Incidence of Crown and Root Rot in Rhododendron simsii Caused by Phytopythium vexans in China and Screening of Endophytic Bacteria for Biocontrol
Abstract
1. Introduction
2. Materials and Methods
2.1. Disease Investigation, Sampling, and Isolation
2.2. Pathogenicity Tests
2.3. Morphological Identification
2.4. DNA Extraction, Amplification, Sequencing, and Phylogenetic Analyses
2.5. Isolation and Screening of Antagonistic Bacteria
2.6. Genome Sequencing
3. Results
3.1. Symptoms Observed in the Wild State
3.2. Pathogenicity of Fungal Isolates
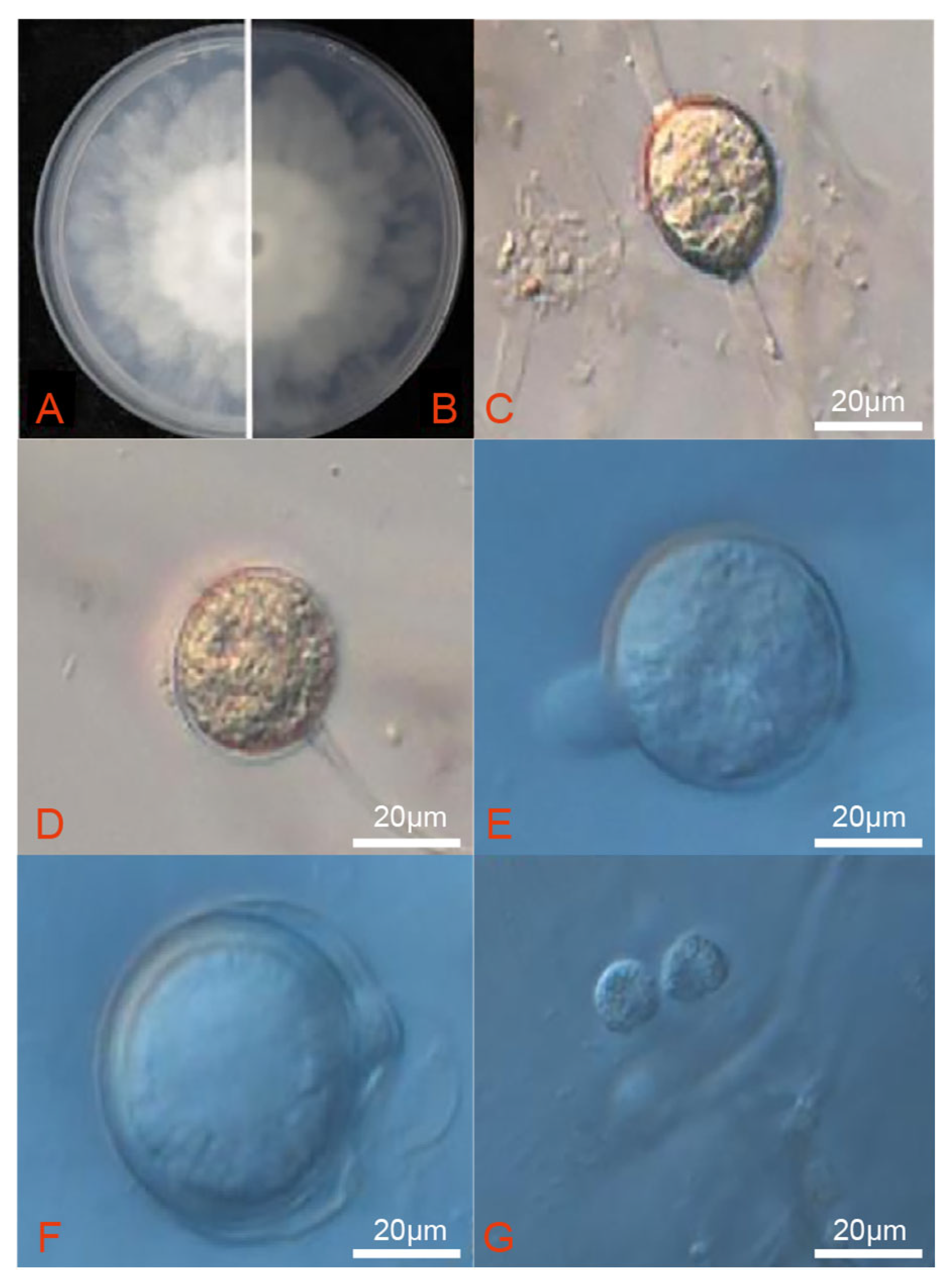
3.3. Morphological Characteristics of the Pathogens
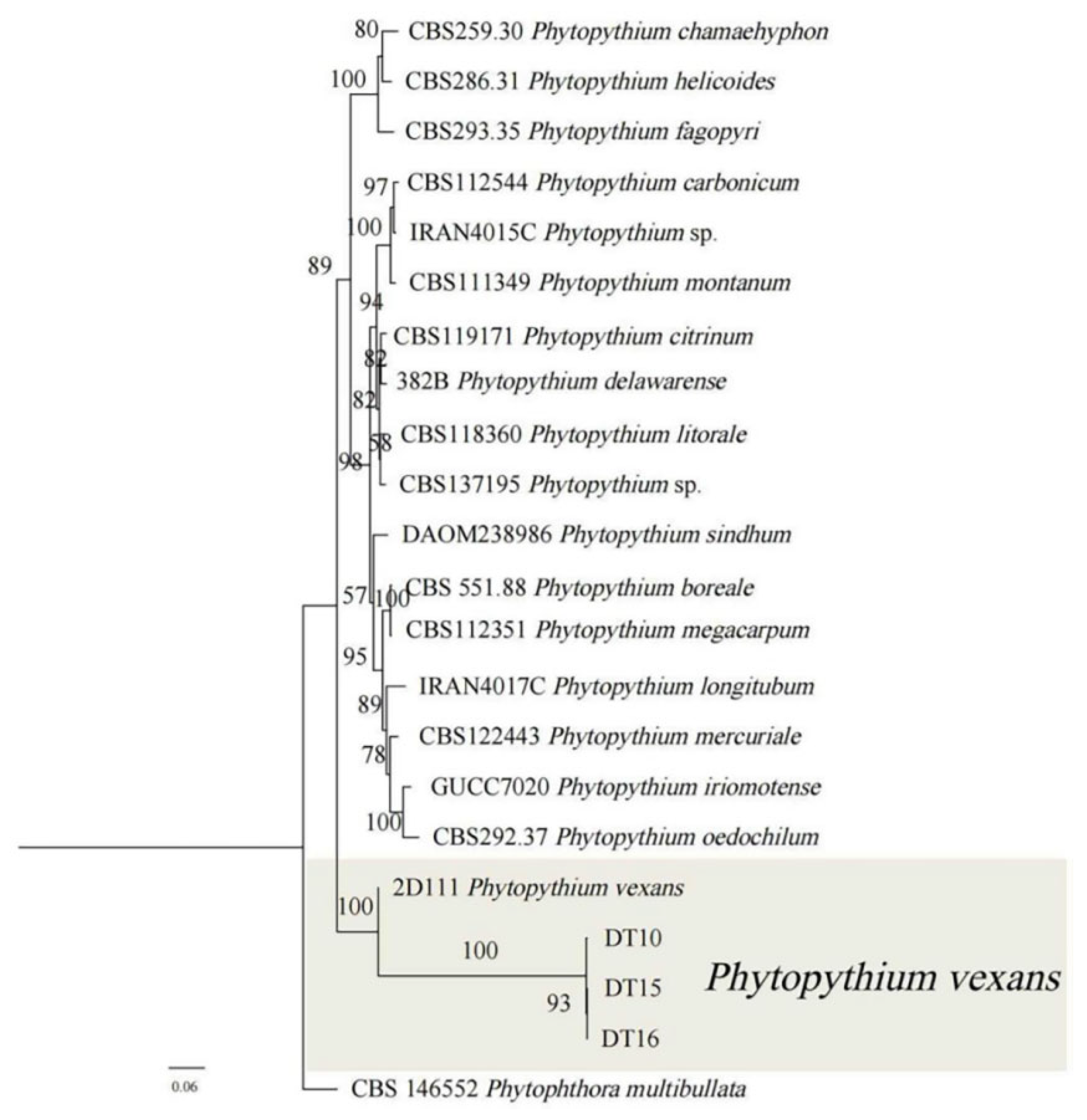
3.4. Phylogenetic Analyses of the Pathogens
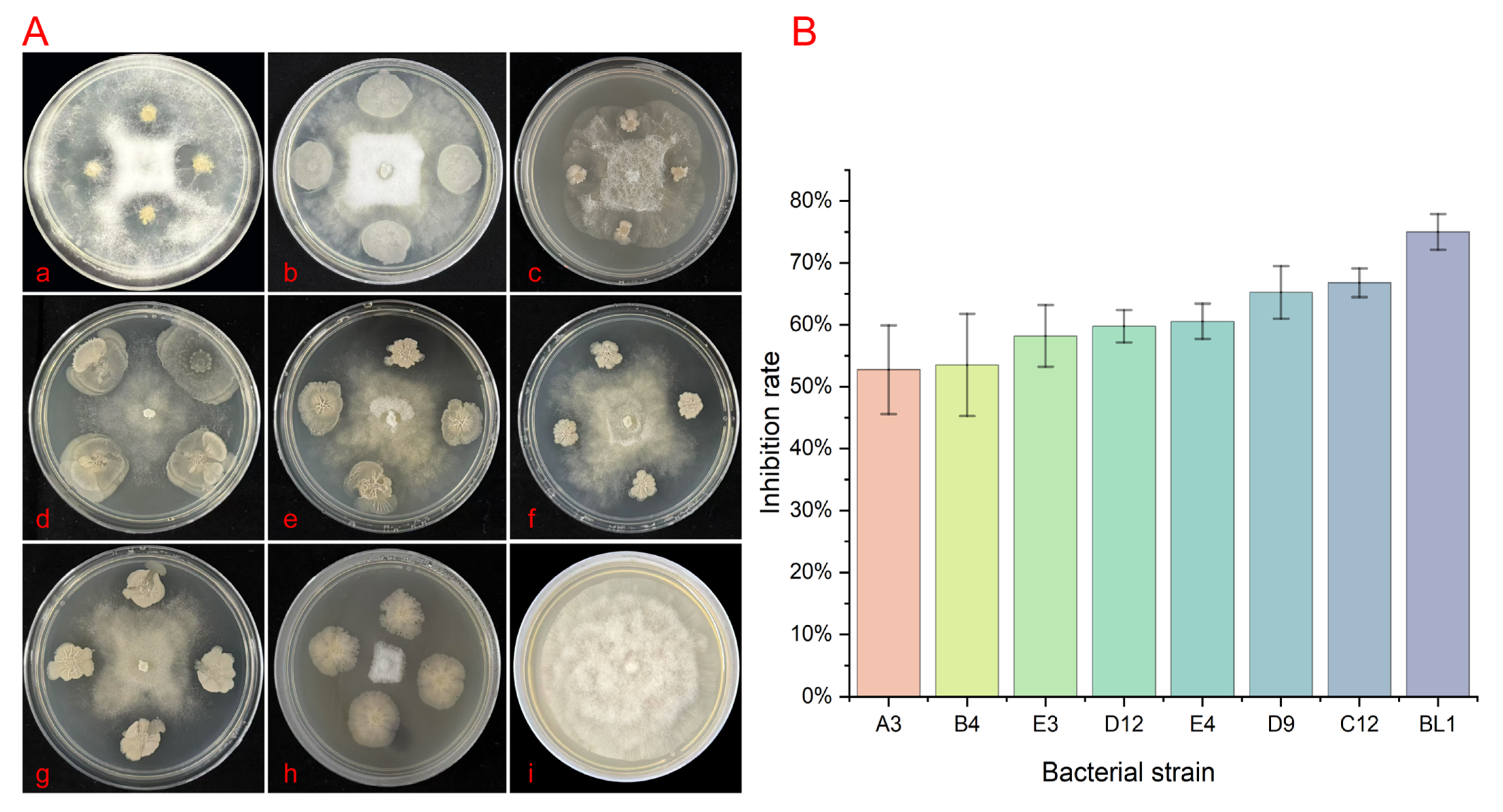
3.5. Screening of Endophytic Bacteria for Biocontrol
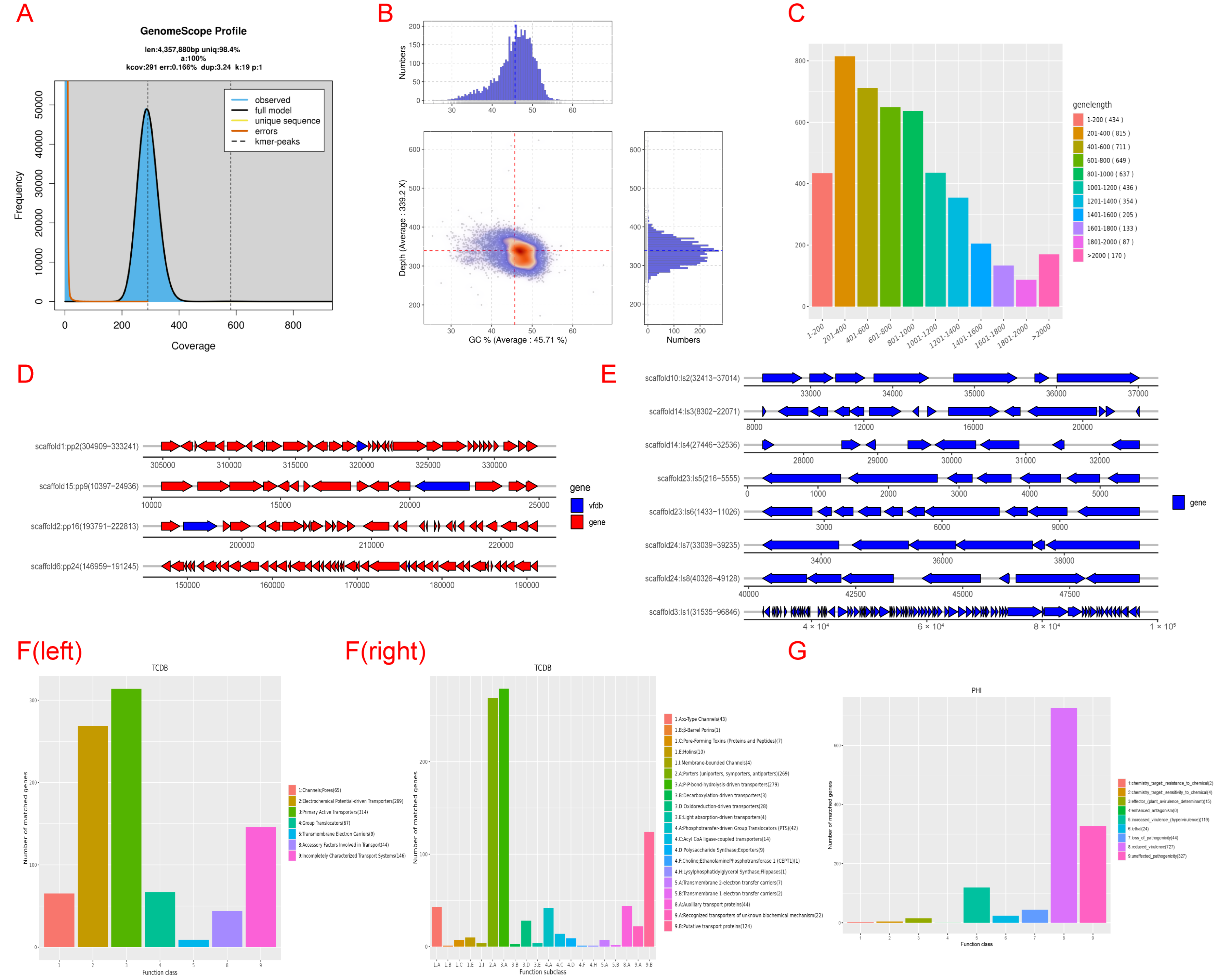
3.6. Whole-Genome Sequencing of Antagonistic Bacteria
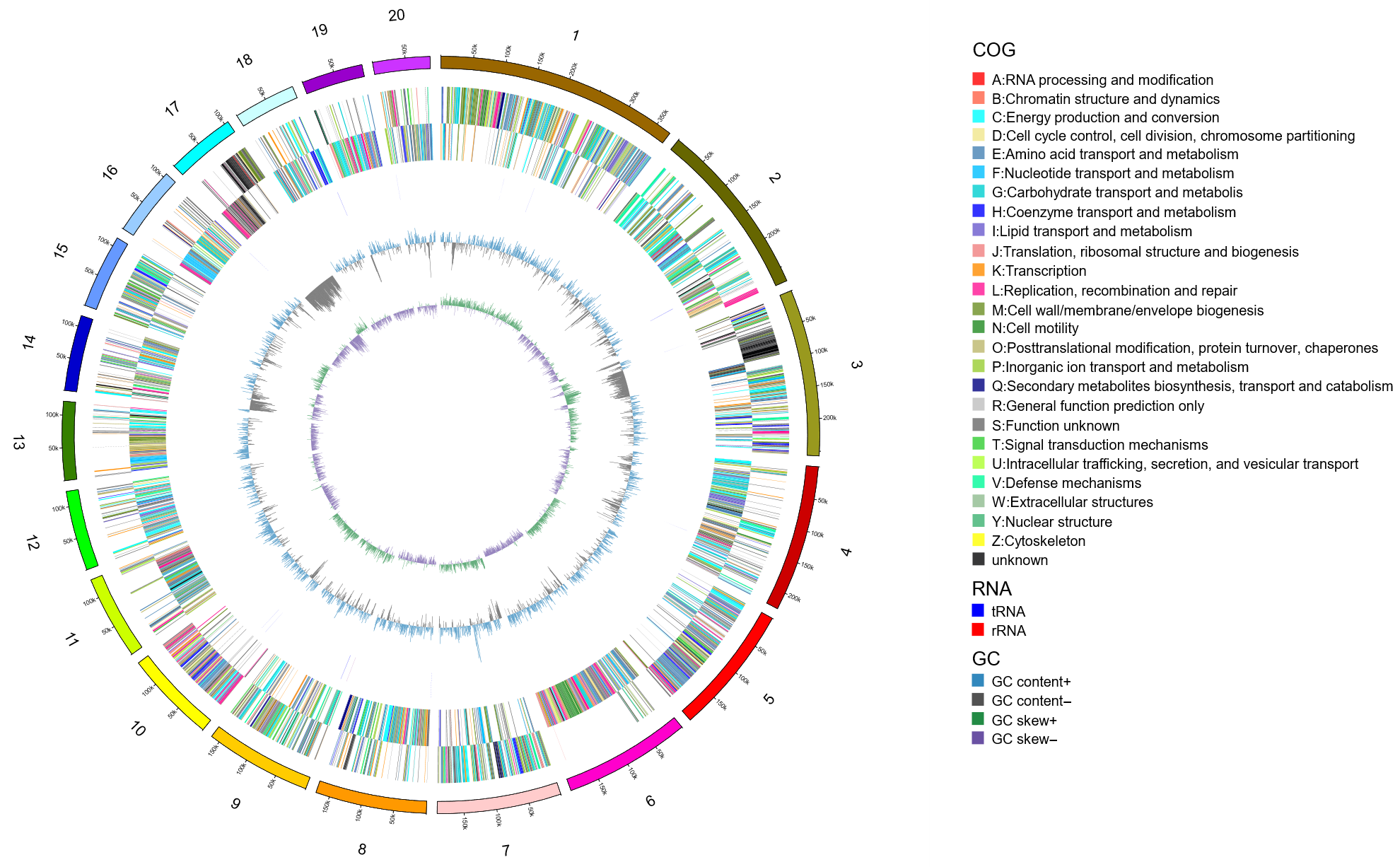
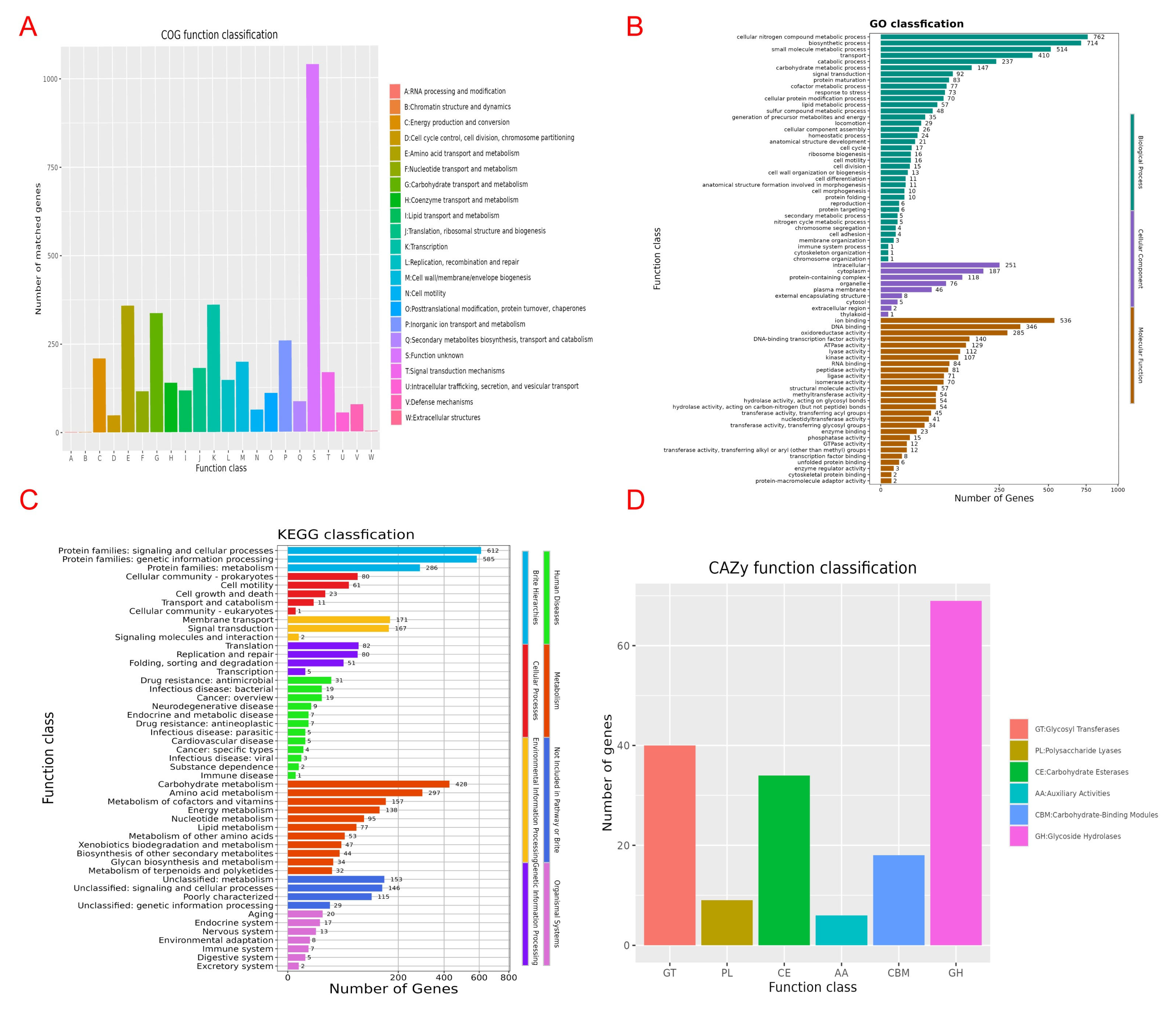
3.7. Genomic Component Analysis and the Coding Gene Annotation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Li, Z.; Ji, N.; Dong, K.; Chen, C.; Chu, H. First Report of Sphaerulina Azaleae Causing Leaf Spot on Rhododendron simsii Sweet in China. Forests 2023, 14, 1459. [Google Scholar]
- Qiu, J.; Gao, C.; Wei, H.; Wang, B.; Hu, Y.; Guo, Z.; Long, L.; Yang, L.; Li, H. Flowering Biology of Rhododendron simsii. Horticulturae 2021, 7, 508. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Zhou, Y.; Tu, M.; Wu, Z.; Gui, D.; Ma, Y.; Wang, J.; Zhang, C. The Rhododendron Plant Genome Database (RPGD): A Comprehensive Online Omics Database for Rhododendron. BMC Genom. 2021, 22, 376. [Google Scholar] [CrossRef]
- Fuqian, W.; Weiguang, S.; Zhou, L.; Zhou, Y.; Lulu, L.; Ziheng, L.; Ling, C.; Qiuyun, Y.; Qunfeng, Y. Chemical constituents and hepatoprotective properties of Rhododendron simsii Planch extract in Con A-induced autoimmune hepatitis—ScienceDirect. Arab. J. Chem. 2023, 16, 104955. [Google Scholar]
- Zhao, C.X.; Liang, Y.Z.; Li, X.N. Chemical components in essential oils from tender branches and leaves of rhododendron. Yao Xue Xue Bao Acta Pharm. Sin. 2005, 40, 854–860. [Google Scholar]
