Abstract
Bacterial outer-membrane vesicles (OMVs) mediate stress tolerance, biofilm formation, and interkingdom communication, but their role in beneficial endophytes remains underexplored. We isolated 11 non-redundant isolates associated with Bacillus, Enterococcus, Kosakonia and Kocuria from Agave tequilana seeds, identified by MALDI-TOF MS and 16S rRNA gene sequencing. We focused on the catalase-negative Enterobacter cloacae SEA01, which exhibits plant-promoting traits and support agave growth under nutrient-poor microcosms. In addition, this endophyte produces OMVs. Time-resolved SEM documented OMV release and cell aggregation within 9 h, followed by mature biofilms at 24 h with continued vesiculation. Purified OMVs (≈80–300 nm) contained extracellular DNA and were characterized by dynamic light scattering and UHPLC–ESI–QTOF-MS lipidomics. The OMV lipidome was dominated by phosphatidylethanolamine (~80%) and was enriched in monounsaturated fatty acids (16:1, 18:1), while the stress-associated cyclopropane fatty acids (17:1, 19:1) were comparatively retained in the whole-cell membranes; OMVs also exhibited reduced ubiquinone-8. SEA01 is catalase-negative, uncommon among plant-associated Enterobacter, suggesting a testable model in which oxidative factors modulate OMV output and biofilm assembly. These may have implications for recognition and redox signaling at the root interface. Future works should combine targeted proteomics/genomics with genetic or chemical disruption of catalase/OMV pathways.
1. Introduction
Bacteria have developed a variety of adaptation mechanisms that enable them to survive in different and often hostile environments [1]. The formation of biofilms and the release of outer membrane vesicles (OMVs) have been shown to be crucial for microbial communication, stress tolerance and host interactions [2,3,4]. Bacterial OMVs—nanoscale, membrane-bound particles ranging in size from 20 to 400 nm—are produced by both Gram-negative and Gram-positive bacteria [5,6]. Current models of OMV biogenesis include (i) outer membrane blebbing, triggered by an imbalance in the envelope (periplasmic compaction, weakened OM-peptidoglycan bonds, LPS remodeling), and (ii) outer-internal membrane vesicles (OIMVs), which are formed after phage infection, prophage infiltration or endolysin-mediated/explosive lysis and can encapsulate periplasmic and cytoplasmic cargo [7,8,9]. Proteomic and biochemical analyses have shown that OMVs contain many bacterial components, including LPS, outer membrane proteins/lipoproteins, periplasmic enzymes, peptidoglycan, small RNAs and extracellular DNA [10,11]. Their role in pathogenesis and immunomodulation is increasingly recognized [12,13,14]. In addition, OMVs help to alleviate membrane stress by removing misfolded proteins, damaged peptidoglycan and excess lipopolysaccharides, while supporting detoxification and outer membrane remodeling in the presence of nutrient deficiency or oxidative stress [15,16,17].
First observed in E. coli in the 1960s [18], bacterial OMVs have only recently attracted attention in the context of plant-associated bacteria [19,20]. For example, in Xylella fastidiosa, a plant pathogen responsible for severe diseases in a number of economically important crops, OMVs reduce cell adhesion in xylem vessels and facilitate systemic movement within the host [19]. Furthermore, OMVs can activate plant immune responses [21]. Such discoveries have led to the conceptualization of bacterial extracellular vesiculation as a type-zero secretion system (T0SS) for selective delivery of bacterial cargoes into the environment [22] such as proteins, peptides, DNA- and RNA-binding proteins along with non-coding small RNAs encoded in genomic islands, suggesting a dual ecological role: enhancing natural bacterial competence and adaptation while supporting host immune defense [23]. Despite recent advances, most studies on bacterial OMVs have focused on pathogens, and their occurrence and function in beneficial plant-associated bacteria, particularly endophytes-remain poorly understood. A few studies have reported OMV production in symbiotic bacteria such as Rhizobium etli and Sinorhizobium fredii HH103, where flavonoids and isoflavones such as naringenin and genistein induce OMV secretion. These vesicles have been shown to modulate genes, suppress plant defense responses, and influence root development in legumes [24,25,26]. However, research on OMVs from non-rhizobial endophytes, especially those inhabiting seeds or associated with non-leguminous plants, remains scarce.
Endophytes are a diverse group of microorganisms that inhabit healthy plant tissues and are key components of the plant microbiome [27,28]. They can promote growth, enhance stress tolerance, and reduce the need for chemical inputs in agriculture [29,30]. In agave plants, previous studies have shown that seed-borne endophytes contribute to early colonization and activate redox signaling pathways involved in root development and defense responses [31,32,33,34]. Understanding how these microbes colonize plant tissues and communicate with their hosts is essential to fully harness their potential for sustainable agriculture. In this study, we investigated the endophytic strain Enterobacter cloacae SEA01 a catalase-negative strain isolated from Agave tequilana seeds and selected for its agronomic properties and previous induction of systemic H2O2 production following colonization of agave roots [35]. Here, we characterize the production of outer membrane vesicles (OMVs) and biofilm formation using scanning electron microscopy (SEM), as well as the lipid composition of both OMVs and bacterial cells. We hypothesize that OMVs and biofilms are complementary strategies used by native endophytes during early plant association. These findings lay the groundwork for future applications in crop microbiome engineering [36].
2. Materials and Methods
2.1. Seeds and Plant Material
Mature seeds were collected from Agave tequilana Weber capsules six months after flowering in 2013. For growth experiments, asexual plantlets (bulbils) were collected from a plantation near Atotonilco el Alto, Jalisco, Mexico (20°34′22.71″ N, 102°32′0.0085″ W; 1900 m a.s.l.). Additionally, commercially micropropagated plants were also obtained from Agromod, Mexico (Tapachula, Chiapas, Mexico).
2.2. Isolation of Endophytic Bacteria from A. tequilana Seeds
Agave seeds were surface sterilized by immersion in 3% sodium hypochlorite for 10 min with constant agitation, followed by rinsing with sterile water and immersed in 85% ethanol for 10 min. The seeds were then rinsed three times with sterile water. To confirm successful disinfection, 100 µL of the last rinse water was plated on tryptic soy agar (TSA; BD Bioxon, Naucalpan, Mexico) and incubated at 32 °C for 15 days to monitor microbial growth. Additionally, ten surface-sterilized seeds were also plated directly onto TSA and incubated at 30 °C. The plates were examined daily for colony development. Emerging colonies were isolated, subcultured on fresh TSA plates, and purified. Morphological characteristics were examined by light microscopy and Gram staining was performed.
2.3. Identification of Bacterial Endophytes by MALDI-TOF MS and 16S rRNA Gene Sequencing
Bacterial strains were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using an Autoflex Speed instrument (Bruker Daltonics, Bremen, Germany). Colonies were grown on tryptic soy agar (TSA) at 30 °C for 18 h and processed according to the manufacturer’s protocol. Mass spectra were acquired in linear positive mode over a mass range of 5–20 kDa, using a 35% laser setting and 2000 laser shots per sample. Identification was carried out with MBT Compass Biotyper software 4.1.100 (Bruker; Bremen, Germany), using a reference spectral database. External calibration was performed using Escherichia coli DH5α as the standard.
For molecular identification, genomic DNA was extracted from overnight cultures, and the 16S rRNA gene was amplified using universal primers 27F (5′-AGAGTTTGATYMTGGCTCAG-3′) and 1525R (5′-GGYTACCTTGTTACGACTT-3′). PCR was performed with an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min, with a final extension step at 72 °C for 10 min. Amplicons were verified by agarose gel electrophoresis and sequenced using a 3130 Series Genetic Analyzer (IPICYT, San Luis Potosí, Mexico). Sequences were edited and aligned using Geneious 8.1, then compared to entries in the NCBI GenBank database using BLAST software v2.16.0 for taxonomic identification.
2.4. Phylogenetic Analysis
Sequences were aligned using ClustalX 2.0 [37] with default parameters (gap opening = 10, gap extension = 1, divergence delay = 30%, and BLOSUM matrix). Alignments were visualized and edited in JalView Version 2 [38]. Phylogenetic analysis was conducted in MEGA11 [39] using the Neighbor-Joining (NJ) with 1000 bootstrap replicates to assess branch support [40]. Evolutionary distances were calculated using the Jukes-Cantor substitution model.
