Presence of Microorganisms in the Environment: One Health Approach
Abstract
1. Introduction
2. Bacteria
2.1. Zoonotic Bacterial Infections
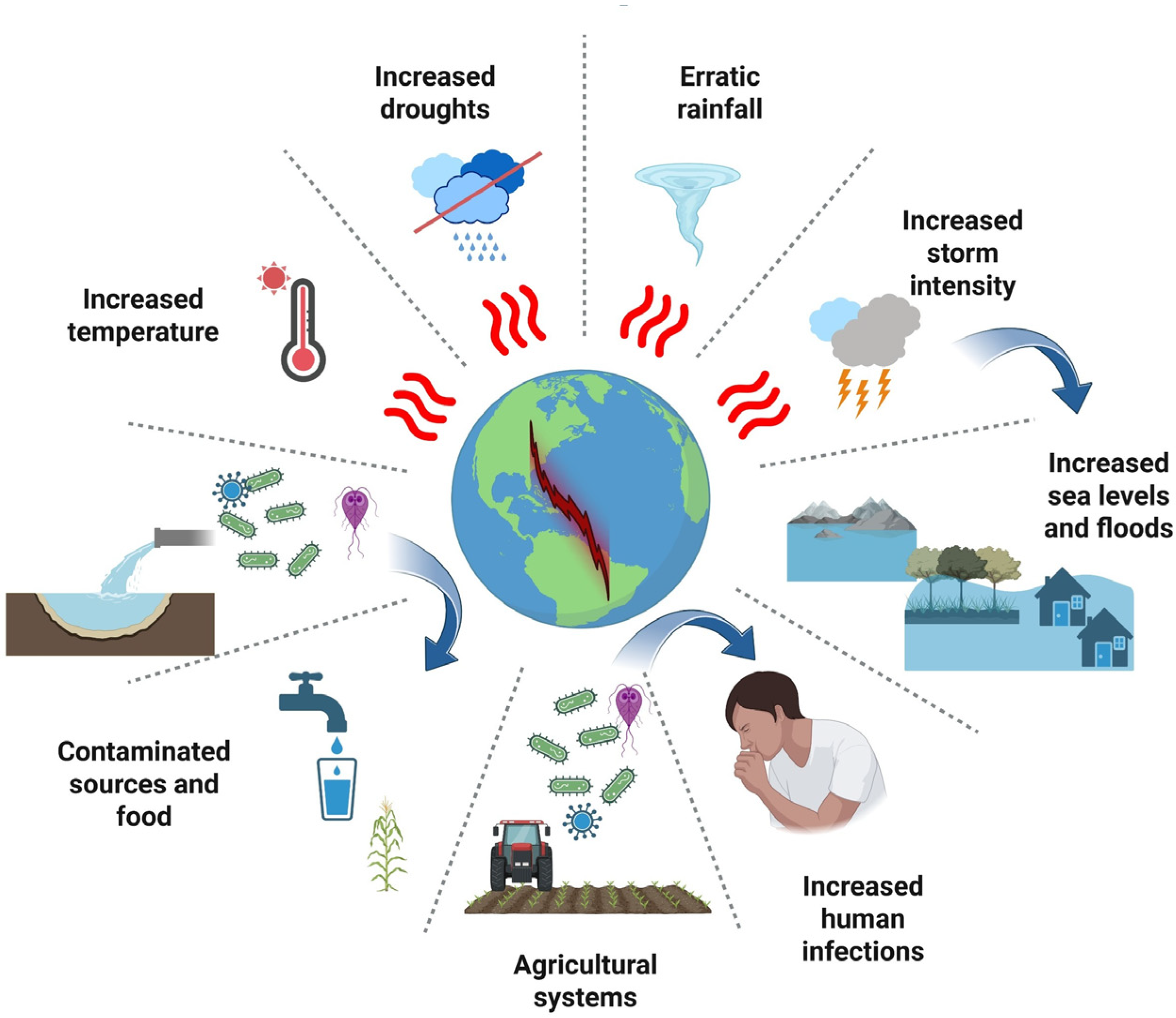
2.2. Effect of the Environment on Bacteria Infections
2.3. Antibiotic Resistance
3. Fungal
3.1. Fungal Infections
3.2. Zoonotic Mycoses
3.3. Effect of the Environment in Fungal Infections
3.4. Surveillance and Diagnostic Challenges in One Health Implementation
3.5. Antifungal Resistant
4. Parasites
4.1. Parasitic Infections
4.2. Parasitic Zoonoses
4.3. Foodborne Parasitic Diseases
4.4. Effect of the Environment in Parasitic Infections
4.5. Antiparasitic Drug Resistance
5. Viruses
5.1. Viral Zoonoses
5.2. Effect of the Environment in Viral Infections
5.3. Antiviral Resistance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Tripartite and UNEP Support OHHLEP’s Definition of “One Health”. Available online: https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health (accessed on 20 June 2025).
- Mackenzie, J.S.; Jeggo, M. The One Health Approach—Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef]
- Weiss, R.A.; Sankaran, N. Emergence of Epidemic Diseases: Zoonoses and Other Origins. Fac. Rev. 2022, 11, 2. [Google Scholar] [CrossRef]
- WOAH. The Importance of the One Health Approach in Tackling Emerging and Re-emerging Zoonotic Epidemics and Pandemics; The animal health perspective; Policy Brief; World Organization for Animal Health (WOAH): Paris, France, 2024. [Google Scholar] [CrossRef]
- Pandey, A.; Mideo, N.; Platt, T.G. Virulence Evolution of Pathogens That Can Grow in Reservoir Environments. Am. Nat. 2022, 199, 141–158. [Google Scholar] [CrossRef]
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991374. [Google Scholar] [CrossRef]
- Kusi, J.; Ojewole, C.O.; Ojewole, A.E.; Nwi-Mozu, I. Antimicrobial Resistance Development Pathways in Surface Waters and Public Health Implications. Antibiotics 2022, 11, 821. [Google Scholar] [CrossRef]
- Cole, J.; Desphande, J. Poultry farming, climate change, and drivers of antimicrobial resistance in India. Lancet Planet. Health 2019, 3, e494–e495. [Google Scholar] [CrossRef]
- Singh, R.; Kim, K. Environmental occurrence of antibiotic resistance, control measures and challenges in finding therapeutic management. Emerg. Contam. 2025, 11, 100440. [Google Scholar] [CrossRef]
- Larson, E. Community factors in the development of antibiotic resistance. Annu. Rev. Public Health 2007, 28, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Zaragoza, M.; Rodriguez-Preciado, S.Y.; Hernández-Ventura, L.; Ortiz-Covarrubias, A.; Castellanos-García, G.; Sifuentes-Franco, S.; Pereira-Suárez, A.L.; Muñoz-Valle, J.F.; Montoya-Buelna, M.; Macias-Barragan, J. Evaluation of Antibiotic Resistance in Escherichia coli Isolated from a Watershed Section of Ameca River in Mexico. Microbiol. Res. 2025, 16, 186. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Tokuda, M.; Shintani, M. Microbial Evolution Through Horizontal Gene Transfer by Mobile Genetic Elements. Microb. Biotechnol. 2024, 17, e14408. [Google Scholar] [CrossRef]
- McKeegan, K.S.; Borges-Walmsley, M.I.; Walmsley, A.R. Microbial and Viral Drug Resistance Mechanisms. Trends Microbiol. 2002, 10, S8–S14. [Google Scholar] [CrossRef]
- Picot, S.; Beugnet, F.; Leboucher, G.; Bienvenu, A.L. Drug Resistant Parasites and Fungi from a One-Health Perspective: A Global Concern That Needs Transdisciplinary Stewardship Programs. One Health 2022, 14, 100368. [Google Scholar] [CrossRef]
- Cantas, L.; Suer, K. Review: The Important Bacterial Zoonoses in “One Health” Concept. Front. Public Health 2014, 2, 144. [Google Scholar] [CrossRef] [PubMed]
- Azimi, T.; Pourmand, M.R.; Fallah, F.; Karimi, A.; Mansour-Ghanaie, R.; Hoseini-Alfatemi, S.M.; Shirdoust, M.; Azimi, L. Serosurvey and Molecular Detection of the Main Zoonotic Parasites Carried by Commensal Rattus norvegicus Population in Tehran, Iran. Trop. Med. Health 2020, 48, 60. [Google Scholar] [CrossRef]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk Factors for Human Disease Emergence. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Lampel, K.A.; Formal, S.B.; Maurelli, A.T. A Brief History of Shigella. EcoSal Plus 2018, 8, 1110–1128. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella Isolates from the Global Enteric Multicenter Study Inform Vaccine Development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of Zoonotic Pathogens and Characterization of Novel Viruses Carried by Commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [PubMed]
- Sabour, S.; Azimi, T.; Nasser, A.; Hadi, N.; Mohsenzadeh, A.; Shariati, A. A Global Overview of the Most Important Zoonotic Bacteria Pathogens Transmitted from Rattus norvegicus to Humans in Urban Environments. Infect. Med. 2022, 1, 192–207. [Google Scholar] [CrossRef]
- Eisenberg, T.; Ewers, C.; Rau, J.; Akimkin, V.; Nicklas, W. Approved and Novel Strategies in Diagnostics of Rat Bite Fever and Other Streptobacillus Infections in Humans and Animals. Virulence 2016, 7, 630–648. [Google Scholar] [CrossRef]
- Rascalou, G.; Pontier, D.; Menu, F.; Gourbière, S. Emergence and Prevalence of Human Vector-Borne Diseases in Sink Vector Populations. PLoS ONE 2012, 7, e36858. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- de Thoisy, B.; Richard-Hansen, C.; Goguillon, B.; Joubert, P.; Obstancias, J.; Winterton, P.; Brosse, S. Rapid Evaluation of Threats to Biodiversity: Human Footprint Score and Large Vertebrate Species Responses in French Guiana. Biodivers. Conserv. 2010, 19, 1567–1584. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Marín, M.; López-Olvera, J.R.; Ayats, T.; Fernandez Aguilar, X.; Lavín, S.; Mentaberre, G.; Cerdà-Cuéllar, M. Zoonotic Campylobacter spp. and Salmonella spp. Carried by Wild Boars in a Metropolitan Area: Occurrence, Antimicrobial Susceptibility and Public Health Relevance. Sci. Total Environ. 2022, 822, 153444. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Scallan Walter, E.J.; Cui, Z.; Tierney, R.; Griffin, P.M.; Hoekstra, R.M.; Payne, D.C.; Rose, E.B.; Devine, C.; Namwase, A.S.; Mirza, S.A.; et al. Foodborne Illness Acquired in the United States—Major Pathogens, 2019. Emerg. Infect. Dis. 2025, 31, 669–677. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2011. EFSA J. 2013, 11, 3129. [Google Scholar] [CrossRef]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; et al. Epidemic Profile of Shiga-Toxin-Producing Escherichia coli O104:H4 Outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Al Noman, Z.; Tasnim, S.; Masud, R.I.; Anika, T.T.; Islam, M.S.; Rahman, A.M.M.T.; Rahman, M.T. A Systematic Review on Reverse-Zoonosis: Global Impact and Changes in Transmission Patterns. J. Adv. Vet. Anim. Res. 2024, 11, 601–617. [Google Scholar] [CrossRef]