- Lai, Y.; Zhong, Y.; Liang, Y. Identification of antibacterial constituents from Rhododendron simsii planch with an ac-tivity-guided method. Front. Pharmacol. 2024, 15, 1490335. [Google Scholar] [CrossRef]
- Álvarez, L.A.; Pérez-Sierra, A.; García-Jiménez, J.; Abad-Campos, P.; Landeras, E.; Alzugaray, R. First Report of Leaf Spot and Twig Blight of Rhododendron spp. Caused by Phytophthora hibernalis in Spain. Plant Dis. 2007, 91, 909. [Google Scholar] [CrossRef]
- Ma, X.F.; Zhai, L.F.; Jiang, Y.C. First Report of Fusarium oxysporum and Fusariumsolani Causing Root Rot on Trifoliate Orange Rootstock in China. Plant Dis. 2023, 107, 944. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Hu, S.; Zhou, Z.C. First report of leaf spot on Rhododendron delavayi caused by Pestalotiopsis scoparia in China. Plant Dis. 2023, 107, 1626. [Google Scholar] [CrossRef]
- Rytkon, A.; Lilha, A.; Vercautere, A. Identity and potential pathogenicity of Phytophthora species found on symptomatic Rhododendron plants in a Finnish nursery. Can. J. Plant Pathol. Rev. Can. Phytopathol. 2012, 34, 13. [Google Scholar]
- Tsopela, P.; Paplomata, E.J.; Tjamos, S.E. First Record of Phytophthora nicotianae Causing Leaf Blight on Rhododendron in Greece. Plant Dis. 2011, 95, 777. [Google Scholar] [CrossRef]
- Browne, Y.E.; Edwards, G.S.; Nellist, F.C. First report of Phytopythium vexans and Phytopythium litorale associated with root rot symptoms on red raspberry (Rubus idaeus). New Dis. Rep. 2023, 48, e12197. [Google Scholar] [CrossRef]
- Wang, K.X.; Xie, Y.L.; Yuan, G.Q.; Li, Q.Q.; Lin, W. First Report of Root and Collar Rot Caused by Phytopythium Helicoides on Kiwifruit (Actinidia chinensis). Plant Dis. 2015, 99, 725. [Google Scholar] [CrossRef]
- Kumar, B.; Choudhary, M.; Kumar, K.; Kumar, P.; Kumar, S.; Bagaria, P.K.; Sharma, M.; Lahkar, C.; Singh, B.K.; Pra-dhan, H. Maydis Leaf Blight of Maize: Update on Status, Sustainable Management and Genetic Architecture of Its Resistance. Physiol. Mol. Plant Pathol. 2022, 121, 101889. [Google Scholar] [CrossRef]
- Marin, M.; Seijo, T.; Mertely, J.; Peres, N. First Report of Crown Rot Caused by Phytopythium helicoides on Strawberry in the Americas. Plant Dis. 2019, 103, 10–1094. [Google Scholar] [CrossRef]