2.5. Putative Plant Growth-Promoting Traits: Nitrogen Fixation, Phosphate Solubilization, IAA Production, Siderophore Secretion, and ACC Deaminase Activity
- (a)
- Nitrogen Fixation
The potential nitrogen-fixing ability of strain SEA01 was assessed by culturing it in nitrogen-free semi-solid Nfb-malate medium, as described by Döbereiner et al. [41]. To confirm its ability to fix atmospheric nitrogen, the strain was transferred seven consecutive transfers in Nfb medium.
- (b)
- Indole-3-Acetic Acid (IAA) Production
Indole-3-acetic acid (IAA) production was assessed following the method described by Ullah et al. [42]. Bacteria were cultured for 48 h in tryptic soy broth supplemented with 0.1% L-tryptophan. One milliliter of the centrifuged supernatant was mixed with 2 mL of Salkowski reagent and incubated in the dark for 30 min. The appearance of a pink coloration indicated IAA production.
- (c)
- Phosphate Solubilization
Phosphate solubilization was tested using the plate assay method on Pikovskaya’s agar [43] containing insoluble phosphate sources such as tricalcium phosphate and hydroxyapatite. Spot-inoculated plates were incubated, and the formation of a clear halo around bacterial colonies was considered a positive result.
- (d)
- ACC Deaminase Activity
The ability to produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase was determined using Dworkin and Foster (DF) minimal salt medium supplemented with ACC as the sole nitrogen source [44]. Growth on DF medium was considered indicative of endogenous ACC deaminase activity.
- (e)
- Siderophore Production
Siderophore production was detected using the chrome azurol S (CAS) agar assay [45]. Bacterial colonies were streaked on nutrient agar plates containing 10% CAS reagent and incubated at 30 °C for one week. The appearance of a yellow-to-pink halo around the colonies indicated positive siderophore production.
2.6. Catalase Activity and Native PAGE Electrophoresis
Catalase activity was evaluated by placing a loopful of 16 h colony growth onto a clean glass slide and adding a drop of 30% hydrogen peroxide (H2O2). The immediate formation of oxygen bubbles indicated catalase activity. For protein-level analysis, native PAGE was performed. Crude protein extracts were loaded onto an 8% non-denaturing polyacrylamide gel and electrophoresed at 150 V for 2 h. After electrophoresis, the gel was incubated in 5% methanol with shaking for 5 min, then treated with 10 mM H2O2 for 10 min. Subsequently, the gel was transferred to a ferricyanide-ferric chloride staining solution (0.3 g potassium ferricyanide in 30 mL distilled water +0.336 g ferric chloride in 30 mL distilled water) for 10 min, and finally incubated in 10% acetic acid for 15 min. Clear bands within a blue background indicated catalase activity, based on H2O2 degradation by catalase and inhibition of blue pigment formation [46].
2.7. OMVs Isolation, Purification, and SEM Visualization
E. cloacae SEA01 was cultured in M9 broth adjusted to OD600 = 0.2 (approximately 5.3 × 105 CFU mL−1) and incubated at 35 °C with agitation (200 rpm). To monitor OMV production, samples were collected at 0, 3, 5, 7, 9, 18, 24, and 48 h. For each time point, 50 mL of culture was centrifuged at 2000× g for 20 min at 4 °C. The supernatant was filtered through 0.45 µm and 0.22 µm PVDF membranes (Millipore, Burlington, MA, USA) to remove bacterial cells and debris. OMVs were pelleted by ultracentrifugation at 150,000× g for 70 min at 4 °C, resuspended in 200 µL of sterile Milli-Q water containing a protease inhibitor cocktail, and re-filtered through 0.22 µm to ensure sterility. Aliquots (10 µL) of each preparation were plated on TSA and incubated at 37 °C for 5 days to confirm the absence of viable bacteria. Purified OMV suspensions were stored at 4 °C for short-term use (≤1 week) or at –80 °C for long-term preservation.
For scanning electron microscopy (SEM), bacterial pellets were washed three times in 0.08% glucose solution and fixed in 2.5% glutaraldehyde for 1 h. Samples were then washed four times with 0.1 M cacodylate buffer (pH 7.2), post-fixed in 1% osmium tetroxide for 1 h, rinsed again, treated with 1% tannic acid for 30 min, and washed twice with deionized water. A second fixation in 1% osmium tetroxide was carried out for 30 min, followed by triple rinsing with Milli-Q water. Dehydration was performed using a graded ethanol series (10–100%) with 10 min steps. Samples were dried at the critical point and sputter-coated with a 10 nm layer of gold. Observations were conducted using a FEI Quanta FEG 250 scanning electron microscope operated at 5 kV (Thermo Fisher Scientific, Whaltam, MA, USA).
2.8. Dynamic Light Scattering (DLS)
Outer membrane vesicles (OMVs) collected at 9 h of culture were used for both DLS and lipidome analyses. Dynamic light scattering (DLS) measurements were performed using a Zetasizer NanoS instrument (Malvern Instruments, Malvern, UK). The average hydrodynamic diameter was calculated from the unimodal size distribution of the vesicles. Samples were diluted 1:500 in 0.2 M NaCl and filtered through a 0.45 µm pore-size membrane to remove aggregates. Measurements were conducted at room temperature, with 40–50 runs per sample over a total duration of 30 min. The intensity-weighted average diameter was recorded, and size values represent the mean of three independent OMV preparations.
2.9. Lipid Extraction and Lipidomic Analysis
Lipid extraction was performed following the protocol described by [47]. Briefly, 50 μL of bacterial cells or vesicles were mixed with 450 μL of sodium phosphate buffer (pH 7.4) and 400 μL of ice-cold methanol. Then, 1.5 mL of chloroform/ethyl acetate (4:1, v/v) was added, and the mixture was vortexed for 30 s. After centrifugation at 1500× g for 2 min at 4 °C, the lower organic phase containing the total lipid extract (TLE) was collected and evaporated under a stream of nitrogen gas. The dried TLE was redissolved in 100 μL of isopropanol and spiked with a mixture of external standards for semi-quantification (Table S1). A 1 μL aliquot was injected for analysis.
Lipid profiling was conducted using electrospray ionization time-of-flight mass spectrometry (ESI-TOFMS; Triple TOF 6600, Sciex, Concord, Framingham, MA, USA) coupled to an ultra-high performance liquid chromatography system (UHPLC Nexera, Shimadzu, Kyoto, Japan), as previously described [48]. The MS was operated in negative ionization mode with a scan range of m/z 200–2000. Data acquisition was performed using Information-Dependent Acquisition (IDA®) mode. Lipid identification was based on retention time, accurate mass, characteristic fragment ions and/or neutral losses [49] using an internally developed Excel-based macro. Peak areas were quantified with MultiQuant® software 3.0.3, and the area ratios were calculated by normalizing to the external standard. The relative abundance (%) of each lipid species was determined within its corresponding lipid class.
2.10. Statistical Analysis
The data were analyzed using SPSS 22.0 (IBM). A one-way ANOVA was performed to determine significant differences between treatments in plant dry biomass accumulation (Figure S3). Values of p ≤ 0.05 were considered statistically significant.
3. Results
3.1. Identification of Bacterial Endophytes from Agave Seeds
A total of 50 bacterial endophytes were isolated from Agave tequilana seeds. Isolates were selected based on colony morphology, including differences in size, pigmentation, and appearance time within the first 72 h of incubation. After eliminating redundant morphotypes (sibling colonies), 11 unique isolates were selected and identified using matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry. These isolates were assigned to the genera Bacillus (B. aerius, B. altitudinis, B. megaterium, B. tequilensis, B. safensis, B. subtilis, and B. pumilus), Enterococcus casseliflavus, Enterobacter cloacae, Kosakonia cowanii, and the actinobacterial genus Kocuria (K. marina). To validate the identifications, 16S rDNA sequencing was performed on all selected isolates, revealing a 95% concordance with the MALDI-TOF results. Notably, the strain identified as Bacillus megaterium by MALDI-TOF was classified as Priestia megaterium based on 16S rDNA, consistent with the reclassification of this species [50]. The 16S rDNA sequences were deposited in GenBank under the following accession numbers: E. cloacae SEA01 (KY625189.1), E. casseliflavus x19 (MF322527.1), K. cowanii Agave3 (KY681445.1), K. marina KM (KY681446.1), B. altitudinis A6-2111 (MF567399.1), B. pumilus A12-212 (MF567388.1), B. safensis ATC9 (KY476353.1), B. tequilensis 29G (MF540451.1), B. aerius PMBG1 (MF288782.1), B. subtilis Rb (MF322532.1), and P. megaterium B511-5 (MF322535.1).