- Soon, J.M.; Seaman, P.; Baines, R.N. Escherichia coli O104:H4 outbreak from sprouted seeds. Int. J. Hyg. Environ. Health 2013, 216, 346–354. [Google Scholar] [CrossRef]
- Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Martak, D.; Henriot, C.P.; Hocquet, D. Environment, Animals, and Food as Reservoirs of Antibiotic-Resistant Bacteria for Humans: One Health or More? Infect. Dis. Now 2024, 54, 104895. [Google Scholar] [CrossRef] [PubMed]
- Julian, T.R.; Pickering, A.J. A Pilot Study on Integrating Videography and Environmental Microbial Sampling to Model Fecal Bacterial Exposures in Peri-Urban Tanzania. PLoS ONE 2015, 10, e0136158. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding Human Health Risks Caused by Antibiotic Resistant Bacteria (ARB) and Antibiotic Resistance Genes (ARG) in Water Environments: Current Knowledge and Questions to Be Answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Lebaron, P.; Cournoyer, B.; Lemarchand, K.; Nazaret, S.; Servais, P. Environmental and Human Pathogenic Microorganisms. In Environmental Microbiology: Fundamentals and Applications: Microbial Ecology; Springer: Dordrecht, The Netherlands, 2014; pp. 619–658. [Google Scholar] [CrossRef]
- Abdul, S.; Adeghe, E.P.; Adegoke, B.O.; Adegoke, A.A.; Udedeh, E.H. Public-Private Partnerships in Health Sector Innovation: Lessons from Around the World. Magna Sci. Adv. Biol. Pharm. 2024, 12, 045–059. [Google Scholar] [CrossRef]
- Onen, O.I.; Aboh, A.A.; Mfam, A.N.; Akor, M.O.; Nweke, C.N.; Osuagwu, A.N. Microbial Diversity: Values and Roles in Ecosystems. Asian J. Biol. 2020, 9, 10–22. [Google Scholar] [CrossRef]
- Sehulster, L.; Chinn, R.Y.; Centers for Disease Control and Prevention(CDC); Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. Recomm. Rep. 2003, 52, 1–42. [Google Scholar]
- Chilambi, G.S.; Nordstrom, H.R.; Evans, D.R.; Ferrolino, J.A.; Hayden, R.T.; Marón, G.M.; Vo, A.N.; Gilmore, M.S.; Wolf, J.; Rosch, J.W.; et al. Evolution of Vancomycin-Resistant Enterococcus faecium during Colonization and Infection in Immunocompromised Pediatric Patients. Proc. Natl. Acad. Sci. USA 2020, 117, 11703–11714. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Hogerwerf, L.; Borlée, F.; Still, K.; Heederik, D.; van Rotterdam, B.; de Bruin, A.; Nielen, M.; Wouters, I.M. Detection of Coxiella burnetii DNA in Inhalable Airborne Dust Samples from Goat Farms after Mandatory Culling. Appl. Environ. Microbiol. 2012, 78, 5410–5412. [Google Scholar] [CrossRef]
- Fan, L.; Chen, C.; Zhang, H.; Zeng, Y.; Li, T.; Gao, R.; Li, J.; Ren, Y.; Wu, Z.; Bi, F.; et al. Atmospheric Detection, Prevalence, Transmission, Health and Ecological Consequences of Antibiotic Resistance Genes and Resistant Bacteria: A Comprehensive Review. Emerg. Contam. 2025, 11, 100514. [Google Scholar] [CrossRef]
- Gamage, S.D.; Ambrose, M.; Kralovic, S.M.; Roselle, G.A. Water Safety and Legionella in Health Care: Priorities, Policy, and Practice. Infect. Dis. Clin. N. Am. 2016, 30, 689–712. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhang, T. Detecting Human Bacterial Pathogens in Wastewater Treatment Plants by a High-Throughput Shotgun Sequencing Technique. Environ. Sci. Technol. 2013, 47, 5433–5441. [Google Scholar] [CrossRef]
- Stevik, T.K.; Aa, K.; Ausland, G.; Hanssen, J.F. Retention and Removal of Pathogenic Bacteria in Wastewater Percolating Through Porous Media: A Review. Water Res. 2004, 38, 1355–1367. [Google Scholar] [CrossRef]
- Makovcova, J.; Slany, M.; Babak, V.; Slana, I.; Kralik, P. The Water Environment as a Source of Potentially Pathogenic Mycobacteria. J. Water Health 2014, 12, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Nadzir, M.M. Multi-Drug Resistant ESKAPE Pathogens and the Uses of Plants as Their Antimicrobial Agents. Arch. Microbiol. 2023, 205, 115. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Smets, W.; Moretti, S.; Denys, S.; Lebeer, S. Airborne Bacteria in the Atmosphere: Presence, Purpose, and Potential. Atmos. Environ. 2016, 139, 214–221. [Google Scholar] [CrossRef]
- Ng, L.S.; Teh, W.T.; Ng, S.K.; Eng, L.C.; Tan, T.Y. Bacterial Contamination of Hands and the Environment in a Microbiology Laboratory. J. Hosp. Infect. 2011, 78, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Gerba, C.P. Environmentally Transmitted Pathogens. In Environmental Microbiology, 2nd ed.; Maier, R.M., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 445–484. [Google Scholar] [CrossRef]
- Fernández Salgueiro, M.; Cernuda Martínez, J.A.; Gan, R.K.; Arcos González, P. Climate Change and Antibiotic Resistance: A Scoping Review. Environ. Microbiol. Rep. 2024, 16, e70008. [Google Scholar] [CrossRef]
- Custer, G.F.; Bresciani, L.; Dini-Andreote, F. Ecological and Evolutionary Implications of Microbial Dispersal. Front. Microbiol. 2022, 13, 855859. [Google Scholar] [CrossRef]
- Aleruchi, O.; Ogbuleka, N.A.C.; Ogbonna, S.I.; Robinson, V.K.; Ajie, P.C.; Harold, I. Impact of Agricultural and Urban Runoff on Waterborne Pathogen Contamination. Eur. J. Sci. Res. Rev. 2025, 2, 104. [Google Scholar] [CrossRef]
- Acosta-España, J.D.; Romero-Alvarez, D.; Luna, C.; Rodriguez-Morales, A.J. Infectious Disease Outbreaks in the Wake of Natural Flood Disasters: Global Patterns and Local Implications. Infez. Med. 2024, 32, 451–462. [Google Scholar] [CrossRef]
- Semenza, J.C.; Ko, A.I. Waterborne Diseases That Are Sensitive to Climate Variability and Climate Change. N. Engl. J. Med. 2023, 389, 2175–2187. [Google Scholar] [CrossRef]
- McLellan, S.L.; Hollis, E.J.; Depas, M.M.; Van Dyke, M.; Harris, J.; Scopel, C.O. Distribution and Fate of Escherichia coli in Lake Michigan Following Contamination with Urban Stormwater and Combined Sewer Overflows. J. Great Lakes Res. 2007, 33, 566–580. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, Y.; Li, P.; Sun, J.; Jiang, Y.; Pan, Z.; Li, Y.Z.; Zhang, Z. Anthropogenic Activity and Climate Change Exacerbate the Spread of Pathogenic Bacteria in the Environment. Sci. Adv. 2025, 11, eads4355. [Google Scholar] [CrossRef]