- Gonçalves, D.R.; de Jesus, A.L.; Pires-Zottarelli, C.L.A. Pythium and Phytopythium Species Associated with Hydroponically Grown Crops around the City of São Paulo, Brazil. Trop. Plant Pathol. 2016, 41, 397–405. [Google Scholar]
- Afandi, A.; Murayama, E.; Yin, L.; Hieno, A.; Suga, H.; Kageyama, K. Population Structures of the Water-Borne Plant Pathogen Phytopythium helicoides Reveal Its Possible Origins and Transmission Modes in Japan. PLoS ONE 2018, 13, e0209667. [Google Scholar] [CrossRef]
- Han, K.-S.; Hong, S.-B.; Lee, S.-C.; Han, Y.-K.; Kim, D.-H. Root Rot of Rose Caused by Pythium helicoides in Korea. Plant Pathol. J. 2010, 26, 429. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, T.; Xu, X.; Dai, T.; Liu, T. The New Report of Root Rot on Fatsia Japonica Caused by Phytophthora nicotianae in China. Forests 2023, 14, 1459. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Noireung, P. First Report of Phytopythium Vexans Causing Root Rot on Mandarin (Citrus reticulate L. Cv. Sainampueng) in Thailand. Plant Pathol. Quar. 2020, 10, 85–90. [Google Scholar] [CrossRef]
- Bala, K.; Robideau, G.P.; Désaulniers, N.; de Cock, A.W.A.M.; Lévesque, C.A. Taxonomy, DNA Barcoding and Phylogeny of Three New Species of Pythium from Canada. Persoonia—Mol. Phylogeny Evol. Fungi 2010, 25, 22–31. [Google Scholar]
- Ahsan, N.; Hayashi, M.; Otsubo, K.; Hieno, A.; Suga, H.; Kageyama, K. First Report of Root and Stem Rot of Sedum Caused by Five Oomycete Species. J. Gen. Plant Pathol. 2023, 89, 244–248. [Google Scholar] [CrossRef]
- Xie, X.Y.; Zhou, H.Z.; Fan, S.S.; Zhang, X.J. First Report of Phytopythium helicoides Causing Stalk Rot on Corn in China. Plant Dis. 2021, 105, 3766. [Google Scholar] [CrossRef]
- Polat, Z.; Awan, Q.N.; Hussain, M.; Akgül, D.S. First Report of Phytopythium vexans Causing Root and Collar Rot of Kiwifruit in Turkey. Plant Dis. 2017, 101, 1058. [Google Scholar] [CrossRef]
- Yang, X.; Richardson, P.A.; Olson, H.A.; Hong, C.X. Root and Stem Rot of Begonia Caused by Phytopythium helicoides in Virginia. Plant Dis. 2013, 97, 1385. [Google Scholar] [CrossRef]