A phylogenetic tree based on the Neighbor-Joining method was constructed to illustrate the evolutionary relationships of the isolates (Figure 1). Most isolates belonged to the phylum Bacillota, distributed among the orders Bacillales, Caryophanales, and Lactobacillales (7 isolates), followed by 2 isolates in the phylum Pseudomonadota (order Gammaproteobacteria), and one isolate from the phylum Actinomycetota (order Micrococcales).
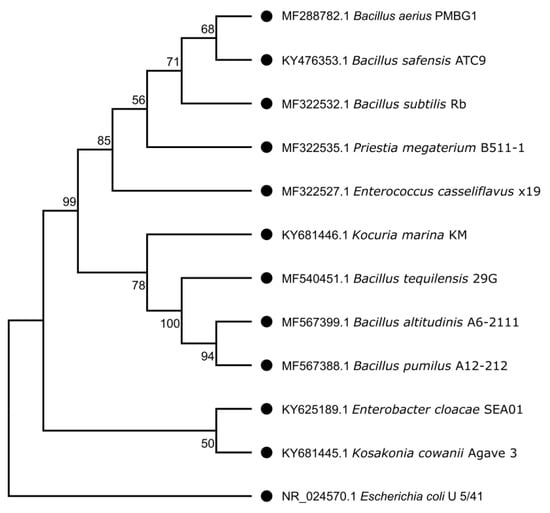
Figure 1.
Phylogenetic relationships of endophytic bacteria isolated from Agave tequilana seeds based on 16S rRNA gene sequences. The phylogenetic tree was constructed using the Neighbor-Joining (NJ) method with E. coli U5/41 16S (NR_024570) [51] as an outgroup. Bootstrap values (1000 replicates) are shown at the nodes to indicate the reliability of the branching. The tree highlights the taxonomic position of isolates within the phyla Bacillota, Pseudomonadota, and Actinomycetota, confirming the diversity of endophytic bacteria colonizing A. tequilana seeds.
3.2. Qualitative Characterization of Agave Endophytes as Plant Growth-Promoting Bacteria (PGPB)
Table 1 summarizes the plant growth-promoting (PGP) traits of seven endophytic bacteria isolated from A. tequilana seeds, based on qualitative and microbiological assessments. Approximately 70% of the isolates exhibited nitrogen fixation activity, except for B. safensis, B. aerius and B. altitudinis. Regarding phosphate solubilization, 63% of the isolates were positive. The production of auxin, particularly indole-3-acetic acid (IAA)-like compounds, was detected in B. tequilensis, K. marina and E. casseliflavus. Regarding the production, siderophores was observed in 54% of the strains. Remarkably, only 45% of the isolates demonstrated 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity a key property associated with alleviating plant stress by degrading the ethylene precursor ACC, thereby reducing ethylene-induced senescence. Among the isolates, E. cloacae and B. pumilus exhibited the most plant growth-promoting properties, although neither produced auxins, as shown in Table 1.

Table 1.
Qualitative assessment of plant growth-promoting traits in seven representative endophytic bacteria isolated from Agave tequilana seeds.
3.3. Visualization of OMVs Release and Biofilm Formation by E. cloacae SEA01
Previous studies have shown that E. cloacae modulate endophytic root colonization [52,53,54]. It has been demonstrated that, upon perceiving bacterial presence, plants secrete hydrogen peroxide to eliminate or restrict microbial access [55,56]. Therefore, the ability to form biofilms confers a survival and colonization advantage to Enterobacter strains, as has already been reported for some isolates [57,58,59,60].
Scanning electron microscopy (SEM) images reveal the dynamics of biofilm formation and outer membrane vesicle (OMV) production by Enterobacter cloacae SEA01over a 48 h time course (Figure 2). At the initial time point (0 h), bacterial cells exhibited a typical rod-shaped morphology (~0.3 × 0.5 μm), with smooth surfaces and uniform distribution (Figure 2A). After 5 h (Figure 2B), cells elongation was observed, suggesting active division and early exponential growth, while 9 h, dense microcolonies had formed (Figure 2C), and numerous extracellular vesicles were visible on the bacterial surface and within the surrounding matrix (Figure 2D). Notably, thin projections or “nanopods” (indicated by yellow arrow) were seen connecting neighboring cells and suggesting long-distance interaction.
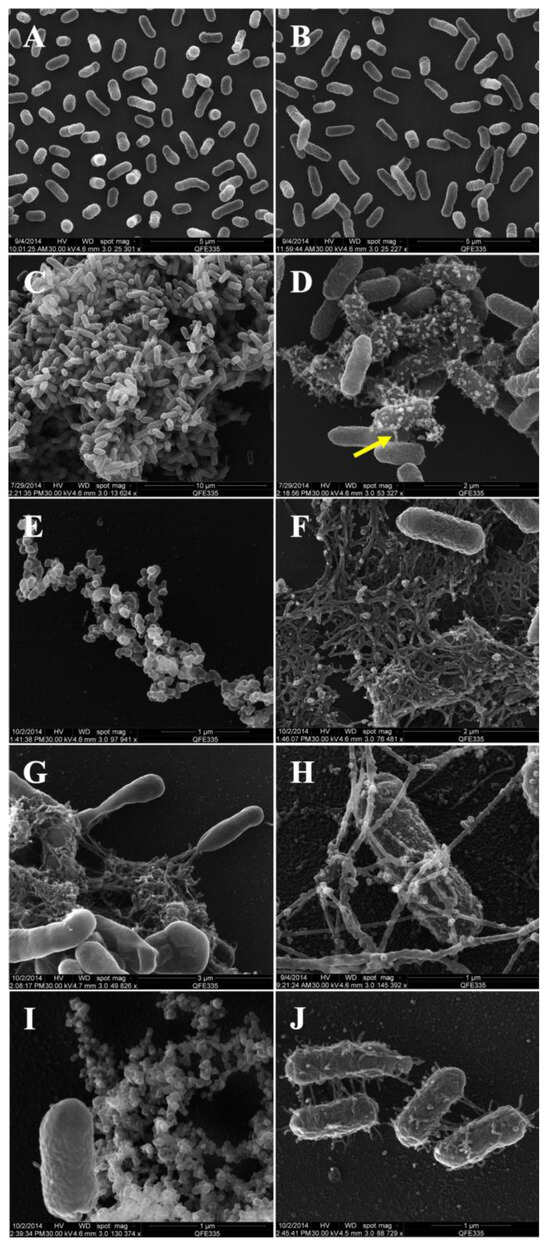
Figure 2.
Scanning electron micrographs illustrating the dynamic process of outer membrane vesicle (OMV) production and biofilm formation by E. cloacae SEA01 over a 48 h period. (A) At 0 h, bacterial cells exhibit typical rod-shaped morphology. (B) At 5 h, elongation of cells indicates active division in liquid M9 medium. (C) At 9 h, microcolony formation begins, accompanied by initial vesicle production on the cell surface. (D) Close-up view of OMVs and intercellular connections via “nanopod”-like projections (yellow arrow). (E) At 18 h, detached OMVs are observed dispersed in the biofilm matrix. (F) Aggregated biofilm-derived extracellular vesicles (OMVs) embedded in a maturing biofilm matrix at 18 h. (G) At 24 h, a mature biofilm structure with actively migrating cells is evident. (H) Filamentous networks, possibly pili, are seen connecting cells and are coated with OMVs. (I) After 48 h, extensive vesicle OMV release occurs and (J) Cells at the late stage are densely covered fimbriae-associated OMVs. Scale bars are indicated in each panel.
At 18 h, extracellular vesicles appeared aggregated into organized chains and clusters (Figure 2E), coinciding with the early development of the biofilm matrix (Figure 2F). The emerging biofilm displayed a complex reticular architecture composed of vesicles and filamentous components. After 24 h (Figure 2G–H), a mature biofilm structure was visible, characterized by dense bacterial aggregates interconnected by filamentous structures, along with signs of bacteria migration away from the matrix. These filamentous structures may correspond to pili or extracellular fibers involved in intercellular communication and vesicle anchoring. OMVs were observed adhered to these filaments (Figure 2H), indicating a potential structural or signaling role.
At 48 h (Figure 2I–J), the number of most bacterial cells had decreased, but vesicle release persisted. Some cells had large amounts of vesicles on their surface and fimbria-like extensions, indicating a final phase of vesiculation and possible late-stage biofilm remodeling (Figure 2J). These observations confirm the ability of E. cloacae SEA01 to produce OMVs in a growth stage-dependent manner and to form structured biofilms, both of which may contribute to its plant-associated lifestyle and plant growth-promoting activities.