- Manaia, C.M.; Vaz-Moreira, I.; Nunes, O.C. Antibiotic Resistance in Waste Water and Surface Water and Human Health Implications. In Emerging Organic Contaminants and Human Health; Barceló, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 173–212. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510, Erratum in Front. Cell. Infect. Microbiol. 2024, 14, 1488430. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling Antibiotic Resistance: The Environmental Framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.; Laxminarayan, R.; Saam, M.; van Belkum, A.; Pittet, D. World Healthcare-Associated Infections Resistance Forum Participants. Antimicrobial Resistance: One World, One Fight! Antimicrob. Resist. Infect. Control 2015, 4, 49. [Google Scholar] [CrossRef]
- Rybak, B.; Krawczyk, B.; Furmanek-Blaszk, B.; Wysocka, M.; Fordon, M.; Ziolkowski, P.; Meissner, W.; Stepniewska, K.; Sikorska, K. Antibiotic Resistance, Virulence, and Phylogenetic Analysis of Escherichia coli Strains Isolated from Free-Living Birds in Human Habitats. PLoS ONE 2022, 17, e0262236. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and Combating Antibiotic Resistance from One Health and Global Health Perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 20 June 2025).
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.; Dantas, G. The shared antibiotic resistome of soil bacteria and human pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Danko, D.; Bezdan, D.; Afshin, E.E.; Ahsanuddin, S.; Bhattacharya, C.; Butler, D.J.; Chng, K.R.; Donnellan, D.; Hecht, J.; Jackson, K.; et al. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell 2021, 184, 3376–3393.e17. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying Drivers of Antibiotic Resistance in Humans: A Systematic Review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef]
- Martinez, J.L. Bottlenecks in the Transferability of Antibiotic Resistance from Natural Ecosystems to Human Bacterial Pathogens. Front. Microbiol. 2012, 3, 265. [Google Scholar] [CrossRef]
- Torres, R.T.; Fernandes, J.; Carvalho, J.; Cunha, M.V.; Caetano, T.; Mendo, S.; Serrano, E.; Fonseca, C. Wild Boar as a Reservoir of Antimicrobial Resistance. Sci. Total Environ. 2020, 717, 135001. [Google Scholar] [CrossRef]
- Vittecoq, M.; Godreuil, S.; Prugnolle, F.; Durand, P.; Brazier, L.; Renaud, N.; Arnal, A.; Aberkane, S.; Jean-Pierre, H.; Gauthier-Clerc, M.; et al. Antimicrobial Resistance in Wildlife. J. Appl. Ecol. 2016, 53, 519–529. [Google Scholar] [CrossRef]
- Li, X.; Mowlaboccus, S.; Jackson, B.; Cai, C.; Coombs, G.W. Antimicrobial Resistance Among Clinically Significant Bacteria in Wildlife: An Overlooked One Health Concern. Int. J. Antimicrob. Agents 2024, 64, 107251. [Google Scholar] [CrossRef]
- Kumar, M.; Sulfikar Chaminda, T.; Patel, A.K.; Sewwandi, H.; Mazumder, P.; Joshi, M.; Honda, R. Prevalence of antibiotic resistance in the tropical rivers of Sri Lanka and India. Environ. Res. 2020, 188, 109765. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.M.; Cole, L.; Daignault, D.; Desruisseau, A.; et al. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef]
- Sudhakari, P.A.; Ramisetty, B.C.M. Resistome Diversity in Escherichia coli Isolates of Global Wastewaters. Microb. Drug Resist. 2024, 30, 37–49. [Google Scholar] [CrossRef]
- Chavarría-Bencomo, C.; Benavides-Montaño, B.; Mosquera-Rebolledo, A.D. Antimicrobial Resistance Genes in Tick Microbiota: Implications for One Health. Clin. Vet. J. 2023, 6, 198–207. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Withdrawal Periods for Veterinary Medicinal Products Containing Antimicrobial Substances; EMA: London, UK, 2019. [Google Scholar]
- Du, X.; Xia, C.; Shen, J.; Wu, B.; Shen, Z. Characterization of Florfenicol Resistance Among Calf Pathogenic Escherichia coli. FEMS Microbiol. Lett. 2004, 236, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Batuman, O.; Britt-Ugartemendia, K.; Kunwar, S.; Yilmaz, S.; Fessler, L.; Redondo, A.; Chumachenko, K.; Chakravarty, S.; Wade, T. The Use and Impact of Antibiotics in Plant Agriculture: A Review. Phytopathology 2024, 114, 885–909. [Google Scholar] [CrossRef] [PubMed]
- Jestin, A.; Artiges, A.; Hache, C. Foreword: One Health, Sharing Microbes and Antibiotic Resistance. Comptes Rendus Biol. 2024, 346, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, K.D.; Barkema, H.W.; Babujee, A.; Forseille, J.; Naum, K.; Buote, P.; Dalton, D.; Checkley, S.L.; Lehman, K.; Morris, T.; et al. One Health and Antimicrobial Stewardship: Where to Go from Here? Can. Vet. J. 2022, 63, 198–200. [Google Scholar]
- Abia, A.L.K.; Ndama Traoré, A.; Potgieter, N. Editorial: Antimicrobial Resistance and One Health: From Culture to Genomics. Front. Cell. Infect. Microbiol. 2023, 13, 1294241. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 21 July 2025).
- Guo, Z.; Tang, X.; Wang, W.; Luo, Z.; Zeng, Y.; Zhou, N.; Yu, Z.; Wang, D.; Song, B.; Zhou, C.; et al. The Photo-Based Treatment Technology Simultaneously Removes Resistant Bacteria and Resistant Genes from Wastewater. J. Environ. Sci. 2025, 148, 243–262. [Google Scholar] [CrossRef]
- Lin, X.; Xu, J.; Keller, A.A.; He, L.; Gu, Y.; Zheng, W.; Sun, D.; Lu, Z.; Huang, J.; Huang, X.; et al. Occurrence and risk assessment of emerging contaminants in a water reclamation and ecological reuse project. Sci. Total Environ. 2020, 744, 140977. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Wong, K.; Xagoraraki, I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011, 45, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Jäger, T.; Hembach, N.; Elpers, C.; Wieland, A.; Alexander, J.; Hiller, C.; Krauter, G.; Schwartz, T. Reduction of Antibiotic Resistant Bacteria During Conventional and Advanced Wastewater Treatment, and the Disseminated Loads Released to the Environment. Front. Microbiol. 2018, 9, 2599. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Fact Sheet. WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 29 August 2025).
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA—Annual Epidemiological Report 2023. ECDC. Available online: https://www.ecdc.europa.eu/en/publications (accessed on 29 August 2025).