- Fichtner, E.J.; Browne, G.T.; Mortaz, M.; Ferguson, L.; Blomquist, C.L. First Report of Root Rot Caused by Phytopythium helicoides on Pistachio Rootstock in California. Plant Dis. 2016, 100, 2337. [Google Scholar] [CrossRef]
- Xie, H.; Yu, T.; Zhou, Q.; Na, K.; Lu, S.; Zhang, L.; Guo, X. Comparative Evaluation of Spores and Vegetative Forms of Bacillus subtilis and Bacillus licheniformis on Probiotic Functionality In Vitro and In Vivo. Probiotics Antimicrob. Proteins 2024, 1–19. [Google Scholar] [CrossRef]
- Sharma, M.; Ghosh, R. A Reliable Method for Phytophthora cajani Isolation, Sporangia, Zoospore Production and in Planta Infection of Pigeonpea. Bio-Protocol 2016, 6, e1706. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, J.; Jiao, B.; Wu, C.; Dai, T. First Report of Crown and Root Rot Caused by Phytopythium helicoides on Photinia × Fraseri Dress in China. Plant Dis. 2022, 104, 1098. [Google Scholar]
- Tkaczyk, M. Phytopythium: Origin, Differences and Meaning in Modern Plant Pathology. Folia For. Pol. 2020, 62, 227–232. [Google Scholar] [CrossRef]
- Lévesque, C.A.; Cock, A.W.A.M.D. Molecular Phylogeny and Taxonomy of the Genus Pythium. Mycol. Res. 2004, 108, 1363–1383. [Google Scholar] [CrossRef] [PubMed]
- Uzuhashi, S.; Kakishima, M.; Tojo, M. Phylogeny of the genus pythium and description of new genera. Mycoscience 2010, 51, 337–365. [Google Scholar] [CrossRef]
- De Cock, A.W.; Lodhi, A.M.; Rintoul, T.L.; Bala, K.; Robideau, G.P.; Abad, Z.G.; Coffey, M.D.; Shahzad, S.; Lévesque, C.A. Phytopythium: Molecular phylogeny and systematics. Persoonia 2015, 34, 25–39. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum gloeosporioides isolates from avocado and almond fruits with molecular and pathogenicity tests. Appl. Environ. Microbiol. 1996, 62, 1014–1120. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Chac, L.D.; Thinh, B.B. Species Identification through DNA Barcoding and Its Applications: A Review. Biol. Bull. Russ. Acad. Sci. 2023, 50, 1143–1156. [Google Scholar] [CrossRef]
- Kwiatkowski, N.; Babiker, W.; Merz, W.; Carroll, K.; Zhang, S. Evaluation of Nucleic Acid Sequencing of the D1/D2 Region of the Large Subunit of the 28S rDNA and the Internal Transcribed Spacer Region Using SmartGene IDNS Software for Identification of Filamentous Fungi in a Clinical Laboratory. J. Mol. Diagn. 2012, 14, 393–401. [Google Scholar] [CrossRef]