3.4. Characterization of DLS and Zeta Potential
Dynamic light scattering (DLS) analysis was conducted on vesicles isolated from a 9 h culture of E. cloacae SEA01 (Figure 3A). The DLS results revealed a unimodal size distribution ranging from 75 to 400 nm, with an average hydrodynamic diameter of 153.1 nm (Figure 3B). To assess the colloidal stability of the vesicle suspensions, Zeta potential measurements were also performed. Typically, absolute Zeta potential values exceeding ±30 mV are indicative of stable dispersions due to strong electrostatic repulsion, while lower values suggest propensity for particle aggregation. The vesicles of E. cloacae SEA01 exhibited an average zeta potential of −26.1 mV and an electrophoretic mobility of −2.041 μm-cm/V-s, suggesting moderate colloidal stability and a tendency toward aggregate.
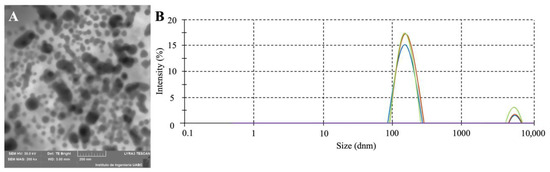
Figure 3.
Characterization of outer membrane vesicles (OMVs) isolated from E. cloacae SEA01. (A) Scanning electron micrograph showing spherical OMVs of varying sizes, characterized by a double-membrane structure and electron-dense filamentous material within the lumen. (B) Dynamic light scattering (DLS) analysis indicating a unimodal size distribution of OMVs ranging from 75 to 400 nm, with an average hydrodynamic diameter of 153.1 nm. Each colored line (green, blue, orange) denotes an independent OMV sample/preparation measured under identical conditions.
3.5. Lipid Analysis of OMVs
Lipid profiling of E. cloacae SEA01 vegetative cells and OMVs was conducted 9 h post-inoculation using ultra-high-performance liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry (UHPLC-ESI-TOF-MS). As shown in Figure 4A, a total of 53 distinct lipid species were identified and classified into five major lipid classes: phosphatidylethanolamines (PE; 23 species), phosphatidylglycerols (PG; 6 species), acyl-phosphatidylglycerols (Acyl-PG; 6 species), cardiolipins (CL; 17 species) and ubiquinone-8 (UbQ8; 1 species). Among these, PE dominated the lipid profile, comprising ~80% of the total lipid content in both cells and OMVs. This predominance of PE is consistent with its known structural role in Gram-negative bacterial membranes, where it contributes to membrane curvature and facilitates vesicle formation (Figure 4B).
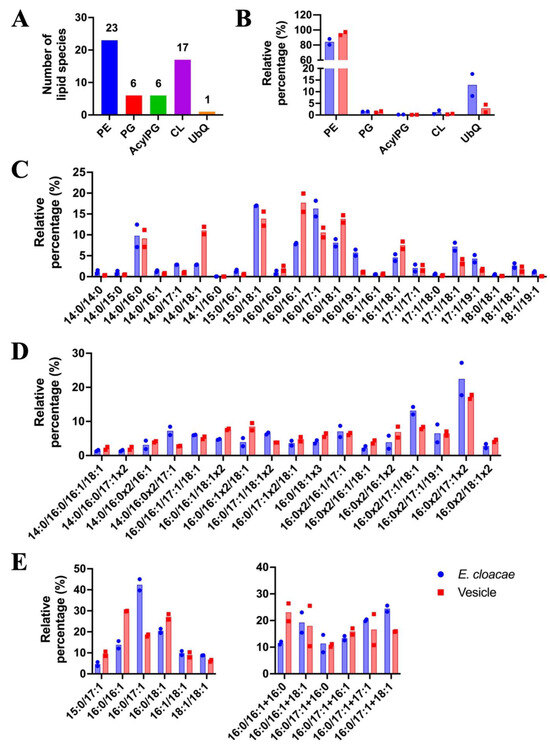
Figure 4.
Lipid composition of E. cloacae SEA01 vegetative cells and their outer membrane vesicles (OMVs). (A) Total number of identified lipid species grouped into five lipid classes: phosphatidylethanolamine (PE), phosphatidylglyceron (PG), acylphosphatidylglycerol (Acyl-PG), cardiolipin (CL), and ubiquinone (UbQ8). (B) Relative abundance (5%) of each lipid class in vegetative cells (blue) and OMVs (red), showing PE as the dominant class in both fractions. (C–E) Relative distribution of individual lipid molecular species within each class (C) PE, (D) CL, and (E) PG and Acyl-PG. OMVs exhibited a enrichment in lipid species containing 16:1 and 18:1 fatty acyl chains-such as PE (16:0/16:1) and PG (16:0/18:1)-while species containing 17:1 and 19:1 chains were more abundant in vegetative cells. Data represent means ± SD of two biological replicates (n = 2). Colored bars represent OMVs (red) and vegetative cells (blue).
The analysis of the percentage contribution of each lipid class indicated differing profiles for vegetative cells and OMVs. Fatty acyl compositions were selectively modulated in OMVs, indicating preferential and likely controlled selection of the lipids composing the OMVs. In the PE class (Figure 4C–E), vesicles exhibited a higher proportion of unsaturated species such as PE (16:0/16:1), PE (16:0/18:1) and PE (14:0/18:1), suggesting preferential sorting of these lipids into the vesicles. Conversely, fatty acyl species with 17:1 or 19:1 (e.g., PE (16:0/17:1), PE (16:0/19:1) were underrepresented in OMVs. In the cardiolipin (CL) profile (Figure 4D), OMVs were enriched with species such as CL (16:0/16:0/17:1/17:1) and CL (16:0/16:0/17:1/18:1), further supporting a reorganization of membrane microdomains during vesicle biogenesis. Similarly, OMVs exhibited distinct PG and acyl-PG profiles (Figure 4E), with increased abundance of PG (16:0/16:1), PG (16:0/18:1) and acyl-PG (16:0/18:1) species, which may contribute to membrane curvature and vesicle release.
4. Discussion
Agave tequilana, the most important species for tequila production, suffers from low genetic diversity due to clonal propagation, which increases both the need for fertilizers and susceptibility to pests and diseases. In this context, endophytic bacteria play an important role by promoting plant growth, nutrient uptake and stress tolerance [29]. Understanding these symbiotic interactions is particularly given the rising global demand for tequila [61]. Here, we isolated endophytic bacteria from mature seeds of A. tequilana, including genera such as Bacillus, Enterobacter, Enterococcus, Kosakonia, Kocuria and Priestia (Figure 1). Although these genera are commonly associated with microbiomes and have been previously detected in various agave tissues [32,33], Enterococcus casseliflavus remains the only species so far reported as a seed endophyte of A. tequilana [62]. Despite the limited number of cultivable isolates obtained, the recovered bacterial community included representatives of the three major phyla typically found in agaves: Firmicutes, Proteobacteria and Actinobacteria [33].
Seed endophytes represent a conserved segment of the plant microbiota and have a significant impact on the initial stages of plant growth and the establishment of the microbial community. To evaluate the plant growth-promoting potential of the isolated strains, we performed a series of functional assays targeting classical plant growth-promoting traits, including atmospheric nitrogen fixation, solubilization of inorganic phosphate, production of indole-3-acetic acid (IAA), secretion of siderophores, and ACC deaminase activity. Our analysis shows that both B. pumilus A12-212 and E. cloacae SEA01 tested positive for nitrogen fixation, phosphate solubilization, siderophore production and ACC deaminase activity. B. pumilus A12-212 is currently being evaluated as part of the development of a bioinoculant formulation for agave and other crops as a biostimulant and for control of fungal pathogens.
Previous work by our group has shown that E. cloacae SEA01 induces systemic H2O2 accumulation in agave seedlings after root inoculation [35]. However, the effects on seedling biomass were not investigated. Here, continuous application of SEA01 for six months in nutrient-poor sand microcosms was shown to increase seedling dry biomass compared to water-irrigated controls (Supplementary Figure S3). This growth-promoting trend could be due in part to rhizophagy-like processes, i.e., internalization of plants and degradation of associated bacteria during colonization [31,63,64]. This phenomenon, termed “rhizophagy” by the James White Group, involves the root-mediated uptake of bacteria through oxidative processes with hydrogen peroxide (H2O2) [65]. The Enterobacter strains have been described as endophytes that can colonize a variety of plant hosts and interact with the microbiome of native plants. Their effective root colonization by E. cloacae has been linked to their competitive ability and their capacity to form biofilms supported by components of the extracellular matrix [55,58,66].