- Fabre, V.; Cosgrove, S.E.; Secaira, C.; Torrez, J.C.T.; Lessa, F.C.; Patel, T.S.; Quiros, R. Antimicrobial Stewardship in Latin America: Past, Present, and Future. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e68. [Google Scholar] [CrossRef]
- Kotay, S.; Chai, W.; Guilford, W.; Barry, K.; Mathers, A.J. Spread from the Sink to the Patient: In Situ Study Using Green Fluorescent Protein (GFP)-Expressing Escherichia coli to Model Bacterial Dispersion from Hand-Washing Sink-Trap Reservoirs. Appl. Environ. Microbiol. 2017, 83, e03327-16. [Google Scholar] [CrossRef]
- Bourigault, C.; Andreo, A.; Mangeant, R.; Le Gallou, F.; Marquot, G.; Demeure Dit Latte, D.; Mahé, P.-J.; Birgand, G.; Bidon, C.; Asehnoune, K.; et al. Hospital Outbreak of NDM-Producing Klebsiella pneumoniae in a Surgical Intensive Care Unit: Sink Traps as the Causing Source of Epidemic Strain Resurgence. Am. J. Infect. Control 2025, 53, 648–651. [Google Scholar] [CrossRef]
- McCallum, G.E.; Hall, J.P.J. The Hospital Sink Drain Microbiome as a Melting Pot for AMR Transmission to Nosocomial Pathogens. NPJ Antimicrob. Resist. 2025, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.C.B.; Epperson, W.B.; Pritchard, R.H. Assessing the Effect of a Single Dose Florfenicol Treatment in Feedlot Cattle on the Antimicrobial Resistance Patterns in Faecal Escherichia coli. Vet. Res. 2005, 36, 723–734. [Google Scholar] [CrossRef][Green Version]
- Aljasham, A.T.; Damra, E.M.; Alkahtani, N.S.; Alouffi, A.; Al Salem, W.S.; Alshabanah, A.O.; Alotaibi, M.; Tanaka, T.; Ali, A.; Almutairi, M.M. Isolation, Identification and Antimicrobial Susceptibility of the Bacteria Isolated from Hyalomma dromedarii Infesting Camels in Al-Jouf Province, Saudi Arabia. Front. Vet. Sci. 2023, 10, 1227908. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254nm Radiation for the Removal of Microorganisms and Antibiotic Resistance Genes from Urban Wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 29 August 2025).
- Chen, S.C.; Chakrabarti, A.; Cornely, O.A.; Meis, J.F.; Perfect, J.R. Informing the World Health Organization Fungal Priority Pathogens List (WHO-FPPL): A collection of systematic reviews. Med. Mycol. 2024, 62, myae046. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. About One Health. Available online: https://www.cdc.gov/one-health/about/index.html (accessed on 29 July 2025).
- Banerjee, S.; Denning, D.W.; Chakrabarti, A. One Health aspects & priority roadmap for fungal diseases: A mini-review. Indian J. Med. Res. 2021, 153, 311–319. [Google Scholar] [CrossRef]
- Segal, E.; Elad, D. Human and zoonotic dermatophytoses: Epidemiological aspects. Front. Microbiol. 2021, 12, 713532. [Google Scholar] [CrossRef]
- Dellière, S.; Jabet, A.; Abdolrasouli, A. Current and emerging issues in dermatophyte infections. PLoS Pathog. 2024, 20, e1012258. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Susmita Talukder, M.; Bakotic, W.L. Global dermatophyte infections linked to human and animal health: A scoping review. Microorganisms 2025, 13, 575. [Google Scholar] [CrossRef]
- Fratti, M.; Bontems, O.; Salamin, K.; Guenova, E.; Monod, M. Prevalence of dermatophyte species in domestic animals and direct mycological examination relevance: A 15-year retrospective study. J. Fungi 2023, 9, 253. [Google Scholar] [CrossRef]
- Piorunek, M.; Kubisiak-Rzepczyk, H.; Dańczak-Pazdrowska, A.; Trafas, T.; Walkowiak, J. Superficial Zoonotic Mycoses in Humans Associated with Cats. J. Fungi 2024, 10, 244. [Google Scholar] [CrossRef]
- Longo, C.L.S.; Hercules, F.M.; Azevedo, F.S.; Ferreira, A.L.P.; Orofino-Costa, R. Tinea corporis caused by Trichophyton benhamiae: Report of the first case transmitted by guinea pig in Brazil. An. Bras. Dermatol. 2024, 99, 475–479. [Google Scholar] [CrossRef] [PubMed]
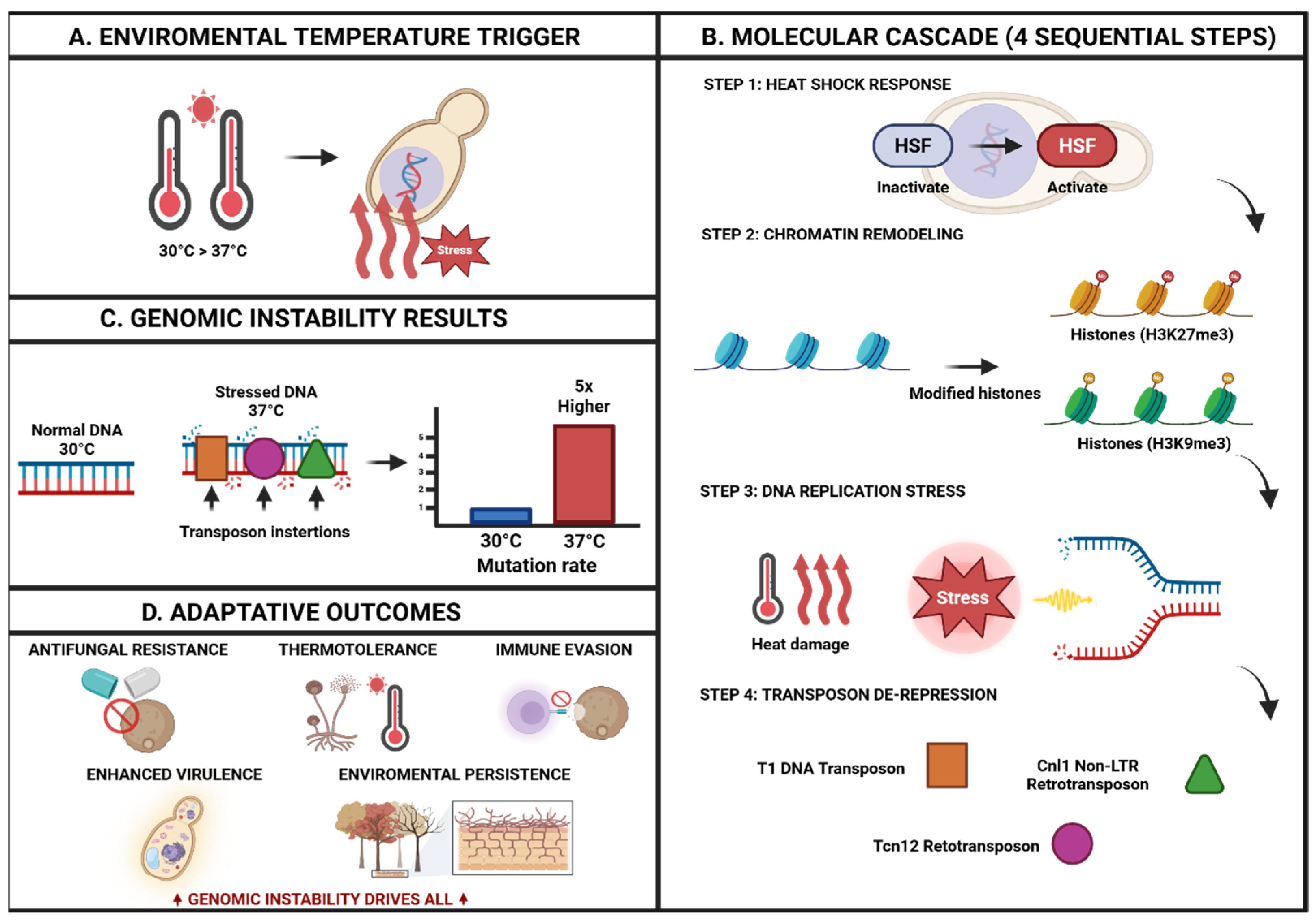
- George, M.E.; Gaitor, T.T.; Cluck, D.B.; Henao-Martínez, A.F.; Sells, N.R.; Chastain, D.B. The impact of climate change on the epidemiology of fungal infections: Implications for diagnosis, treatment, and public health strategies. Ther. Adv. Infect. Dis. 2025, 12, 20499361251313841. [Google Scholar] [CrossRef]
- Gusa, A.; Yadav, V.; Roth, C.; Williams, J.D.; Shouse, E.M.; Magwene, P.; Heitman, J.; Jinks-Robertson, S. Genome-wide analysis of heat stress-stimulated transposon mobility in the human fungal pathogen Cryptococcus deneoformans. Proc. Natl. Acad. Sci. USA 2023, 120, e2209831120. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Surveillance for coccidioidomycosis, histoplasmosis, and blastomycosis-United States, 2019. MMWR Surveill. Summ. 2022, 71, 1–14. [Google Scholar] [CrossRef]
- Mazi, P.B.; Sahrmann, J.M.; Olsen, M.A.; Coler-Reilly, A.; Liang, S.Y.; Powderly, W.G.; Spec, A. The geographic distribution of dimorphic mycoses in the United States for the modern era. Clin. Infect. Dis. 2022, 76, 1295–1301. [Google Scholar] [CrossRef]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of climate change and natural disasters on fungal infections. Lancet Microbe 2024, 5, e594–e605. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; White, P.L.; Kidd, S.E.; Goshia, T.; Fraley, S.I.; Hoenigl, M.; Thompson, G.R. An update on current and novel molecular diagnostics for the diagnosis of invasive fungal infections. Expert Rev. Mol. Diagn. 2023, 23, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Gudisa, R.; Harchand, R.; Rudramurthy, S.M. Nucleic-acid-based molecular fungal diagnostics: A way to a better future. Diagnostics 2024, 14, 520. [Google Scholar] [CrossRef]
- Pham, D.; Sivalingam, V.; Tang, H.M.; Montgomery, J.M.; Chen, S.C.; Halliday, C.L. Molecular diagnostics for invasive fungal diseases: Current and future approaches. J. Fungi 2024, 10, 447. [Google Scholar] [CrossRef]
- Pashootan, N.; Shams-Ghahfarokhi, M.; Chaichi Nusrati, A.; Salehi, Z.; Asmar, M.; Razzaghi-Abyaneh, M. Phylogeny, antifungal susceptibility, and point mutations of SQLE gene in major pathogenic dermatophytes isolated from clinical dermatophytosis. Front. Cell. Infect. Microbiol. 2022, 12, 851769. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Chowdhary, A.; Dannaoui, E. In vitro antifungal combination of terbinafine with itraconazole against isolates of Trichophyton species. Antimicrob. Agents Chemother. 2022, 66, e01449-21. [Google Scholar] [CrossRef]
- Siopi, M.; Efstathiou, I.; Theodoropoulos, K.; Pournaras, S.; Meletiadis, J. Molecular epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: Emergence of terbinafine-resistant Trichophyton mentagrophytes type VIII locally and globally. J. Fungi 2021, 7, 419. [Google Scholar] [CrossRef]
- Noel, Z.A.; Longley, R.; Benucci, G.M.N.; Trail, F.; Chilvers, M.I.; Bonito, G. Non-target impacts of fungicide disturbance on phyllosphere yeasts in conventional and no-till management. ISME Commun. 2022, 2, 19. [Google Scholar] [CrossRef]