- Brænne, I.; Willenborg, C.; Tragante, V.; Kessler, T.; Zeng, L.; Reiz, B.; Kleinecke, M.; von Ameln, S.; Willer, C.J.; Laakso, M.; et al. A Genomic Exploration Identifies Mechanisms That May Explain Adverse Cardiovascular Effects of COX-2 Inhibitors. Sci. Rep. 2017, 7, 10252. [Google Scholar] [CrossRef]
- Robideau, G.P.; Brouwer, H.; Bala, K. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol. Ecol. Resour. 2011, 11, 1002–1011. [Google Scholar] [CrossRef]
- Liang, C.H.; Bo, J.W.; Hua, W. Molecular cloning and expression analysis of cytochrome c oxidase subunit II from Sitophilus zeamais. Biochem. Biophys. Res. Commun. 2016, 478, 1660–1666. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Bakker, F.T.; van den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic Analysis of Phytophthora Species Based on Mitochondrial and Nuclear DNA Sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. Bacteriol 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Yang, J.X.; Lian, S.W.; Xie, Z.H.; Luo, J.Q.; Cheng, P.Y.; Xie, J.J.; Li, Z.J. First Phylogenetic Analyses Showing Pseudocercospora rhododendricola Causing Leaf Dark Spot on Rhododendron simsii. Plant Dis. 2023, 107, 953. [Google Scholar] [CrossRef]
- Baten, M.A.; Asano, T.; Motohashi, K.; Ishiguro, Y.; Rahman, M.Z.; Inaba, S.; Suga, H.; Kageyama, K. Phylogenetic relationships among Phytopythium species, and re-evaluation of Phytopythium fagopyri comb. nov., recovered from damped-off buckwheat seedlings in Japan. Mycol. Prog. 2014, 13, 1003. [Google Scholar] [CrossRef]
- Villanueva, O.; Ellouze, W. First report of a Canadian isolate of Phytopythium vexans causing root rot disease on apple and peach under laboratory conditions. New Dis. Rep. 2023, 48, e12195. [Google Scholar] [CrossRef]
- Drizou, F.; Hubbuck, K.; Barry, C. First report of Phytophthora occultans infecting euphorbia amygdaloides in the United Kingdom. New Dis. Rep. 2024, 49, e12242. [Google Scholar] [CrossRef]