Outer membrane vesicles (OMVs) produced by Gram-negative bacteria play a role in cell-to-cell signaling, biofilm formation and stress responses [8,12,13]. Herein, we describe the release dynamics and structural features of OMVs produced by E. cloacae SEA01. These vesicles exhibit a size distribution of 75–400 nm and show a zeta potential indicating moderate colloidal stability and a tendency to aggregate (Figure 2). Vesicle production begins around the 9th hour of growth, which coincides with the logarithmic phase and early stages of biofilm formation. Notably, nanopod-like structures have been observed connecting the bacterial cells (Figure 2D), possibly facilitating the transport of OMVs and promoting local metabolic interactions within the biofilm microenvironment [67]. As the culture transitions to stationary phase (after 18 h), biofilm formation comes to the fore, with filamentous networks and aggregated OMVs clearly visible in the matrix (Figure 2F). This pattern of OMV production is like the vesicle formation observed in other bacterial species such as E. cloacae ATCC 13047 [68], Ferrividacidithiobacillus caldus [69], the Vibrio predator Pseudoalteromonas piscicida [70] and Pseudomonas chlororaphis under abiotic stress conditions [71].
During the first 24 h of culture, all stages of the biofilm life cycle were observed, including the maturation and subsequent spreading of the bacterial cells (Figure 2G). These spreading cells exhibited adherent OMVs on their surface, along with filamentous structures associated with fimbriae, suggesting active OMV deposition. This observation suggests a possible second wave of vesicle release. Even after 48 h, some cells continued to release OMVs despite a decrease in bacterial density. These results emphasize the central role of OMVs in the development of the biofilm matrix and in supporting bacterial persistence under conditions of nutrient limitation and environmental stress. Electrophoretic analysis of intact OMVs and cells of E. cloacae SEA01 (Supplementary Figure S1) revealed that the OMVs contain aggregated extracellular DNA (eDNA) and were compared to OMVs of E. cloacae C2 strain, a banana endophyte [51]. This eDNA, together with other components of the biofilm matrix, has an important structural function that promotes both bacterial survival under nutrient-poor conditions and intercellular communication [4,72,73,74]. In addition, during biofilm maturation, eDNA also contributes to the degradation of the scaffold and facilitates cell spreading [75], as shown by the bacterial escape observed in Figure 2G.
Comparative lipidomics of purified OMVs and whole cells of E. cloacae SEA01 revealed five major classes. Phosphatidylethanolamine (PE) dominated in both profiles (~80%), consistent with its known role as the major phospholipid in Gram-negative bacteria, where it facilitates membrane expansion and vesicle formation (Figure 4B) [76]. Compared to whole cells, OMV tended to accumulate monounsaturated acyl chains (16:1, 18:1) and PE (14:0/18:1, 16:0/16:1, 16:0/18:1), which likely increase membrane flexibility and promote vesicle formation (Figure 4C) [7,77]. A lower abundance of 17:1 and 19:1 fatty acid species was observed in OMVs, possibly corresponding to cyclopropane fatty acids (CPAs), which have the same mass as their monounsaturated fatty acid counterparts. CPAs are widely distributed in bacteria, from E. coli to H. pylori. Although the functions of CPAs are not yet fully understood, they are associated with stress responses and may be selectively retained in the cell membrane to preserve structural integrity while being excluded from vesicles, consistent with trends toward selective lipid sorting that preserves envelope rigidity under stress [78,79,80,81]. Lower classes (PG and acyl-PG) may support vesicle stabilization and host interactions, while cardiolipin (CL) likely reflects the bacterial response to stress [77]. OMVs also showed a trend towards reduced ubiquinone-8 (Q8) compared to whole cells, consistent with reports that Q8 increases membrane stiffness and Q8 deficiency increases superoxide generation in E. coli [82,83,84]. Altogether, these trending remodeling processes support a model in which OMV production accompanies early biofilm development, as has been proposed [85]. However, targeted lipidomics is required to understand how E. cloacae SEA01 shapes OMV for interaction with plants as a colonizer endophyte.
Finally, we would like to emphasize that E. cloacae SEA01 is a catalase-negative strain (Supplementary Figure S2), an unusual characteristic among plant-associated Enterobacter. As far as we are aware, no endophyte has been reported to combine catalase deficiency with OMV production during association with the host. This observation highlights a potential functional role of OMVs in the maintenance of an endophytic lifestyle and makes them a useful tool as secretory system in various environmental situations [86,87,88]. We therefore hypothesize that catalase deficiency triggers OMV biogenesis and early biofilm development, which could provide a physiological advantage at the onset of plant association. Definitive testing will require comparison with catalase-positive closely related to Enterobacter endophytes and/or catalase complementation in SEA01, as well as omics analyzes and assays in plants to define the specific roles of OMVs during root interaction, nutrient transfer and colonization of the blue agave.
5. Conclusions
This study shows that the agave-seed endophyte Enterobacter cloacae SEA01 supports Agave tequilana growth in nutrient-poor microcosms and produces outer-membrane vesicles (OMVs) associated with early biofilm development and lipid signature approach consistent with vesiculation. Notably, SEA01 is catalase-negative, an uncommon feature in plant-associated Enterobacter, leading to a testable model in which oxidative factors influence OMV output and biofilm assembly. This raises questions about their roles in recognition and redox signaling at the root interface.
As SEA01 belongs to the E. cloacae complex containing ESKAPE lineages, any translational application must be preceded by rigorous biosafety characterization (antibiotic susceptibility testing, genome-based AMR/virulence screening). Future works should combine targeted proteomics/genomics with genetic or chemical disruption of catalase/OMV pathways. These results will provide testable opportunities to develop safe OMV-based strategies as cell-free biostimulants for agave and other crops while clarifying the mechanistic role of OMVs during early plant association.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13112432/s1. Figure S1: Detection of DNA associated with outer membrane vesicles (OMVs) from Enterobacter cloacae strains SEA01 and C2; Figure S2. Representative native PAGE gel showing in-gel catalase activity; Figure S3. Effect of E. cloacae SEA01 on the total dry biomass of A. tequilana plantlets after six months of treatment; Figure S4. Principal component analysis of lipidomics data from whole Bacteria and bacteria’s Outer membrane vesicles. Table S1. External standards used for the semi quantification in the lipidomic analysis.
Author Contributions
Conceptualization, supervision, formal analysis and data curation M.H.G.M., S.M., P.D.M., M.J.B.-G. and M.Y.Y.; methodology and Investigation K.R.P., H.P.V., F.M.P., A.M.-R., I.Z.-C., P.M.-S., A.B.C.-F., M.Y.Y. and M.C.; writing—original draft preparation, M.J.B.-G., P.D.M., F.V.W., M.H.G.M., S.M. and M.Y.Y.; editing figures, A.M.-R., P.M.-S., S.M. and M.Y.Y.; project administration, M.J.B.-G. and P.D.M.; funding Acquisition, P.D.M., S.M. and F.V.W., M.J.B.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Mexican research grant FODECIJAL program of COECYT-JAL Grant 10602-2023 and CONHACYT grants 2013-205520 and 2016-269607 for support in the acquisition of the MALDI-TOF mass spectrometer. Brazilian research funding institutions; P. Di Mascio were funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Grant/Award Number: 2013/07937-8; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant/Award Number: 304350/2023-0, 304945/2021-8; CAPES; PRPUSP, Grant/Award Number: 2011.1.9352.1.8; John Simon Guggenheim Memorial Foundation; Sayuri Miyamoto was funded by the São Paulo Research Foundation (FAPESP; grant #2013/07937-8) and the National Council for Scientific and Technological Development (CNPq; grant #313926/2021-2) and Flavia V. Winck was funded by FAPESP (grant#2025/05512-7, São Paulo Research Foundation–FAPESP), National Council for Scientific and Technological Development-CNPq (grant#421447/2023–0), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil -CAPES (Grant number 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article and Supplementary Materials; and all the 16SrRNA gene sequences of the identified endophytic strains are openly available in the National Center for Biotechnology Information (NCBI) data base under accession numbers: E. cloacae SEA01 (KY625189.1), E. casseliflavus x19 (MF322527.1), K. cowanii Agave3 (KY681445.1), K. marina KM (KY681446.1), B. altitudinis A6-2111 (MF567399.1), B. pumilus A12-212 (MF567388.1), B. safensis ATC9 (KY476353.1), B. tequilensis 29G (MF540451.1), B. aerius.