- Toda, M.; Beer, K.D.; Kuivila, K.M.; Chiller, T.M.; Jackson, B.R. Trends in agricultural triazole fungicide use in the United States, 1992–2016 and possible implications for antifungal-resistant fungi in human disease. Environ. Health Perspect. 2021, 129, 057001. [Google Scholar] [CrossRef]
- Pham, T.H.T.; Ho, T.T.; Nguyen, N.L. Isolation and characterization of triazole fungicide-degrading bacterial strains and evaluation of their potential for bioremediation and plant growth promotion. J. Microbiol. Biotechnol. 2024, 34, 56–64. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris pan-drug-resistant to four classes of antifungal agents. Antimicrob. Agents Chemother. 2022, 66, e00053-22. [Google Scholar] [CrossRef]
- Zulli, A.; Chan, E.M.G.; Shelden, B.; Duong, D.; Xu, X.S.; White, B.J.; Wolfe, M.K.; Boehm, A.B. Prospective study of Candida auris nucleic acids in wastewater solids in 190 wastewater treatment plants in the United States suggests widespread occurrence. mBio 2024, 15, e00908-24. [Google Scholar] [CrossRef]
- Riera, F.; Cortes Luna, J.; Rabagliatti, R.; Scapellato, P.; Caeiro, J.P.; Chaves Magri, M.M.; Sotomayor, C.E.; Rodrigues Falci, D. Antifungal stewardship: The Latin American experience. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e217. [Google Scholar] [CrossRef]
- Biswas Mondal, S. Antimicrobial stewardship program in perspective to One Health approach. J. Compr. Health 2024, 12, 86–88. [Google Scholar] [CrossRef]
- Ahmed, M. Intestinal Parasitic Infections in 2023. Gastroenterol. Res. 2023, 16, 127–140. [Google Scholar] [CrossRef]
- Abdalal, S.A.; Niyazi, H.A.; Alsulami, S.M.; Azhari, A.A.; Niyazi, H.A.; Mokhtar, J.A.; Attallah, D.M.; Al Braikan, F.A.; Aldarmasi, M.A. Prevalence and Predictors of Intestinal Parasitic Infections at King Abdulaziz University Hospital, Jeddah, Saudi Arabia, from 2019 to 2023: A Retrospective Study. Infect. Drug Resist. 2024, 17, 2793–2801. [Google Scholar] [CrossRef]
- Kaminsky, R.; Mäser, P. Global Impact of Parasitic Infections and the Importance of Parasite Control. Front. Parasitol. 2025, 4, 1546195. [Google Scholar] [CrossRef]
- Pisarski, K. The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Simon, A.; Bachand, N.; Stephen, C. Wildlife Parasites in a One Health World. Trends Parasitol. 2015, 31, 174–180. [Google Scholar] [CrossRef]
- Robertson, L.J.; Utaaker, K.S.; Goyal, K.; Sehgal, R. Keeping Parasitology under the One Health Umbrella. Trends Parasitol. 2014, 30, 369–372. [Google Scholar] [CrossRef]
- Nag, V.L.; Kalita, J.M. Epidemiology of Parasitic Infections. In Textbook of Parasitic Zoonoses. Microbial Zoonoses; Parija, S.C., Chaudhury, A., Eds.; Springer: Singapore, 2022; pp. 69–90. [Google Scholar] [CrossRef]
- Barry, A.E. Grand Challenges in Parasite Epidemiology and Ecology. Front. Parasitol. 2022, 1, 1034819. [Google Scholar] [CrossRef]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar] [CrossRef]
- Weiss, L.M. Zoonotic Parasitic Diseases: Emerging Issues and Problems. Int. J. Parasitol. 2008, 38, 1209–1210. [Google Scholar] [CrossRef]
- Chávez-Ruvalcaba, F.; Chávez-Ruvalcaba, M.I.; Moran Santibañez, K.; Muñoz-Carrillo, J.L.; León Coria, A.; Reyna Martínez, R. Foodborne Parasitic Diseases in the Neotropics—A Review. Helminthologia 2021, 58, 119–133. [Google Scholar] [CrossRef]
- Torgerson, P.R.; de Silva, N.R.; Fèvre, E.M.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Sripa, B.; Gargouri, N.; Willingham, A.L.; Stein, C. The Global Burden of Foodborne Parasitic Diseases: An Update. Trends Parasitol. 2014, 30, 20–26. [Google Scholar] [CrossRef]
- Johansen, M.V.; Trevisan, C.; Braae, U.C.; Magnussen, P.; Ertel, R.L.; Mejer, H.; Saarnak, C.F. The Vicious Worm: A Computer-Based Taenia solium Education Tool. Trends Parasitol. 2014, 30, 372–374. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Scientific Opinion on the public health risks associated with food-borne parasites. EFSA J. 2018, 16, e05495. [Google Scholar] [CrossRef]
- Dorny, P.; Praet, N.; Deckers, N.; Gabriel, S. Emerging food-borne parasites. Vet. Parasitol. 2009, 163, 196–206. [Google Scholar] [CrossRef]
- Slifko, T.R.; Smith, H.V.; Rose, J.B. Emerging parasite zoonoses associated with water and food. Int. J. Parasitol. 2000, 30, 1379–1393. [Google Scholar] [CrossRef]
- Sures, B.; Nachev, M.; Selbach, C.; Marcogliese, D.J. Parasite Responses to Pollution: What We Know and Where We Go in ‘Environmental Parasitology’. Parasites Vectors 2017, 10, 65. [Google Scholar] [CrossRef]
- Gomez de Leon, C.T.; Ostoa-Saloma, P.; Segovia-Mendoza, M.; Del Rio-Araiza, V.H.; Morales-Montor, J. Environmental Parasitology and Its Impact on the Host Neuroimmunoendocrine Network. Front. Biosci. 2021, 26, 431–443. [Google Scholar] [CrossRef]
- Turner, W.C.; Kamath, P.L.; van Heerden, H.; Huang, Y.H.; Barandongo, Z.R.; Bruce, S.A.; Kausrud, K. The Roles of Environmental Variation and Parasite Survival in Virulence-Transmission Relationships. R. Soc. Open Sci. 2021, 8, 210088. [Google Scholar] [CrossRef]
- Ramadan, M.E. The Growing Global Health Threat of Parasitic Infections Due to Climate Change. Int. J. Med. Parasitol. Epidemiol. Sci. 2024, 5, 104–105. [Google Scholar] [CrossRef]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of Environmental Change on Emerging Parasitic Diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- Smith, H.V. Detection of Parasites in the Environment. Parasitology 1998, 117, S113–S141. [Google Scholar] [CrossRef]
- Cui, L.; Mharakurwa, S.; Ndiaye, D.; Rathod, P.K.; Rosenthal, P.J. Antimalarial Drug Resistance: Literature Review and Activities and Findings of the ICEMR Network. Am. J. Trop. Med. Hyg. 2015, 93 (Suppl. 3), 57–68. [Google Scholar] [CrossRef]
- Kalra, S.P.; Naithani, N.; Mehta, S.R.; Kumar, R. Resistant Malaria: Current Concepts and Therapeutic Strategies. Med. J. Armed Forces India 2002, 58, 228–233. [Google Scholar] [CrossRef]
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery. Infect. Drug Resist. 2020, 13, 4047–4060. [Google Scholar] [CrossRef]
- Argüello-García, R.; Cruz-Soto, M.; Romero-Montoya, L.; Ortega-Pierres, G. In Vitro Resistance to 5-Nitroimidazoles and Benzimidazoles in Giardia duodenalis: Variability and Variation in Gene Expression. Infect. Genet. Evol. 2009, 9, 1057–1064. [Google Scholar] [CrossRef]
- Geerts, S.; Gryseels, B. Drug Resistance in Human Helminths: Current Situation and Lessons from Livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Understanding Zoonotic Diseases: A Global Health Challenge. Available online: https://www.fao.org/one-health/highlights/understanding-zoonotic-diseases/en (accessed on 7 July 2025).