- Li, Z.; Guo, L. Three New Species of Exobasidiu (Exobasidiale) from China. Mycotaxon 2009, 107, 215–220. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; Conti Taguali, S.; Pane, A.; Franco, M.; Cacciola, S.O. Phytophthora × cambivora as a Major Factor Inciting the Decline of European Beech in a Stand within the Southernmost Limit of Its Natural Range in Europe. J. Fungi. 2022, 8, 973. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Li, Y.; Chen, Z.; Dai, T. First Report of Phytophthora pini Causing Foliage Blight and Shoot Dieback of Rhododendron simsii in China. Plant Dis. 2021, 105, 1229. [Google Scholar] [CrossRef]
- Jabiri, S.; Lahlali, R.; Bahra, C.; Bendriss Amraoui, M.; Tahiri, A.; Amiri, S. First Report of Phytopythium vexans Associated with Dieback Disease of Apple Trees in Morocco. J. Plant Pathol. 2020, 102, 1319. [Google Scholar] [CrossRef]
- Park, M.-J.; Back, C.-G.; Park, J.-H. Occurrence of Phytopythium vexans Causing Stem Rot on Anthurium andraeanum in Korea. Korean J. Mycol. 2019, 47, 443–446. [Google Scholar]
- Liyanapathiranage, P.; Avin, F.A.; Oksel, C.; Parajuli, M.; Scott, M.; Simmons, T.; Baysal-Gurel, F. First Report of Root Rot of Redbud Caused by Phytopythium vexans in Tennessee and the United States. Plant Dis. 2023, 107, 4036. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Chen, F. Root Rot of Cinnamomum camphora (Linn) Presl Caused by Phytopythium vexans in China. Plants 2023, 12, 1072. [Google Scholar] [CrossRef]
- Galvez, A.; Maqueda, M.; Cordovilla, P. Characterization and biological activity against naegleria fowleri of amoebicins produced by bacillus licheniformis D-13. Antimicrob. Agents Chemother. 1994, 38, 1314–1319. [Google Scholar] [CrossRef]
- Hudáková, N.; Kacírová, J.; Sondorová, M. Inhibitory effect of bacillus licheniformis strains isolated from canine oral cavity. Life 2022, 12, 1238. [Google Scholar] [CrossRef]
- Nannan, C.; Vu, H.; Gillis, A. Bacilysin within the bacillus subtilis group: Gene prevalence versus antagonistic activity against gram-negative foodborne pathogens. J. Biotechnol. 2021, 327, 28–35. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Pan, X.; Hu, M.Y.; Wang, Z.W. Genome-wide identification and expression analysis of chitinase gene family in rice. Plant Physiol. J. 2022, 58, 746–756. [Google Scholar]