Acknowledgments
M.J.B.-G. thanks Plan de Carrera from Autonomous University of Guadalajara and the FODECIJAL program of COECYT-JAL for the economic support of the project 10602-2023, acquisition of the MALDI-TOF mass spectrometer the projects 2013-205520 and 2016-269607, and I grateful to FAPESP grant#2025/05512-7 for their support for my visiting scholar to CENA-USP. P.D.M. acknowledges the support of the Brazilian Research Foundation, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (FAPESP CEPID Redoxoma no. 2013/07937- 8, no. 2016/00696-3, no. 2023/00995-4, no. 2023/170318, no. 2022/03833-2), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 304350/2023-0), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), PRPUSP (Pro- Reitoria de Pesquisa da Universidade de São Paulo; NAP Redoxoma (no. 2011.1.9352.1.8), and the John Simon Guggenheim Memorial Foundation. A.M.-R. thanks the CONHACYT for the PhD fellowship 720754, the FODECIJAL Grant 10602-2023 for the postdoctoral fellowship and the Fondo Semilla for supporting her academic mobility to CENA-USP in Piracicaba. P.M.-S. thanks CONHACYT their PhD fellowship 730994 and FODECIJAL Grant 10602-2023 for postdoctoral fellowship. F.V.W. acknowledges the National Council for Scientific and Technological Development-CNPq (Grant number 421447/2023–0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior- Brasil -CAPES (Grant number 001) and acknowledges FAPESP (grant#2025/05512-7, São Paulo Research Foundation-FAPESP). The authors thank Greice Kelle Saraiva and Iolanda Midea Cuccovia from the Institute of Chemistry, University of São Paulo, for performing the DLS analysis of the purified OMVs. We gratefully acknowledge the support of ChatGPT 4 (OpenAI) for its assistance in refining the English language and enhancing the clarity and readability of the manuscript. The authors thank the Fondo Semilla- UAG for supporting the APC payment.
Conflicts of Interest
The authors declare no conflicts of interest. The use of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation.
References
- Federica, D.; Cosimato, I.; Salzano, F.; Mensitieri, F.; Andretta, V.; Santoro, E.; Boccia, G.; Folliero, V.; Franci, G. Adaptations of bacterial extracellular vesicles in response to antibiotic pressure. Int. J. Mol. Sci. 2025, 26, 5025. [Google Scholar] [CrossRef] [PubMed]
- Fauzia, K.A.; Effendi, W.I.; Alfaray, R.I.; Malaty, H.M.; Yamaoka, Y.; Mifthussurur, M. Molecular mechanisms of biofilm formation in Helicobacter pylori. Antibiotics 2024, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- Potapova, A.; Garvey, W.; Dahl, P.; Guo, S.; Chang, Y.; Schwechheimer, C.; Trebino, M.A.; Floyd, K.A.; Phinney, B.S.; Liu, J.; et al. Outer membrane vesicles and the outer membrane protein OmpU govern Vibrio cholerae biofilm matrix assembly. mBio 2024, 15, e03304-23. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Di Marcantonio, M.C.; Robuffo, I.; Pompilio, A.; Celia, C.; Di Marzio, L.; Paolino, D.; Codagnone, M.; Muraro, R.; Stoodley, P.; et al. Helicobacter pylori ATCC 43629/NCTC 11639 outer membrane vesicles (OMVs) from biofilm and planktonic phase associated with extracellular DNA (eDNA). Front. Microbiol. 2015, 6, 1369. [Google Scholar] [CrossRef]
- Jan, A.T. Outer membrane vesicles (OMVs) of Gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, M.J.; Park, Y.; Chung, J.; Kweon, H.S.; Kang, N.G.; Hwang, S.J.; Youn, S.H.; Hwang, B.K.; Kim, D. Visualizing extracellular vesicle biogenesis in gram-positive bacteria using super-resolution microscopy. BMC Biol. 2022, 20, 270. [Google Scholar] [CrossRef]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Bu, Y.; Ren, X.; Dong, Z. Review on bacterial outer membrane vesicles: Structure, vesicle formation, separation and biotechnological applications. Microb. Cell Fact. 2025, 24, 27. [Google Scholar] [CrossRef]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef]
- Lee, E.Y.; Choi, D.S.; Kim, K.P.; Gho, Y.S. Proteomics in gram-negative bacterial outer membrane vesicles. Mass. Spectrom. Rev. 2008, 27, 535–555. [Google Scholar] [CrossRef]
- Baquero, D.P.; Borrel, G.; Gazi, A.; Martin-Gallausiaux, C.; Cvirkaite-Krupovic, V.; Commere, P.H.; Pende, N.; Tachon, S.; Sartori-Rupp, A.; Douché, T.; et al. Biogenesis of DNA-carrying extracellular vesicles by the dominant human gut methanogenic archaeon. Nat. Commun. 2025, 16, 5093. [Google Scholar] [CrossRef] [PubMed]
- Puca, V.; Marinacci, B.; Pellegrini, B.; Campanile, F.; Santagati, M.; Grande, R. Biofilm and bacterial membrane vesicles: Recent advances. Expert Opin. Ther. Pat. 2024, 34, 475–491. [Google Scholar] [CrossRef]
- Mozaheb, N.; Mingeot-Leclercq, M.P. Membrane vesicle production as a bacterial defense against stress. Front. Microbiol. 2020, 11, 600221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Zhao, L.; Dickey, S.W.; Wang, H.; Xu, R.; Chen, T.; Jian, Y.; Wang, X.; Lv, H.; et al. Essential role of membrane vesicles for biological activity of the bacteriocin micrococcin PL. J. Extracell. Vesicles 2022, 11, e12212. [Google Scholar] [CrossRef]
- Lima, S.; Matinha-Cardoso, J.; Giner-Lamia, J.; Couto, N.; Pacheco, C.; Florencio, F.J.; Wright, P.C.; Tamagnini, P.; Oliveira, P. Extracellular vesicles as an alternative copper-secretion mechanism in bacteria. J. Hazard. Mater. 2022, 431, 128594. [Google Scholar] [CrossRef]
- Macdonald, L.A.; Kuehn, M.J. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 2971–2981. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Maas, R.H.W.; van den Ijssel, J.; van Keulen, L.; Martens, D.E.; Wijffels, R.H.; Stork, M. High dissolved oxygen tension triggers outer membrane vesicle formation by Neisseria meningitidis. Microb. Cell Fact. 2018, 17, 157. [Google Scholar] [CrossRef]
- Work, E.; Knox, K.W.; Vesk, M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann. N. Y. Acad. Sci. 1966, 133, 438–449. [Google Scholar] [CrossRef]
- Ionescu, M.; Zaini, P.A.; Baccari, C.; Tran, S.; da Silva, A.M.; Lindow, S.E. Xylella fastidiosa outer membrane vesicles modulate plant colonization by blocking attachment to surfaces. Proc. Natl. Acad. Sci. USA 2014, 111, E3910–E3918. [Google Scholar] [CrossRef]
- Salvachúa, D.; Werner, A.Z.; Pardo, I.; Michalska, M.; Black, B.A.; Donohoe, B.S.; Haugen, S.J.; Katahira, R.; Notonier, S.; Ramirez, K.J.; et al. Outer membrane vesicles catabolize lignin-derived aromatic compounds in Pseudomonas putida KT2440. Proc. Natl. Acad. Sci. USA 2020, 117, 9302–9310. [Google Scholar] [CrossRef]
- Chalupowicz, L.; Mordukhovich, G.; Assouline, N.; Kats, L.; Sela, N.; Bahar, O. Bacterial outer membrane vesicles induce a transcriptional shift in Arabidopsis towards immune system activation leading to suppression of pathogen growth in planta. J. Extracell. Vesicles 2023, 12, e12286. [Google Scholar] [CrossRef]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Ruf, A.; Blumenkamp, P.; Ludwig, C.; Lippegaus, A.; Brachmann, A.; Klingl, A.; Goesmann, A.; Brinkrolf, K.; Papenfort, K.; Robatzek, S. Extracellular Vesicles from Xylella fastidiosa Carry sRNAs and Genomic Islands, Suggesting Roles in Recipient Cells. J. Extracell. Vesicles 2025, 14, e70102. [Google Scholar] [CrossRef] [PubMed]