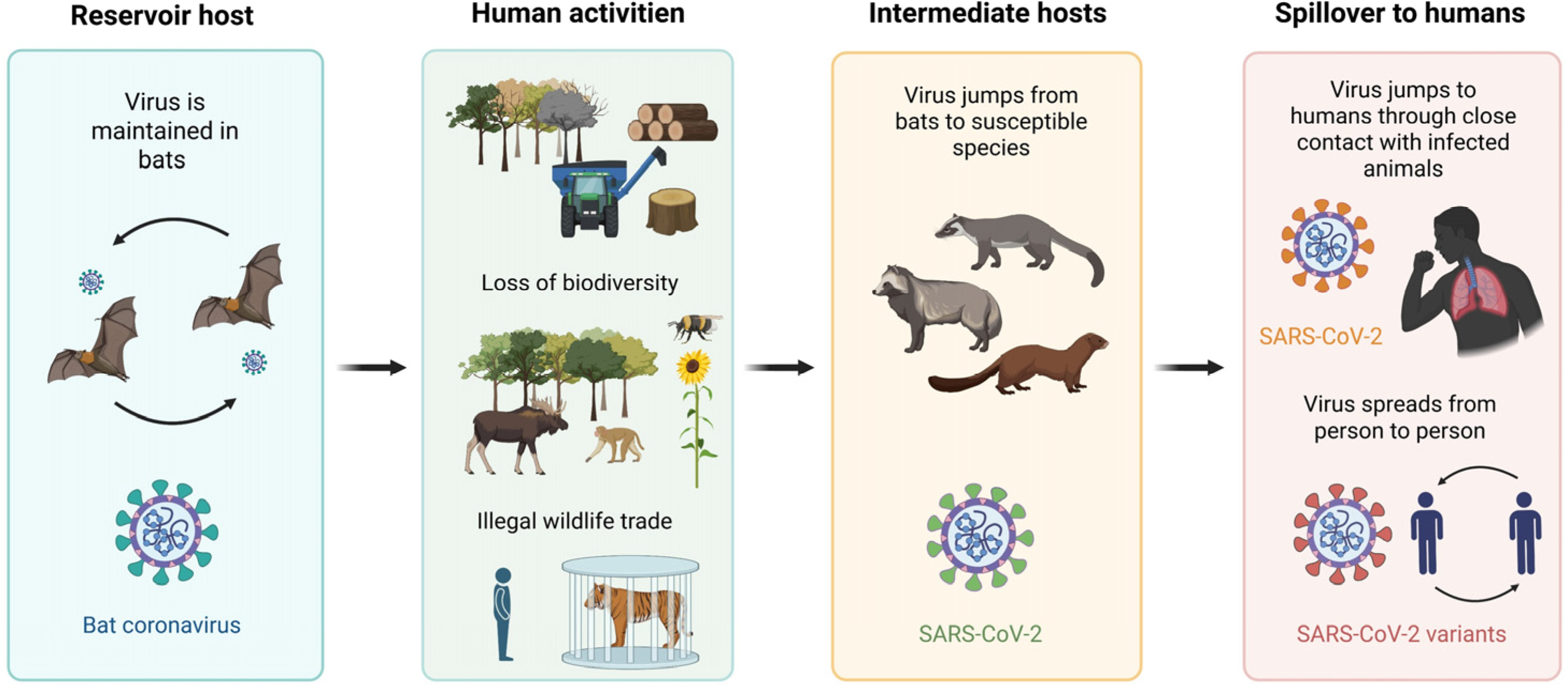
- Tajudeen, Y.A.; Oladunjoye, I.O.; Bajinka, O.; Oladipo, H.J. Zoonotic Spillover in an Era of Rapid Deforestation of Tropical Areas and Unprecedented Wildlife Trafficking: Into the Wild. Challenges 2022, 13, 41. [Google Scholar] [CrossRef]
- Klestova, Z. Possible Spread of SARS-CoV-2 in Domestic and Wild Animals and Body Temperature Role. Virus Res. 2023, 327, 199066. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaton, B.T. Bats, civets and the emergence of SARS. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 315, pp. 325–344. [Google Scholar] [CrossRef]
- Mohd, H.A.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016, 13, 87. [Google Scholar] [CrossRef]
- Hoffmann, M.; Nehlmeier, I.; Brinkmann, C.; Krähling, V.; Behner, L.; Moldenhauer, A.S.; Krüger, N.; Nehls, J.; Schindler, M.; Hoenen, T.; et al. Tetherin inhibits Nipah virus but not Ebola virus replication in fruit bat cells. J. Virol. 2019, 93, e01821-18. [Google Scholar] [CrossRef]
- Baudel, H.; De Nys, H.; Mpoudi Ngole, E.; Peeters, M.; Desclaux, A. Understanding Ebola virus and other zoonotic transmission risks through human-bat contacts: Exploratory study on knowledge, attitudes and practices in Southern Cameroon. Zoonoses Public Health 2019, 66, 288–295. [Google Scholar] [CrossRef]
- Schnitzler, S.U.; Schnitzler, P. An update on swine-origin influenza virus A/H1N1: A review. Virus Genes 2009, 39, 279–292. [Google Scholar] [CrossRef]
- Galindo-González, J. Live animal markets: Identifying the origins of emerging infectious diseases. Curr. Opin. Environ. Sci. Health 2022, 25, 100310. [Google Scholar] [CrossRef]
- Escudero-Pérez, B.; Lalande, A.; Mathieu, C.; Lawrence, P. Host-pathogen interactions influencing zoonotic spillover potential and transmission in humans. Viruses 2023, 15, 599. [Google Scholar] [CrossRef]
- Tian, J.; Sun, J.; Li, D.; Wang, N.; Wang, L.; Zhang, C.; Meng, X.; Ji, X.; Suchard, M.A.; Zhang, X.; et al. Emerging viruses: Cross-species transmission of coronaviruses, filoviruses, henipaviruses, and rotaviruses from bats. Cell Rep. 2022, 39, 110969. [Google Scholar] [CrossRef]
- Aleebrahim-Dehkordi, E.; Soveyzi, F.; Deravi, N.; Rabbani, Z.; Saghazadeh, A.; Rezaei, N. Human coronaviruses SARS-CoV, MERS-CoV, and SARS-CoV-2 in children. J. Pediatr. Nurs. 2021, 56, 70–79. [Google Scholar] [CrossRef]
- Sajini, A.A.; Alkayyal, A.A.; Mubaraki, F.A. The recombination potential between SARS-CoV-2 and MERS-CoV from cross-species spill-over infections. J. Epidemiol. Glob. Health 2021, 11, 155–159. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.A.L.; Andrade, A.C.D.S.P.; Boratto, P.V.M.; Trindade, G.S.; Kroon, E.G.; Abrahão, J.S. An Anthropocentric View of the Virosphere-Host Relationship. Front. Microbiol. 2017, 8, 1673. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Dubovi, E.J. The Nature of Viruses. In Fenner’s Veterinary Virology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–16. [Google Scholar] [CrossRef]
- Prosdocimi, F.; Cortines, J.R.; José, M.V.; Farias, S.T. Decoding Viruses: An Alternative Perspective on Their History, Origins and Role in Nature. BioSystems 2023, 231, 104960. [Google Scholar] [CrossRef]
- Chan, K.H.; Peiris, J.S.; Lam, S.Y.; Poon, L.L.; Yuen, K.Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef] [PubMed]
- Correia, G.; Rodrigues, L.; Afonso, M.; Mota, M.; Oliveira, J.; Soares, R.; Tomás, A.L.; Reichel, A.; Silva, P.M.; Costa, J.J.; et al. SARS-CoV-2 Air and Surface Contamination in Residential Settings. Sci. Rep. 2022, 12, 18058. [Google Scholar] [CrossRef] [PubMed]
- Dargahi, A.; Jeddi, F.; Vosoughi, M.; Karami, C.; Hadisi, A.; Mokhtari, S.A.; Ghobadi, H.; Alighadri, M.; Haghighi, S.B.; Sadeghi, H. Investigation of SARS CoV-2 Virus in Environmental Surface. Environ. Res. 2021, 195, 110765. [Google Scholar] [CrossRef]
- Brittain, O.S.; Wood, H.; Kumar, P. Prioritising Indoor Air Quality in Building Design Can Mitigate Future Airborne Viral Outbreaks. Cities Health 2020, 5 (Suppl. 1), S162–S165. [Google Scholar] [CrossRef]
- Xiao, A.; Wu, F.; Bushman, M.; Zhang, J.; Imakaev, M.; Chai, P.R.; Duvallet, C.; Endo, N.; Erickson, T.B.; Armas, F.; et al. Metrics to Relate COVID-19 Wastewater Data to Clinical Testing Dynamics. Water Res. 2022, 212, 118070. [Google Scholar] [CrossRef]
- Hopkins, L.; Persse, D.; Caton, K.; Ensor, K.; Schneider, R.; McCall, C.; Stadler, L.B. Citywide Wastewater SARS-CoV-2 Levels Strongly Correlated with Multiple Disease Surveillance Indicators and Outcomes over Three COVID-19 Waves. Sci. Total Environ. 2023, 855, 158967. [Google Scholar] [CrossRef]
- Diamond, M.B.; Keshaviah, A.; Bento, A.I.; Conroy-Ben, O.; Driver, E.M.; Ensor, K.B.; Halden, R.U.; Hopkins, L.P.; Kuhn, K.G.; Moe, C.L.; et al. Wastewater Surveillance of Pathogens Can Inform Public Health Responses. Nat. Med. 2022, 28, 1992–1995. [Google Scholar] [CrossRef]
- Reyne, M.I.; Allen, D.M.; Levickas, A.; Allingham, P.; Lock, J.; Fitzgerald, A.; McSparron, C.; Nejad, B.F.; McKinley, J.; Lee, A.; et al. Detection of Human Adenovirus F41 in Wastewater and Its Relationship to Clinical Cases of Acute Hepatitis of Unknown Aetiology. Sci. Total Environ. 2023, 857, 159579. [Google Scholar] [CrossRef] [PubMed]
- Toancha, K.; Borges, A.; Lázaro, L.; Teixeira, N.; Lima, A.K.; Gonçalves, A.; Winter, D.; Santos, A.; do Nascimento, M.; de Sousa, A.B.; et al. Wastewater-Based Surveillance for Hepatitis A Virus, Enterovirus, Poliovirus, and SARS-CoV-2 in São Tomé and Príncipe: A Pilot Study. Sci. Total Environ. 2024, 955, 176923. [Google Scholar] [CrossRef]
