Microbial Interventions for Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis of Atopic Dermatitis and Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Research Question
2.3. Eligibility Criteria
2.3.1. Inclusion Criteria
- Clinical efficacy: investigator-assessed improvement, disease severity scores (e.g., SCORAD, EASI, PASI, IGA), time to clinical response, or prevention of recurrence.
- Physiological markers: indicators of skin barrier function (e.g., TEWL, stratum corneum hydration) or inflammatory biomarkers.
- Patient-reported outcomes: quality of life measures (e.g., DLQI, CDLQI).
- Microbiological parameters: data on microbiome diversity.
- Safety: the incidence of adverse events.
2.3.2. Exclusion Criteria
2.4. Data Sources and Search Strategy
2.5. Selection and Data Extraction
2.6. Risk of Bias Assessment
2.7. Assessment of the Quality of Evidence
2.8. Data Analysis
- Atopic dermatitis (12 studies, n = 817 participants): the primary outcome was the change in disease severity measured by the SCORing Atopic Dermatitis (SCORAD) index.
- Psoriasis (5 studies, n = 287 participants): outcomes included change in disease severity measured by the Psoriasis Area and Severity Index (PASI) and change in quality of life measured by the Dermatology Life Quality Index (DLQI).
3. Results
3.1. Studies Identified for the Review
3.2. Participant Characteristics
| Study Reference | Participants | Size (n) | Age (Years) | BMI (kg/m2) | Baseline Parameters |
|---|---|---|---|---|---|
| Gerasimov et al., 2010 [28] | Children with moderate-to-severe AD | 90 | 1–3 | Not Reported | SCORAD, IDQOL, DFI, topical steroid use, lymphocyte subsets |
| Rather et al., 2021 [29] | Children and adolescents with AD | 90 | 3–18 | Not Reported | SCORAD, IGA, IgE, ECP, eosinophils, CCL17, CCL27, skin moisture/sebum |