- Meneses, N.; Taboada, H.; Dunn, M.F.; Vargas, M.D.C.; Buchs, N.; Heller, M.; Encarnacion, S. The naringenin-induced exoproteome of Rhizobium etli CE3. Arch. Microbiol. 2017, 199, 737–755. [Google Scholar] [CrossRef]
- Taboada, H.; Dunn, M.F.; Meneses, N.; Vargas-Lagunas, C.; Buchs, N.; Andrade-Dominguez, A.; Encarnacion, S. Qualitative changes in proteins contained in outer membrane vesicles produced by Rhizobium etli grown in the presence of the nod gene inducer naringenin. Arch. Microbiol. 2019, 201, 1173–1194. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Wu, J.; Tang, Z.; Xie, F.; Chen, D.; Lin, H.; Li, Y. Analysis of outer membrane vesicles indicates that glycerophospholipid metabolism contributes to early symbiosis between Sinorhizobium fredii HH103 and soybean. Mol. Plant Microbe Interact. 2022, 35, 311–322. [Google Scholar] [CrossRef]
- Lei, J.; Shi, Y.; Li, H.; Wang, R. Characterizing the endophytic microbiome and microbial functional assemblages associated with Fengtang plum (Prunus salicina Lindl.) development and resistance. Horticulturae 2025, 11, 483. [Google Scholar] [CrossRef]
- Dėlkus, M.; Lukša-Žebelovič, J.; Žižytė-Eidetienė, M.; Ivanauskas, A.; Valiūnas, D.; Servienė, E. Comparative analysis of endophytic bacterial microbiomes in healthy and phytoplasma-infected European blueberry plants. Forests 2025, 16, 758. [Google Scholar] [CrossRef]
- Das, D.; Sharma, P.L.; Paul, P.; Baruah, N.R.; Choudhury, J.; Begum, T.; Karmakar, R.; Khan, T.; Kalita, J. Harnessing endophytes: Innovative strategies for sustainable agricultural practices. Discov. Bact. 2025, 2, 1. [Google Scholar] [CrossRef]
- Khaskheli, M.A.; Nizamani, M.M.; Tarafder, E.; Das, D.; Muhae-Ud-Din, G.; Khaskheli, R.A.; Wang, Y. Manipulation of root-associated bacterial endophytes for sustainable crop production system: A review. Rhizosphere 2025, 33, 101044. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; White, J.F., Jr.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Torres, M.S.; Kato, M.J.; Medeiros, M.H.G.; Di Mascio, P. Nitrogen acquisition in Agave tequilana from degradation of endophytic bacteria. Sci. Rep. 2014, 4, 6938. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, J.C.; De la Mora-Amutio, M.; Plascencia-Correa, L.A.; Audelo-Regalado, E.; Guardado, F.R.; Hernández-Sánchez, E.; Peña-Ramírez, Y.J.; Escalante, A.; Beltrán-García, M.J.; Ogura, T. Cultivable endophytic bacteria from leaf bases of Agave tequilana and their role as plant growth promoters. Braz. J. Microbiol. 2015, 45, 1333–1339. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, A.; Macedo-Raygoza, G.; Huerta-Robles, A.X.; Reyes-Sepulveda, I.; Lozano-Lopez, J.; García-Ochoa, E.Y.; Fierro-Kong, L.; Medeiros, M.H.G.; Di Mascio, P.; White, J.F.; et al. Agave seed endophytes: Ecology and Impacts on Root Architecture, Nutrient Acquisition, and Cold Stress Tolerance. In Seed Endophytes; Verma, S., White, J., Jr., Eds.; Springer: Cham, Switzerland, 2019; pp. 195–214. [Google Scholar]
- Martinez-Rodriguez, A.; Beltran-Garcia, C.; Valdez-Salas, B.; Santacruz-Ruvalcaba, F.; Di Mascio, P.; Beltran-Garcia, M.J. Micropropagation of seed-derived clonal lines of the endangered Agave marmorata Roezl and their compatibility with endophytes. Biology 2022, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.; Prieto, K.R.; Santos, C.S.; Valerio, P.H.; Garcia-Ochoa, E.Y.; Huerta-Robles, A.; Beltran-Garcia, M.J.; Di Mascio, P.; Bertotti, M. In-vivo electrochemical monitoring of H2O2 production induced by root-inoculated endophytic bacteria in Agave tequilana leaves. Biosens. Bioelectron. 2018, 99, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Echeverri, L.M.; Benavides-López, S.; Geiger, O.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A. Bacterial extracellular vesicles: Biotechnological perspective for enhanced productivity. World J. Microbiol. Biotechnol. 2024, 40, 174. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Döbereiner, J.; Baldani, V.L.D.; Baldani, J.I. Como Isolar e Identificar Bactérias Diazotróficas de Plantas Não-Leguminosas; Embrapa-SPI: Itaguaí, Brazil, 1995. [Google Scholar]
- Ullah, I.; Khan, A.R.; Park, G.S.; Lim, J.H.; Waqas, M.; Lee, I.J.; Shin, J.-H. Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci. Biotechnol. 2013, 22, 25–31. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wayne, L.G.; Diaz, G.A. A double staining method for differentiating between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 1986, 157, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kodai, S.; Takemura, S.; Minamiyama, Y.; Niki, E. Simultaneous measurement of F2-isoprostane, hydroxyoctadecadienoic acid, hydroxyeicosatetraenoic acid, and hydroxycholesterols from physiological samples. Anal. Biochem. 2008, 379, 105–115. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef]
- Han, X. Lipidomics: Comprehensive Mass Spectrometry of Lipids. Part II: Characterization of Lipids; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Biedendieck, R.; Knuuti, T.; Moore, S.J.; Jahn, D. The “beauty in the beast”—The multiple uses of Priestia megaterium in biotechnology. Appl. Microbiol. Biotechnol. 2021, 105, 5719–5737. [Google Scholar] [CrossRef]
- Cilia, V.; Lafay, B.; Christen, R. Sequence heterogeneities among 16S ribosomal RNA sequences, and their effect on phylogenetic analyses at the species level. Mol. Biol. Evol. 1996, 13, 451–461. [Google Scholar] [CrossRef]
- Macedo-Raygoza, G.M.; Valdez-Salas, B.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Kato, M.J.; Canto-Canché, B.B.; Carrillo-Beltrán, M.; Di Mascio, P.; White, J.F.; et al. Enterobacter cloacae, an Endophyte That Establishes a Nutrient-Transfer Symbiosis with Banana Plants and Protects Against the Black Sigatoka Pathogen. Front. Microbiol. 2019, 10, 804. [Google Scholar] [CrossRef]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and Representative Bacterial Community of Maize Roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef]
- Zenebe, A.; Hailemichael, F.; Beshah, A.; Giray, R.; Oner, E.T.; Tesfaw, A. The nitrogen-fixing strains of Enterobacter cloacae isolated from mung bean (Vigna radiata L.) enhance mung bean nodulation and growth. Discov. Appl. Sci. 2025, 7, 329. [Google Scholar] [CrossRef]
- Synek, L.; Rawat, A.; L’Haridon, F.; Weisskopf, L.; Saad, M.M.; Hirt, H. Multiple strategies of plant colonization by beneficial endophytic Enterobacter sp. SA187. Environ. Microbiol. 2021, 23, 6223–6240. [Google Scholar] [CrossRef]
- Naher, K.; Miwa, H.; Okazaki, S.; Yasuda, M. Effects of Different Sources of Nitrogen on Endophytic Colonization of Rice Plants by Azospirillum sp. B510. Microbes Environ. 2018, 33, 301–308. [Google Scholar] [CrossRef]
- Liebrenz, K.; Gómez, C.; Brambilla, S.; Frare, R.; Stritzler, M.; Maguire, V.; Ruiz, O.; Soldini, D.; Pascuan, C.; Soto, G.; et al. Whole-Genome Resequencing of Spontaneous Oxidative Stress-Resistant Mutants Reveals an Antioxidant System of Bradyrhizobium japonicum Involved in Soybean Colonization. Microb. Ecol. 2022, 84, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Ponraj, P.; Illakkiam, D.; Rajendhran, J.; Gunasekaran, P. Inactivation of the transcriptional regulator-encoding gene sdiA enhances rice root colonization and biofilm formation in Enterobacter cloacae GS1. J. Bacteriol. 2013, 195, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, L.; Ge, J.; Feng, F.; Wan, Q.; Zeng, D.; Yu, X. Colonization Mechanism of Endophytic Enterobacter cloacae TMX-6 on Rice Seedlings Mediated by Organic Acids Exudated from Roots. J. Agric. Food Chem. 2023, 71, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.K.; Mandal, S.; Barman, A.; Mondal, S.; Chatterjee, S.; Mandal, N.C. Genomic insight of phosphate solubilization and plant growth promotion of two taxonomically distinct winter crops by Enterobacter sp. DRP3. J. Appl. Microbiol. 2024, 135, lxae146. [Google Scholar] [CrossRef]