- Gazecka, M.; Sniezek, J.; Maciolek, K.; Kowala-Piaskowska, A.; Zmora, P. Mpox Virus Detection in the Wastewater and the Number of Hospitalized Patients in the Poznan Metropolitan Area, Poland. Int. J. Infect. Dis. 2023, 133, 75–77. [Google Scholar] [CrossRef]
- Wolfe, M.K.; Duong, D.; Shelden, B.; Chan, E.M.G.; Chan-Herur, V.; Hilton, S.; Paulos, A.H.; Xu, X.R.S.; Zulli, A.; White, B.J.; et al. Detection of Hemagglutinin H5 Influenza A Virus Sequence in Municipal Wastewater Solids at Wastewater Treatment Plants with Increases in Influenza A in Spring, 2024. Environ. Sci. Technol. Lett. 2024, 11, 526–532. [Google Scholar] [CrossRef]
- Dinata, R.; Baindara, P.; Mandal, S.M. Evolution of Antiviral Drug Resistance in SARS-CoV-2. Viruses 2025, 17, 722. [Google Scholar] [CrossRef]
- Irwin, K.K.; Renzette, N.; Kowalik, T.F.; Jensen, J.D. Antiviral Drug Resistance as an Adaptive Process. Virus Evol. 2016, 2, vew014. [Google Scholar] [CrossRef]
- Jiménez-Pérez, M.; González-Grande, R.; España Contreras, P.; Pinazo Martínez, I.; de la Cruz Lombardo, J.; Olmedo Martín, R. Treatment of Chronic Hepatitis C with Direct-Acting Antivirals: The Role of Resistance. World J. Gastroenterol. 2016, 22, 6573–6581. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, Z.; Tahaghoghi-Hajghorbani, S.; Baesi, K.; Ghazvini, K.; Amel-Jamehdar, S.; Youssefi, M. A Survey of Resistance Mutations to Reverse Transcriptase Inhibitors (RTIs) among HIV-1 Patients in Northeast of Iran. Mol. Biol. Res. Commun. 2024, 13, 117–125. [Google Scholar] [CrossRef]
- Lee, L.Y.; Zhou, J.; Koszalka, P.; Frise, R.; Farrukee, R.; Baba, K.; Miah, S.; Shishido, T.; Galiano, M.; Hashimoto, T.; et al. Evaluating the Fitness of PA/I38T-Substituted Influenza A Viruses with Reduced Baloxavir Susceptibility in a Competitive Mixtures Ferret Model. PLoS Pathog. 2021, 17, e1009527. [Google Scholar] [CrossRef]
- Omoto, S.; Speranzini, V.; Hashimoto, T.; Noshi, T.; Yamaguchi, H.; Kawai, M.; Kawaguchi, K.; Uehara, T.; Shishido, T.; Naito, A.; et al. Characterization of Influenza Virus Variants Induced by Treatment with the Endonuclease Inhibitor Baloxavir Marboxil. Sci. Rep. 2018, 8, 9633. [Google Scholar] [CrossRef] [PubMed]
- Iketani, S.; Mohri, H.; Culbertson, B.; Hong, S.J.; Duan, Y.; Luck, M.I.; Annavajhala, M.K.; Guo, Y.; Sheng, Z.; Uhlemann, A.C.; et al. Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir. Nature 2023, 613, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M. Antivirals against Hepatitis Viruses: Basic Mechanisms. In Comprehensive Guide to Hepatitis Advances; Academic Press: Cambridge, MA, USA, 2023; pp. 137–152. [Google Scholar] [CrossRef]
- Sarrazin, C. The Importance of Resistance to Direct Antiviral Drugs in HCV Infection in Clinical Practice. J. Hepatol. 2016, 64, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Khodadad, N.; Hashempour, A.; Nazar, M.M.K.A.; Ghasabi, F. Evaluating HIV Drug Resistance in the Middle East and North Africa and Its Associated Factors: A Systematic Review. Virol. J. 2025, 22, 112. [Google Scholar] [CrossRef] [PubMed]
| Biological System | Setting/Pathogen | Period/Region | Metric/Trend | Implication | Reference |
|---|---|---|---|---|---|
| Human (global) | GLASS indicators (E. coli 3GC-R; MRSA) | WHO GLASS data (2023) | Global median: 42% E. coli resistant to 3rd-gen cephalosporins; 35% MRSA | High pressure on carbapenems; urgent need for stewardship and surveillance | WHO 2023 [97] |
| Human (EU/EEA) | K. pneumoniae bloodstream infections/carbapenems | EARS-Net 2019–2023 | Incidence of 57.5% since 2019 for carbapenem-resistant K. pneumoniae | Rising trend in Gram-negatives; strengthen hospital infection control | ECDC 2023 [98] |
| Human (Latin America) | ICU: A. baumannii and P. aeruginosa/carbapenems | Regional review (2005–2015) | A. baumannii CR often >50%; P. aeruginosa CR 20–60% | Need to optimize β-lactam use and improve infection control in ICUs | Fabre et al., 2022 [99] |
| Human (hospital environment) | Sinks/drains as reservoirs; K. pneumoniae NDM | 2017–2025 (multiple reports) | NDM outbreaks linked to sink traps and drains | Plumbing redesign and biofilm-targeted cleaning are critical | Kotay et al., 2017 [100]; Bourigault et al., 2025 [101]; McCallum et al., 2025 [102] |
| Poultry | Ceftiofur metaphylaxis—Salmonella Heidelberg 3GC-R | Canada 2003–2008 | Resistance rose from 5% to 35%; withdrawal reduced to 7% | Ban non-therapeutic use; respect withdrawal periods | Dutil et al., 2010 [82] |
| Young cattle | Florfenicol sub-therapeutic dosing—gut resistome | USA 2024 | Shifts in resistome and microbiota profiles | Avoid sub-therapeutic dosing; monitor resistome changes | Berge et al., 2005 [103] |
| Animal–environment (vectors) | Ticks from livestock—ARGs (ESBL, aminoglycosides, etc.) | Saudi Arabia 2023 | ARGs detected in camel tick microbiomes | Arthropods are overlooked AMR conduits | Aljasham et al., 2023 [104] |
| Environment (wastewater) | AOPs (ozone, UV/ozone) against ARGs | Europe 2018 | Ozone: 85–98% ARG reduction; UV + O3: 84–99% | Advanced AOPs effective; dose and by-product risks need management | Jäger et al., 2018 [96] |
| Ozonation/UV254 nm—effectiveness and risks | Portugal 2017 | Substantial ARG removal; risk of transient selection | Optimize exposure times and combine with biofiltration | Sousa et al., 2017 [105] | |