| Navarro et al., 2017 [30] | Children with moderate AD | 50 | 4–17 | Not Reported | SCORAD, topical steroid use |
| Wu et al., 2015 [24] | Children with AD (SCORAD ≥ 15) | 66 | 0.3–4 | Not Reported | SCORAD, steroid use, flare frequency, symptom-free duration |
| Jeong et al., 2020 [31] | Children with moderate AD | 100 (66 analyzed) | 1–12 | Not Reported | SCORAD, ECP, IL-31, safety parameters |
| Michelotti et al., 2021 [32] | Adults with mild-to-severe AD | 80 | 18–50 | Not Reported | SCORAD, inflammatory cytokines (tape stripping), skin hydration, smoothness |
| Umborowati et al., 2024 [25] | Adults with mild-to-moderate psoriasis | 49 | 18–70 | 24.8–27.4 | PASI, DLQI, IL-10, IL-17, Foxp3 |
| Cukrowska et al., 2021 [33] | Children with AD + CMPA | 151 | <2 | Not Reported | SCORAD, specific and total IgE |
| Eguren et al., 2024 [34] | Patients with mild-to-moderate acne | 81 | 12–30 | Not Reported | AGSS, GAGS, lesion counts |
| Akbarzadeh et al., 2022 [35] | Adults with psoriasis (PASI > 2%) | 52 | 18–60 | Not Reported | PASI, DLQI, VAS |
| Albuquerque et al., 2022 [36] | Children/adolescents with AD | 60 | 0.5–19 | Not Reported | SCORAD, Hanifin & Rajka criteria, IgE, SPT |
| Carucci et al., 2022 [37] | Children with AD (ProPAD trial) | 100 | 0.5–3 | Not Reported | SCORAD, IDQOL, microbiome |
| Moludi et al., 2021 [38] | Adults with plaque psoriasis | 50 | 18–50 | Not Reported | PASI, PSS, DLQI, BDI-II, inflammatory and oxidative stress markers |
| D’Auria et al., 2021 [39] | Infants with moderate–severe AD | 58 | 0.5–3 | Not Reported | SCORAD, gut microbiota, cytokines, steroid use |
| Ahn et al., 2020 [40] | Children with mild-to-moderate AD | 82 | 2–13 | Not Reported | SCORAD, TEWL, IgE, cytokines, gut microbiome |
