, 20, 5454. [Google Scholar] [CrossRef]
- Spandidos, D.A.; Graham, A.F. Physical and chemical characterization of an avian reovirus. J. Virol. 1976, 19, 968–976. [Google Scholar] [CrossRef]
- Benavente, J.; Martínez-Costas, J. Early steps in avian reovirus morphogenesis. Curr. Top. Microbiol. Immunol. 2006, 309, 67–85. [Google Scholar] [PubMed]
- Martinez-Costas, J.; Grande, A.; Varela, R.; Garcia-Martinez, C.; Benavente, J. Protein architecture of avian reovirus S1133 and identification of the cell attachment protein. J. Virol. 1997, 71, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Lee, L.H.; Hsu, H.W.; Kuo, L.C.; Liao, M.H. Molecular evolution of avian reovirus: Evidence for genetic diversity and reassortment of the S-class genome segments and multiple cocirculating lineages. Virology 2003, 314, 336–349. [Google Scholar] [CrossRef]
- Petek, M.; Felluga, B.; Borghi, G.; Baroni, A. The Crawley agent: An avian reovirus. Arch. Gesamte Virusforsch. 1967, 21, 413–424. [Google Scholar] [CrossRef]
- Farkas, S.L.; Marton, S.; Dandár, E.; Kugler, R.; Gál, B.; Jakab, F.; Bálint, Á.; Kecskeméti, S.; Bányai, K. Lineage diversification, homo- and heterologous reassortment and recombination shape the evolution of chicken orthoreoviruses. Sci. Rep. 2016, 6, 36960. [Google Scholar] [CrossRef]
- Hellal Kort, Y.; Bourogâa, H.; Gribaa, L.; Scott-Algara, D.; Ghram, A. Molecular characterization of avian reovirus isolates in Tunisia. Virol. J. 2013, 10, 12. [Google Scholar] [CrossRef]
- De Carli, S.; Wolf, J.M.; Gräf, T.; Lehmann, F.K.M.; Fonseca, A.S.K.; Canal, C.W.; Lunge, V.R.; Ikuta, N. Genotypic characterization and molecular evolution of avian reovirus in poultry flocks from Brazil. Avian Pathol. 2020, 49, 611–620. [Google Scholar] [CrossRef]
- Lu, H.; Tang, Y.; Dunn, P.A.; Wallner-Pendleton, E.A.; Lin, L.; Knoll, E.A. Isolation and molecular characterization of newly emerging avian reovirus variants and novel strains in Pennsylvania, USA, 2011–2014. Sci. Rep. 2015, 5, 14727. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Ueda, H.; Kikuchi, N.; Kirisawa, R. Isolation and genomic characterization of a novel orthoreovirus from a brown-eared bulbul (Hypsipetes amaurotis) in Japan. J. Gen. Virol. 2015, 96 Pt 7, 1777–1786. [Google Scholar] [CrossRef]
- Vibin, J.; Chamings, A.; Klaassen, M.; Alexandersen, S. Metagenomic characterisation of additional and novel avian viruses from Australian wild ducks. Sci. Rep. 2020, 10, 22284. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H.; Sebastian, A.; Yeh, Y.T.; Praul, C.A.; Albert, I.U.; Zheng, S.Y. Genomic characterization of a turkey reovirus field strain by Next-Generation Sequencing. Infect. Genet. Evol. 2015, 32, 313–321. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H. Genomic characterization of a broiler reovirus field strain detected in Pennsylvania. Infect. Genet. Evol. 2015, 31, 177–182. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, H. Genomic characterization of a novel avian arthritis orthoreovirus variant by next-generation sequencing. Arch. Virol. 2015, 160, 2629–2632. [Google Scholar] [CrossRef]