- Kanehisa, M.; Sato, Y. KEGG mapper for inferring cellular functions from protein sequences. Protein Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef]
- Altman, T.; Travers, M.; Kothari, A. A systematic comparison of the MetaCyc and KEGG pathway databases. BMC Bioinform. 2013, 14, 112. [Google Scholar] [CrossRef]
- Nersisyan, L.; Samsonyan, R.; Arakelyan, A. CyKEGGParser: Tailoring KEGG pathways to fit into systems biology analysis workflows. F1000Research 2014, 3, 145. [Google Scholar] [CrossRef]


| Blast Match Sequence | Sequence Identity (%) | |||
|---|---|---|---|---|
| Isolate | DNA Target | GenBank Accession Numbers | Reference Accession No. | |
| DT10 | LSU | ON627804.1 | P. vexans FBG2017010 (MT533451.1) | 100% |
| COXI | ON623508.1 | P. vexans FBG20181 (MT076052.1) | 99.20% | |
| COXII | ON623510.1 | P. vexans STE-U6741 (GU133560.1) | 99.49% | |
| DT15 | LSU | OQ754108 | P. vexans FBG2017010 (MT533451.1) | 100% |
| COXI | ON623509.1 | P. vexans STE-U6708 (GU133476.1) | 98.81% | |
| COXII | ON623511.1 | P. vexans STE-U6741 (GU133560.1) | 99.83% | |
| DT16 | LSU | MZ519893 | P. vexans KACC:410989 (PV473534.1) | 100% |
| COXI | OQ753101 | P. vexans CBS119.80 (CBS119.80) | 99.32% | |
| COXII | OQ753097 | P. vexans STE-U6741 (GU133560.1) | 99.49% | |
| Phytophthora Species | GenBank Accession Numbers | |||
|---|---|---|---|---|
| Isolate | LSU | COXI | COXII | |
| P. vexans | DT10 | ON627804.1 | ON623508.1 | ON623510.1 |
| P. vexans | DT15 | OQ754108 | ON623509 | ON623511 |
| P. vexans | DT16 | MZ519893 | OQ753101 | OQ753097 |
| P. vexans | 2D111 | AB856796.1 | AB856784.1 | AB948193.1 |
| Phytopythium sp. | CBS137195 | AB948194.1 | AB948191.1 | AB948191.1 |
| Phytopythium sp. | IRAN40150 | MT729975.1 | MT720654.1 | MT720670.1 |
| Ph. boreale | CBS 551.88 | HO665261.1 | EF408872.1 | EF408876.1 |
| Ph. chamaehyphon | CBS259.30 | AB690593.1 | AB690644.1 | AB690674.1 |
| Ph. citrinum | CBS119171 | AB690597.1 | AB690649.1 | AB690679.1 |
| P. helicoides | CBS286.31 | AB690594.1 | AB690645.1 | AB690675.1 |
| Ph.chamaehyphon | CBS259.30 | AB690593.1 | AB690644.1 | AB690674.1 |
| Ph. iriomotense | GUCC7020 | AB690607.1 | AB690659.1 | AB690689.1 |