| Gilli et al., 2023 [26] | Adults with plaque psoriasis | 35 | >18 (Mean: 52.5) | Mean: 30.1 | PASI, BSA, DLQI, IL-17, IL-23 |
| Jacobson et al., 2024 [23] | Children and adults with AD | 154 | ≥2 | Not Reported | IGA, EASI, BSA, POEM, Pruritus NRS |
| Prakoeswa et al., 2020 [22] | Adults with AD | 30 | >14 (Mean: 37.9) | Not Reported | SCORAD, IgE, IL-4, IFN-γ, Foxp3+, IL-17 |
| Suriano et al., 2023 [27] | Adults with plaque psoriasis | 103 | >18 (Mean: 51) | Mean: 29 | PASI, DLQI |
3.3. Intervention Characteristics
3.4. Summary of Outcomes, Adherence, and Safety
3.5. Methodological Quality Assessment

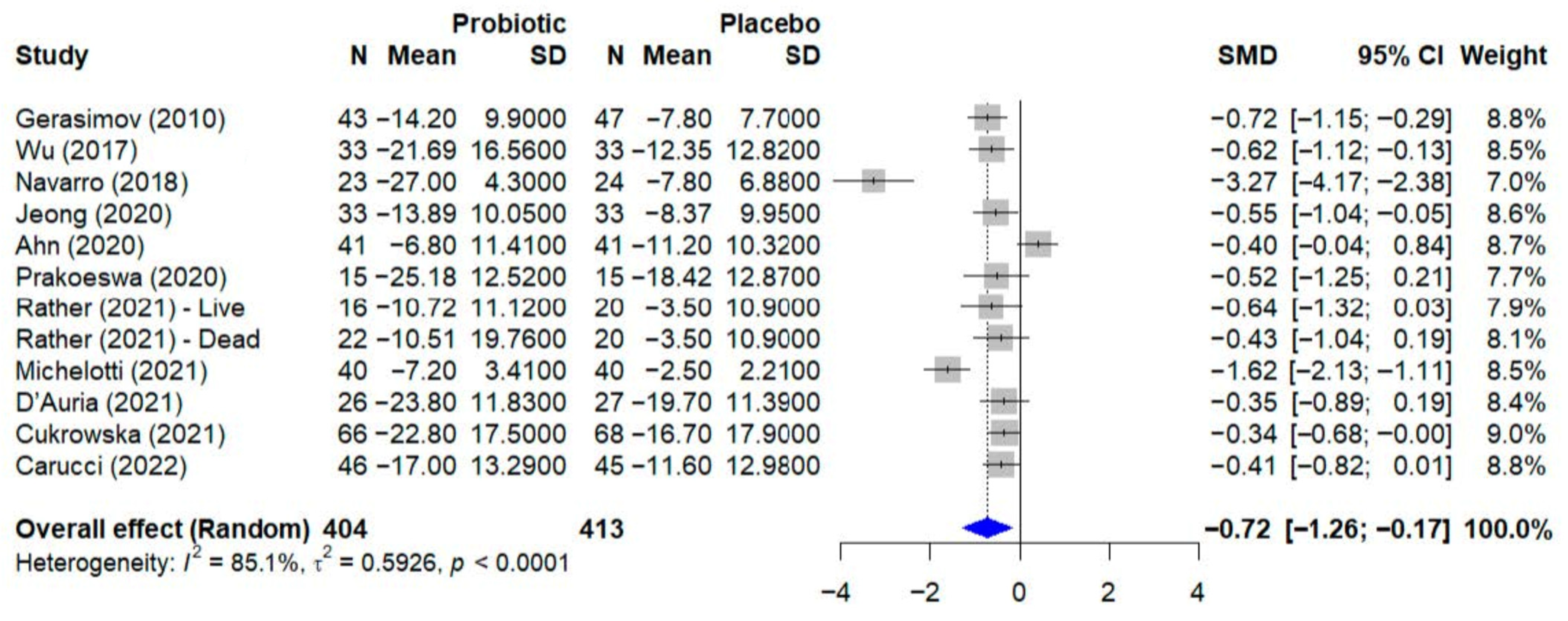
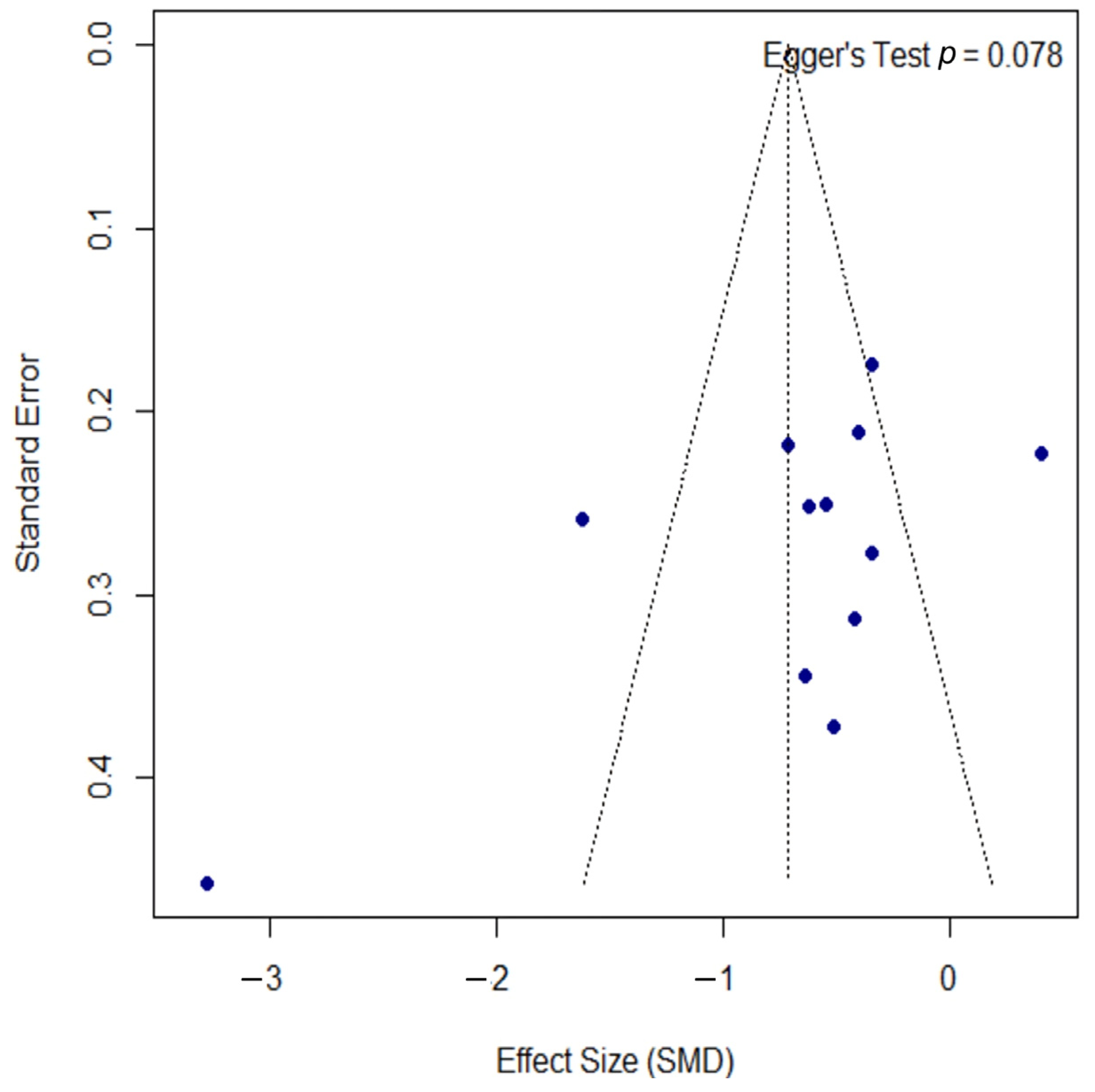
3.6. Quantitative Analysis—Atopic Dermatitis
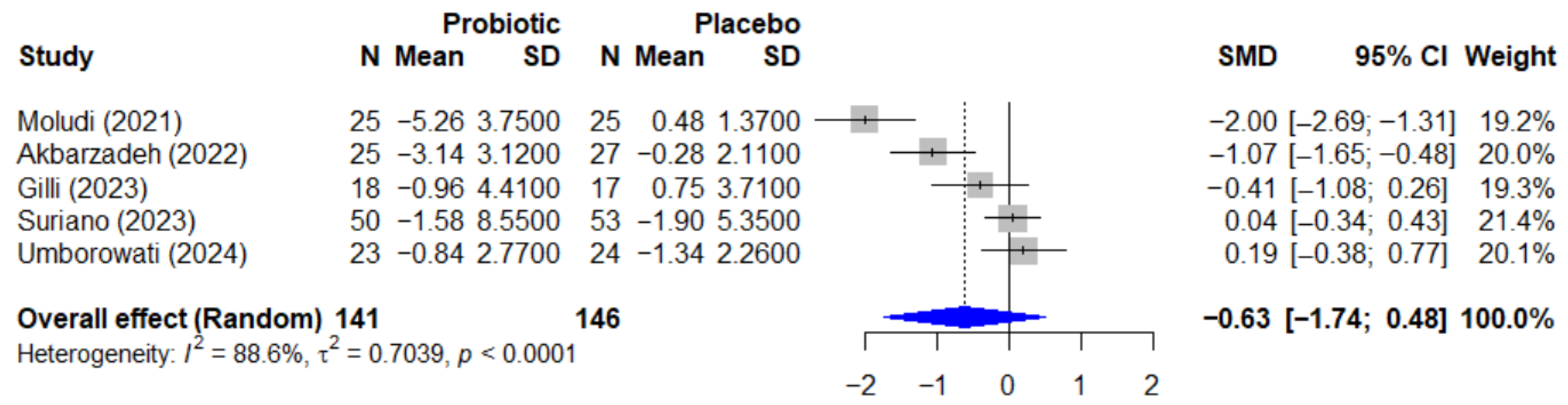
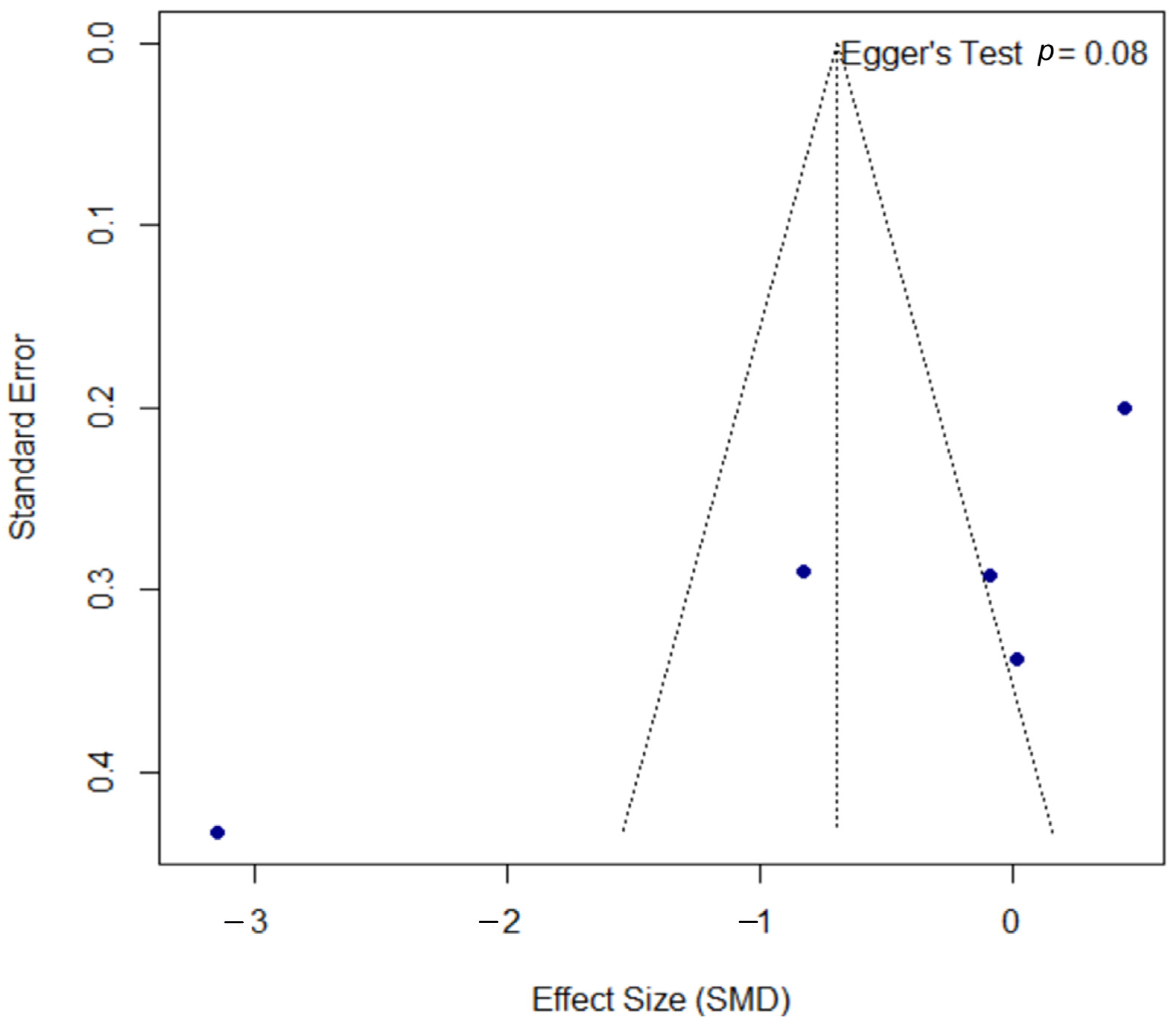
3.7. Quantitative Analysis—Psoriasis
3.8. Quantitative Analysis—Analysis of Probiotics on Quality of Life
4. Discussion
4.1. Main Findings
4.2. Mechanisms of Action of Probiotics, Prebiotics, and Synbiotics in Dermatology
4.3. Comparison with the Existing Literature
4.4. Limitations of the Included Studies
4.5. Limitations of the Review
4.6. Clinical Implications
4.7. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic Dermatitis |
| AGSS | Acne Global Severity Scale |
| AhR | Aryl Hydrocarbon Receptor |
| BMI | Body Mass Index |
| BSA | Body Surface Area |
| CCL17/27 | Chemokines |
| CFUs | Colony-Forming Units |
| CI | Confidence Interval |
| CMPA | Cow’s Milk Protein Allergy |
| CSU | Chronic Spontaneous Urticaria |
| DLQI | Dermatology Life Quality Index |
| EASI | Eczema Area and Severity Index |
| ECP | Eosinophil Cationic Protein |
| FFAR2/3 | Free Fatty Acid Receptor 2/3 |
| FLG | Filaggrin |
| FOS | Fructo-oligosaccharide |
| GAGS | Global Acne Grading System |