- Markis, M. Evaluation of Pathogenicity and Antigenicity of Avian Reoviruses and Disease Control Through Vaccination. Avian Dis. 2022, 66, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, S.A.; Harrell, T.L.; Oluwayinka, O.; Sellers, H.S.; Khalid, Z.; Hauck, R.; Chowdhury, E.U.; Conrad, S.J. Optimizing the Conditions for Whole-Genome Sequencing of Avian Reoviruses. Viruses 2023, 15, 1938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, X.; Ma, L.; Wu, J.; Bao, E. Genetic and pathogenic characteristics of newly emerging avian reovirus from infected chickens with clinical arthritis in China. Poult. Sci. 2019, 98, 5321–5329. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, L.E.; Gupta, A.; Fricke, J.; Ahmed, K.A.; Popowich, S.; Lockerbie, B.; Tikoo, S.K.; Ojkic, D.; Gomis, S. Phenotypic, genotypic and antigenic characterization of emerging avian reoviruses isolated from clinical cases of arthritis in broilers in Saskatchewan, Canada. Sci. Rep. 2017, 7, 3565. [Google Scholar] [CrossRef]
- Vera-Hernández, P.F.; Morales-Garzón, A.; Cortés-Espinosa, D.V.; Galiote-Flores, A.; García-Barrera, L.J.; Rodríguez-Galindo, E.T.; Toscano-Contreras, A.; Lucio-Decanini, E.; Absalón, A.E. Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol. 2016, 45, 73–81. [Google Scholar] [CrossRef]
- Sanger, F.; Coulson, A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975, 94, 441–448. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, Z.; He, D.; Wu, B.; Wei, F.; Li, G.; Wu, Q.; Tang, Y.; Diao, Y. Genetic and pathogenic characteristics of two novel/recombinant avian orthoreovirus. Vet. Microbiol. 2022, 275, 109601. [Google Scholar] [CrossRef]
- Liang, Z.; Guo, J.; Yuan, S.; Cheng, Q.; Zhang, X.; Liu, Z.; Wang, C.; Li, Z.; Hou, B.; Huang, S.; et al. Pathological and Molecular Characterization of a Duck Plague Outbreak in Southern China in 2021. Animals 2022, 12, 3523. [Google Scholar] [CrossRef]
- Kugler, R.; Dandár, E.; Fehér, E.; Jakab, F.; Mató, T.; Palya, V.; Bányai, K.; Farkas, S.L. Phylogenetic analysis of a novel reassortant orthoreovirus strain detected in partridge (Perdix perdix). Virus Res. 2016, 215, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, S.A.; Hosseini, H.; Ghalyanchilangeroudi, A. Molecular characterization of avian reovirus causing tenosynovitis outbreaks in broiler flocks, Iran. Avian Pathol. 2020, 49, 15–20. [Google Scholar] [CrossRef]
- Mase, M.; Gotou, M.; Inoue, D.; Masuda, T.; Watanabe, S.; Iseki, H. Genetic Analysis of Avian Reovirus Isolated from Chickens in Japan. Avian Dis. 2021, 65, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Choi, Y.R.; Park, J.Y.; Wei, B.; Shang, K.; Zhang, J.F.; Jang, H.K.; Cha, S.Y.; Kang, M. Isolation and Genomic Characterization of Avian Reovirus From Wild Birds in South Korea. Front. Vet. Sci. 2022, 9, 794934. [Google Scholar] [CrossRef]
- Liu, L.; Lu, X.; Guo, X.; Gong, X.; Hu, F.; Jiang, Y.; Gao, Y.; Ma, X.; Li, Y.; Huang, B.; et al. Phylogenetic Analysis and Pathogenicity of Avian Reoviruses Isolated from Viral Arthritis Cases in China 2010–2024. Vet. Sci. 2025, 12, 307. [Google Scholar] [CrossRef]
- Shapouri, M.R.; Arella, M.; Silim, A. Evidence for the multimeric nature and cell binding ability of avian reovirus sigma 3 protein. J. Gen. Virol. 1996, 77 Pt 6, 1203–1210. [Google Scholar] [CrossRef]
- Wickramasinghe, R.; Meanger, J.; Enriquez, C.E.; Wilcox, G.E. Avian reovirus proteins associated with neutralization of virus infectivity. Virology 1993, 194, 688–696. [Google Scholar] [CrossRef]