- Guardado-Fierros, B.G.; Tuesta-Popolizio, D.A.; Lorenzo-Santiago, M.A.; Rubio-Cortés, R.; Camacho-Ruíz, R.M.; Castañeda-Nava, J.J.; Gutiérrez-Mora, A.; Contreras-Ramos, S.M. PGPB consortium formulation to increase fermentable sugar in Agave tequilana Weber var. blue: A study in the field. Plants 2024, 13, 1371. [Google Scholar] [CrossRef]
- Desgarennes, D.; Garrido, E.; Torres-Gomez, M.J.; Peña-Cabriales, J.J.; Partida-Martinez, L.P. Diazotrophic potential among bacterial communities associated with wild and cultivated Agave species. FEMS Microbiol. Ecol. 2014, 90, 844–857. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Rentsch, D.; Robatzek, S.; Webb, R.I.; Sagulenko, E.; Näsholm, T.; Schmidt, S.; Lonhienne, T.G.A. Turning the table: Plants consume microbes as a source of nutrients. PLoS ONE 2010, 5, e11915. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.J.; Singh, R.K.; Singh, P.; Li, D.P.; Sharma, A.; Xing, Y.X.; Song, X.P.; Yang, L.T.; Li, Y.R. Complete Genome Sequence of Enterobacter roggenkampii ED5, a Nitrogen Fixing Plant Growth Promoting Endophytic Bacterium with Biocontrol and Stress Tolerance Properties, Isolated from Sugarcane Root. Front. Microbiol. 2020, 11, 580081. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Chen, S.; Tocheva, E.I.; Jensen, G.J.; Hickey, W.J. Nanopods: A new bacterial structure and mechanism for deployment of outer membrane vesicles. PLoS ONE 2011, 6, e20725. [Google Scholar] [CrossRef]
- Bhar, S.; Edelmann, M.J.; Jones, M.K. Characterization and proteomic analysis of outer membrane vesicles from a commensal microbe, Enterobacter cloacae. J. Proteom. 2021, 231, 103994. [Google Scholar] [CrossRef]
- Rossoni, S.; Beard, S.; Segura-Bidermann, M.I.; Duarte-Ramírez, J.; Osorio, F.K.; Varas-Godoy, M.; Martínez-Bellange, P.; Vera, M.; Quatrini, R.; Castro, M. Membrane vesicles in Acidithiobacillia class extreme acidophiles: Influence on collective behaviors of Fervidacidithiobacillus caldus. Front. Microbiol. 2024, 14, 1331363. [Google Scholar] [CrossRef]
- Richards, G.P.; Uknalis, J.; Watson, M.A. Highly Pleomorphic Strains of the Vibrio Predator Pseudoalteromonas piscicida and Their Outer Membrane Vesicles: A Scanning Electron Micrographic Study. Microorganisms 2025, 13, 365. [Google Scholar] [CrossRef]
- Potter, M.; Hanson, C.; Anderson, A.J.; Vargis, E.; Britt, D.W. Abiotic stressors impact outer membrane vesicle composition in a beneficial rhizobacterium: Raman spectroscopy characterization. Sci. Rep. 2020, 10, 21289. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.J.; Qian, H.; Tan, L.; Zhang, Z.; Liu, H.; Pan, Y.; Zhao, Y. Insights into the role of extracellular DNA and extracellular proteins in biofilm formation of Vibrio parahaemolyticus. Front. Microbiol. 2020, 11, 813. [Google Scholar] [CrossRef]
- Mugunthan, S.; Wong, L.L.; Winnerdy, F.R.; Summers, S.; Bin Ismail, M.H.; Foo, Y.H.; Jaggi, T.K.; Meldrum, O.W.; Tiew, P.Y.; Chotirmall, S.H.; et al. RNA is a key component of extracellular DNA networks in Pseudomonas aeruginosa biofilms. Nat. Commun. 2023, 14, 7772. [Google Scholar] [CrossRef]
- Rath, S.; Fatma, S.; Das, S. Unraveling the multifaceted role of extracellular DNA (eDNA) of biofilm in bacterial physiology, biofilm formation, and matrixome architecture. Crit. Rev. Biochem. Mol. Biol. 2025, 60, 1–32. [Google Scholar] [CrossRef]
- Mlynek, K.D.; Bozue, J.A. Why vary what’s working? Phase variation and biofilm formation in Francisella tularensis. Front. Microbiol. 2022, 13, 1076694. [Google Scholar] [CrossRef]
- Bogdanov, M.; Pyrshev, K.; Yesylevskyy, S.; Ryabichko, S.; Boiko, V.; Ivanchenko, P.; Kiyamova, R.; Guan, Z.; Ramseyer, C.; Dowhan, W. Phospholipid distribution in the cytoplasmic membrane of Gram-negative bacteria is highly asymmetric, dynamic, and cell shape dependent. Sci. Adv. 2020, 6, eaaz6333. [Google Scholar] [CrossRef] [PubMed]
- Nagakubo, T.; Nomura, N.; Toyofuku, M. Cracking Open Bacterial Membrane Vesicles. Front. Microbiol. 2020, 10, 3026. [Google Scholar] [CrossRef] [PubMed]
- Grogan, D.W.; Cronan, J.E. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 1997, 61, 429–441. [Google Scholar] [PubMed]
- Shabala, L.; Ross, T. Cyclopropane fatty acids improve Escherichia coli survival in acidified minimal media by reducing membrane permeability to H+ and enhanced ability to extrude H+. Res. Microbiol. 2008, 159, 458–461. [Google Scholar] [CrossRef]
- Maiti, A.; Kumar, A.; Daschakraborty, S. How do cyclopropane fatty acids protect the cell membrane of Escherichia coli in cold shock? J. Phys. Chem. B 2023, 127, 1607–1617. [Google Scholar] [CrossRef]
- Geng, J.; Long, J.; Hu, Q.; Liu, M.; Ge, A.; Du, Y.; Zhang, T.; Jin, Y.; Yang, H.; Chen, S.; et al. Current status of cyclopropane fatty acids on bacterial cell membranes characteristics and physiological functions. Microb. Pathog. 2025, 200, 107295. [Google Scholar] [CrossRef]
- Tempelhagen, L.; Ayer, A.; Culham, D.E.; Stocker, R.; Wood, J.M. Cultivation at high osmotic pressure confers ubiquinone 8-independent protection of respiration on Escherichia coli. J. Biol. Chem. 2020, 295, 981–993. [Google Scholar] [CrossRef]
- Aussel, L.; Pierrel, F.; Loiseau, L.; Lombard, M.; Fontecave, M.; Barras, F. Biosynthesis and physiology of coenzyme Q in bacteria. Biochim. Biophys. Acta 2014, 1837, 1004–1011. [Google Scholar] [CrossRef]
- Søballe, B.; Poole, R.K. Ubiquinone limits oxidative stress in Escherichia coli. Microbiology 2000, 146, 787–796. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Liang, X.; Qin, K.; Zhang, Y.; Wang, J.; Wu, Q.; Gupta, T.B.; Ding, Y. Bacterial extracellular vesicle: A non-negligible component in biofilm life cycle and challenges in biofilm treatments. Biofilm 2024, 8, 100216. [Google Scholar] [CrossRef]
- Rudnicka, M.; Noszczyńska, M.; Malicka, M.; Kasperkiewicz, K.; Pawlik, M.; Piotrowska-Seget, Z. Outer membrane vesicles as mediators of plant-bacterial interactions. Front. Microbiol. 2022, 13, 902181. [Google Scholar] [CrossRef]
- Pawlik, M.; Rudnicka, M.; Bondaruk, I.; Kasperkiewicz, K.; Noszczyńska, M.; Malicka, M.; Siupka, P.; Piotrowska-Seget, Z. Assessment of the Effect of Outer Membrane Vesicles of Endophytic Bacteria on the Growth and Physiological Response of Arabidopsis thaliana. Biol. Life Sci. Forum. 2022, 16, 36. [Google Scholar]
- Li, P.; Luo, W.; Xiang, T.X.; Jiang, Y.; Liu, P.; Wei, D.D.; Fan, L.; Huang, S.; Liao, W.; Liu, Y.; et al. Horizontal gene transfer via OMVs co-carrying virulence and antimicrobial-resistant genes is a novel way for the dissemination of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 945972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).