| GPR41/43/109A | G Protein-Coupled Receptor 41/43/109A |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| HDAC | Histone Deacetylase |
| I-FABP | Intestinal Fatty Acid-Binding Protein |
| IGA | Investigator’s Global Assessment |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| LBP | Lipopolysaccharide-Binding Protein |
| LGG | Lactobacillus rhamnosus GG |
| MCID | Minimum Clinically Important Difference |
| NRS | Numeric Rating Scale |
| PASI | Psoriasis Area and Severity Index |
| PICO | Population, Intervention, Comparison, Outcomes |
| POEM | Patient-Oriented Eczema Measure |
| PPARγ | Peroxisome Proliferator-Activated Receptor γ |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
| Reg3A | Regenerating Islet-Derived Protein 3-Alpha |
| REML | Restricted Maximum-Likelihood |
| RoB 2 | Risk of Bias tool version 2 |
| SCFA | Short-Chain Fatty Acid |
| SCORAD | SCORing Atopic Dermatitis |
| SMD | Standardized Mean Difference |
| SPT | Skin Prick Test |
| TEWL | Transepidermal Water Loss |
| Th1/Th2/Th17 | T Helper Cell Type 1/2/17 |
| TNF-α | Tumor Necrosis Factor-alpha |
| Treg | Regulatory T Cell |
| VAS | Visual Analog Scale |
| WoSCC | Web of Science Core Collection |
References
- Roberts, W. Air Pollution and Skin Disorders. Int. J. Womens Dermatol. 2021, 7, 91–97. [Google Scholar] [CrossRef]
- Shepard, Z.; Rios, M.M.; Solis, J.; Wand, T.; Henao-Martínez, A.F.; Franco-Paredes, C.; Suárez, J.A. Common Dermato-logic Conditions in Returning Travelers. Current Tropical Medicine Reports 2021, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Das, A.; Kumar, P.; Datta, D. Skin and Metabolic Syndrome: An Evidence Based Comprehensive Review. Indian J. Dermatol. 2021, 66, 302–307. [Google Scholar] [CrossRef]