- Shabbir, M.Z.; Yu, H.; Lighty, M.E.; Dunn, P.A.; Wallner-Pendleton, E.A.; Lu, H. Diagnostic investigation of avian reovirus field variants circulating in broiler chickens in Pennsylvania of United States between 2017 and 2022. Avian Pathol. 2024, 53, 400–407. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Faustini, G.; Poletto, F.; Baston, R.; Cecchinato, M.; Legnardi, M. Reconstruction of Avian Reovirus History and Dispersal Patterns: A Phylodynamic Study. Viruses 2024, 16, 796. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, L.E.; Ahmed, K.A.; Mekuria, Z.H.; Lockerbie, B.; Popowich, S.; Tikoo, S.K.; Ojkic, D.; Gomis, S. The dynamics of molecular evolution of emerging avian reoviruses through accumulation of point mutations and genetic re-assortment. Virus Evol. 2020, 6, veaa025. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Guo, L.; Jiang, X.; Wang, H.; Yao, Z.; Zhu, S.; Diao, Y.; Tang, Y. Discovery of a novel recombinant avian orthoreovirus in China. Vet. Microbiol. 2021, 260, 109094. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, Y.; Yang, J.; Wang, H.; Li, C.; Teng, X.; Tang, Y.; Diao, Y. Genetic characterization and pathogenicity of a divergent broiler-origin orthoreovirus causing arthritis in China. Transbound. Emerg. Dis. 2021, 68, 3552–3562. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Luo, D.; Gao, J.; Li, K.; Liu, C.; Qi, X.; Cui, H.; Zhang, Y.; Wang, S.; Wang, X.; et al. A Novel Variant of Avian Reovirus Is Pathogenic to Vaccinated Chickens. Viruses 2023, 15, 1800. [Google Scholar] [CrossRef]
- Chen, H.; Yan, M.; Tang, Y.; Diao, Y. Pathogenicity and genomic characterization of a novel avian orthoreovius variant isolated from a vaccinated broiler flock in China. Avian Pathol. 2019, 48, 334–342. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Y.; Wu, Q.; Zhao, W.; Wang, Y.; Wang, D.; Lu, H.; Diao, Y.; Li, Y.; Tang, Y. Pathogenicity of avian reovirus variant in the immune organs of broiler chicks. Virus Res. 2025, 353, 199538. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, S.; Wu, Z.; Pan, S.; Ma, T.; Zhang, Y.; Xu, B.; Yan, D.; Teng, Q.; Yuan, C.; Pan, X.; et al. Genetic and Pathogenic Characteristics of Variant Avian Reovirus Strains Isolated from Diseased Chickens in China. Microorganisms 2025, 13, 2450. https://doi.org/10.3390/microorganisms13112450
Niu S, Wu Z, Pan S, Ma T, Zhang Y, Xu B, Yan D, Teng Q, Yuan C, Pan X, et al. Genetic and Pathogenic Characteristics of Variant Avian Reovirus Strains Isolated from Diseased Chickens in China. Microorganisms. 2025; 13(11):2450. https://doi.org/10.3390/microorganisms13112450
Chicago/Turabian StyleNiu, Shiqi, Zihua Wu, Shenghui Pan, Tianxin Ma, Yunxiang Zhang, Bangfeng Xu, Dawei Yan, Qiaoyang Teng, Chunxiu Yuan, Xue Pan, and et al. 2025. "Genetic and Pathogenic Characteristics of Variant Avian Reovirus Strains Isolated from Diseased Chickens in China" Microorganisms 13, no. 11: 2450. https://doi.org/10.3390/microorganisms13112450
APA StyleNiu, S., Wu, Z., Pan, S., Ma, T., Zhang, Y., Xu, B., Yan, D., Teng, Q., Yuan, C., Pan, X., Zhang, Z., Yan, M., Shi, X., Li, Z., & Liu, Q. (2025). Genetic and Pathogenic Characteristics of Variant Avian Reovirus Strains Isolated from Diseased Chickens in China. Microorganisms, 13(11), 2450. https://doi.org/10.3390/microorganisms13112450