| Ph. sindhum | DAOM238986 | HO643396.2 | HO708443.1 | |
| Ph. mercuriale | CBS122443 | AB690585.1 | AB690636.1 | AB690666.1 |
| Gene | Primer | Sequence (5′–3′) | PCR Conditions |
|---|---|---|---|
| LUS | NL1 | ACCCGCTGAACTYAAGC | 94 °C, 3 min; (94 °C, 30 s; 58 °C, 30 s; 72 °C, 45 s) × 35; 72 °C, 10 min |
| NL4 | CGCCAGACGAGCTTACC | ||
| COXI | OomCoxI-Levup | TCAWCWMGATGGCTTTTTTCAAC | 94 °C, 3 min; (94 °C, 30 s; 56 °C, 30 s; 72 °C, 45 s) × 35; 72 °C, 10 min |
| FM85mod | RRHWACKTGACTDATRATACAAA | ||
| COXII | FM58 | GTATTTCTTCTTTATTAGGTGC | 94 °C, 3 min; (94 °C, 30 s; 56 °C, 30 s; 72 °C, 45 s) × 35; 72 °C, 10 min |
| FM66 | CGTGAACTAATGTTACATATAC |
| Locus | Primer | Sequence (5′–3′) | PCR Conditions |
|---|---|---|---|
| 16S ribosomal DNA (16S rDNA) | 27F | AGAGTTTGATCCTGGCTCAG | 94 °C, 3 min; (94 °C, 30 s, 58 °C, 30 s; 72 °C, 15 s) × 30; 72 °C, 10 min |
| 1492R | TACGGYTACCTTGTTACGACTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Sun, Y.; Yongcuo, Z.; Zhang, X.; Wang, G.; Liu, Y.; Dai, T. Incidence of Crown and Root Rot in Rhododendron simsii Caused by Phytopythium vexans in China and Screening of Endophytic Bacteria for Biocontrol. Microorganisms 2025, 13, 2417. https://doi.org/10.3390/microorganisms13112417
Liu Z, Sun Y, Yongcuo Z, Zhang X, Wang G, Liu Y, Dai T. Incidence of Crown and Root Rot in Rhododendron simsii Caused by Phytopythium vexans in China and Screening of Endophytic Bacteria for Biocontrol. Microorganisms. 2025; 13(11):2417. https://doi.org/10.3390/microorganisms13112417
Chicago/Turabian StyleLiu, Zhuo, Yang Sun, Zhuoma Yongcuo, Xiaorui Zhang, Guibin Wang, Yuhua Liu, and Tingting Dai. 2025. "Incidence of Crown and Root Rot in Rhododendron simsii Caused by Phytopythium vexans in China and Screening of Endophytic Bacteria for Biocontrol" Microorganisms 13, no. 11: 2417. https://doi.org/10.3390/microorganisms13112417
APA StyleLiu, Z., Sun, Y., Yongcuo, Z., Zhang, X., Wang, G., Liu, Y., & Dai, T. (2025). Incidence of Crown and Root Rot in Rhododendron simsii Caused by Phytopythium vexans in China and Screening of Endophytic Bacteria for Biocontrol. Microorganisms, 13(11), 2417. https://doi.org/10.3390/microorganisms13112417