- Mohseni Afshar, Z.; Goodarzi, A.; Emadi, S.N.; Miladi, R.; Shakoei, S.; Janbakhsh, A.; Aryanian, Z.; Hatami, P. A Comprehensive Review on HIV-Associated Dermatologic Manifestations: From Epidemiology to Clinical Management. Int. J. Microbiol. 2023, 2023, 6203193. [Google Scholar] [CrossRef]
- Whiting, C.; Schwartzman, G.; Khachemoune, A. Syphilis in Dermatology: Recognition and Management. Am. J. Clin. Dermatol. 2023, 24, 287–297. [Google Scholar] [CrossRef]
- Huai, P.; Xing, P.; Yang, Y.; Kong, Y.; Zhang, F. Global Burden of Skin and Subcutaneous Diseases: An Update from the Global Burden of Disease Study 2021. Br. J. Dermatol. 2025, 192, 1136–1138. [Google Scholar] [CrossRef]
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The Burden of Skin and Subcutaneous Diseases: Findings from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef]
- Gowda, V.; Sarkar, R.; Verma, D.; Das, A. Probiotics in Dermatology: An Evidence-Based Approach. Indian Dermatol. Online J. 2024, 15, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Olunoiki, E.; Rehner, J.; Bischoff, M.; Koshel, E.; Vogt, T.; Reichrath, J.; Becker, S.L. Characteristics of the Skin Micro-biome in Selected Dermatological Conditions: A Narrative Review. Life 2022, 12, 1420. [Google Scholar] [CrossRef]
- Ryguła, I.; Pikiewicz, W.; Grabarek, B.O.; Wójcik, M.; Kaminiów, K. The Role of the Gut Microbiome and Microbial Dysbiosis in Common Skin Diseases. Int. J. Mol. Sci. 2024, 25, 1984. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, S.; Jahani, M.; Moazzen, N.; Ahanchian, H.; Khameneh, B. The Potential of Probiotics for Treating Skin Disorders: A Concise Review. Curr. Pharm. Biotechnol. 2022, 23, 1851–1863. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and Postbiotics in Skin Health. Biomedicines 2025, 13, 791. [Google Scholar] [CrossRef]