| Environment–urban | Urban sewage—global resistome | Global 2019 | Resistome reflects human antimicrobial use | Wastewater metagenomics as a surveillance tool | Hendriksen et al., 2019 [73] |
| MetaSUB project—urban microbiomes | 60 cities, 2015–2017 | AMR markers vary by city and climate | Spatial mapping for targeted interventions | Danko et al., 2021 [74] |
| Parasite | Parasite |
|---|---|
| Cryptosporidium spp. | Fruit and vegetables: contaminated with feces of animals, contaminated cultivation water, infected handlers during production process, contaminated wash water during packaging and sale Fruit and vegetable juice: contaminated with feces of animals, contaminated water used for dilution, infected handlers during production process Dairy products: contaminated with feces of infected animals during milking, infected handlers during production process Molluscan shellfish: contaminated by seawater during growing, infected handlers during production process Meat: contaminated by feces/intestinal content of infected animals at the abattoir during slaughter, infected handlers, infected surfaces |
| Toxoplasma gondii | Fruit, vegetables and herbs: contaminated with feces of animals, contaminated cultivation water Fruit and vegetable juice: contaminated with feces of infected felids during cultivation of the crop Dairy products: contaminated by the transfer of tachyzoites to milk of lactating infected mammals such as goats Molluscan shellfish: contaminated by seawater during growing, cross contamination during depuration Meat: that is not adequately treated prior to consumption, infected surfaces, infected handlers |
| Echinococcus spp. | Fruit, vegetables and herbs: contaminated with feces of dogs, foxes and other canids during cultivation, contaminated water used for irrigation Fruit and vegetable juice: contaminated with feces of infected felids during cultivation of the crop, contaminated water used for dilution Drinking water |
| Giardia lamblia | Fruits and vegetables: contaminated with feces of animals and humans, contaminated cultivation water Molluscan shellfish: contaminated by seawater during growing Drinking water |
| Fasciola hepatica | Aquatic plants: such as watercress contaminated with feces, contaminated cultivation water Meat: that is not adequately treated prior to consumption, infected handlers, infected surfaces Drinking water |
| Trypanosoma cruzi | Fruit and vegetable juice: contaminated with feces from infected bugs. |
| Paragonimus westermani | Snails and crustacean: infected with metacercariae |
| Diphyllobothrium latum | Salmonid and other freshwater/sea fish: infected with plerocercoid |
| Taenia soilium | Meat: (pig, camel, rabbit, bear) infected with cyst stages of the parasite, infected surfaces, infected handlers |
| Taenia saginata | Meat: (bovine and cervine) infected with cyst stages of the parasite, infected surfaces, infected handlers |
| Cyclospora cayetanensis | Fruit: specially raspberries contaminated with feces, contaminated cultivation water |
| Trichinella spp. | Meat: (pigs, bears, wild boar, warthog, walrus, seal) infected with cyst stages of the parasites, infected surfaces, infected handlers |
| Virus | Gene targets | Analysis Method | Location | Autor |
|---|---|---|---|---|
| SARS-CoV-2 | N1, N2 | RT-qPCR | Boston, MA, USA | Xiao et al. (2022) [184] |
| SARS-CoV-2 | N1, N2 | RT-ddPCR/RT-qPCR | Houston, TX, USA | Hopkins et al. (2023) [185] |
| HAdV-F41 | HAdV-F40/41 | RT-ddPCR/RT-qPCR | Northern Irish, UK | Reyne et al. (2023) [187] |
| SARS-CoV-2 | N1, Env | RT-qPCR | São Tomé, São Tomé and Príncipe | Toancha et al. (2024) [188] |
| Hepatitis A | Target gene unspecified | |||
| Enterovirus | panEV | |||
| Poliovirus | panPV | |||
| Mpox Virus (MPV) | Vi07922155_s1 (TaqMan) | qPCR | Poznan, Polonia | Gazecka et al. (2023) [189] |
| Influenza A Virus (IAV) | M-gene | dd-RT-PCR | Texas, North Carolina, and Hawaii, USA | Wolfe et al. (2024) [190] |
| Influenza A H5 Subtype | H5-gene (Hemagglutinin) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Plascencia, H.H.F.; Colima-Fausto, A.G.; Licona-Lasteros, K.C.; Díaz-Zaragoza, M.; Cazarez-Navarro, G.; Macias-Barragan, J.G.; Rodriguez-Preciado, S.Y. Presence of Microorganisms in the Environment: One Health Approach. Microorganisms 2025, 13, 2435. https://doi.org/10.3390/microorganisms13112435
Ramirez-Plascencia HHF, Colima-Fausto AG, Licona-Lasteros KC, Díaz-Zaragoza M, Cazarez-Navarro G, Macias-Barragan JG, Rodriguez-Preciado SY. Presence of Microorganisms in the Environment: One Health Approach. Microorganisms. 2025; 13(11):2435. https://doi.org/10.3390/microorganisms13112435
Chicago/Turabian StyleRamirez-Plascencia, Helen Haydee Fernanda, Ana Gabriela Colima-Fausto, Karel Cesar Licona-Lasteros, Mariana Díaz-Zaragoza, Gerardo Cazarez-Navarro, Jose Guadalupe Macias-Barragan, and Sergio Yair Rodriguez-Preciado. 2025. "Presence of Microorganisms in the Environment: One Health Approach" Microorganisms 13, no. 11: 2435. https://doi.org/10.3390/microorganisms13112435
APA StyleRamirez-Plascencia, H. H. F., Colima-Fausto, A. G., Licona-Lasteros, K. C., Díaz-Zaragoza, M., Cazarez-Navarro, G., Macias-Barragan, J. G., & Rodriguez-Preciado, S. Y. (2025). Presence of Microorganisms in the Environment: One Health Approach. Microorganisms, 13(11), 2435. https://doi.org/10.3390/microorganisms13112435