- Da Silva Vale, A.; De Melo Pereira, G.V.; De Oliveira, A.C.; De Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, Formulation, and Application of Postbiotics in the Treatment of Skin Conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Ocampo-Ibáñez, I.D.; Liscano, Y.; Rivera-Sánchez, S.P.; Oñate-Garzón, J.; Lugo-Guevara, A.D.; Flórez-Elvira, L.J.; Lesmes, M.C. A Novel Cecropin D-Derived Short Cationic Antimicrobial Peptide Exhibits Antibacterial Activity Against Wild-Type and Multidrug-Resistant Strains of Klebsiella Pneumoniae and Pseudomonas Aeruginosa. Evol. Bioinform. Online 2020, 16, 1176934320936266. [Google Scholar] [CrossRef]
- Rivera-Sanchez, S.P.; Ocampo-Ibáñez, I.D.; Liscano, Y.; Martínez, N.; Muñoz, I.; Manrique-Moreno, M.; Martinez-Martinez, L.; Oñate-Garzon, J. Integrating In Vitro and In Silico Analysis of a Cationic Antimicrobial Peptide Interaction with Model Membranes of Colistin-Resistant Pseudomonas Aeruginosa Strains. Pharmaceutics 2022, 14, 1248. [Google Scholar] [CrossRef]
- Trejos, M.; Aristizabal, Y.; Aragón-Muriel, A.; Oñate-Garzón, J.; Liscano, Y. Characterization and Classification In Silico of Peptides with Dual Activity (Antimicrobial and Wound Healing). IJMS 2023, 24, 13091. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics in Skin Conditions: A Systematic Review of Animal and Human Studies and Implications for Future Therapies. Exp. Dermatol. 2020, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Bonita, L.; Karim, A.; Herwanto, N.; Umborowati, M.A.; Setyaningrum, T.; Hidayati, A.N.; Surono, I.S. Beneficial Effect of Lactobacillus Plantarum IS-10506 Supplementation in Adults with Atopic Dermatitis: A Randomized Controlled Trial. J. Dermatol. Treat. 2020, 33, 1491–1498. [Google Scholar] [CrossRef]
- Jacobson, M.E.; Myles, I.A.; Paller, A.S.; Eichenfield, L.F.; Simpson, E.L. A Randomized, Double-Blind, Placebo-Controlled, Multicenter, 16-Week Trial to Evaluate the Efficacy and Safety of FB-401 in Children, Adolescents, and Adult Subjects (Ages 2 Years and Older) with Mild-to-Moderate Atopic Dermatitis. Dermatology 2024, 240, 85–94. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Wu, W.-F.; Hung, C.-W.; Ku, M.-S.; Liao, P.-F.; Sun, H.-L.; Lu, K.-H.; Sheu, J.-N.; Lue, K.-H. Evaluation of Efficacy and Safety of Lactobacillus Rhamnosus in Children Aged 4–48 Months with Atopic Dermatitis: An 8-Week, Double-Blind, Randomized, Placebo-Controlled Study. J. Microbiol. Immunol. Infect. 2017, 50, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Umborowati, M.A.; Hasna, I.H.; Endaryanto, A.; Surono, I.S.; Prakoeswa, C.R.S. Lactiplantibacillus Plantarum IS-10506 Supplementation Improves Clinical Outcome and Immunology Markers in Psoriasis Vulgaris Patients: A Randomized Controlled Trial. Indones. Biomed. J. 2024, 16, 353–362. [Google Scholar] [CrossRef]
- Gilli, I.O.; Da Silva, G.C.; Mendes, V.; Duarte, M.G.; Tanaka, A.A. The Role of Probiotics as an Adjunctive Therapy in Psoriasis. J. Psoriasis Psoriatic Arthritis 2023, 8, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Suriano, E.S.; Souza, M.D.M.; Kobata, C.M.; Santos, F.H.Y.; Mimica, M.J. Efficacy of an Adjuvant Lactobacillus Rhamnosus Formula in Improving Skin Lesions as Assessed by PASI in Patients with Plaque Psoriasis from a University-Affiliated, Tertiary-Referral Hospital in São Paulo (Brazil): A Parallel, Double-Blind, Randomized Clinical Trial. Arch. Dermatol. Res. 2023, 315, 1621–1629. [Google Scholar] [CrossRef]
- Gerasimov, S.V.; Vasjuta, V.V.; Myhovych, O.O.; Bondarchuk, L.I. Probiotic Supplement Reduces Atopic Dermatitis in Preschool Children: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Am. J. Clin. Dermatol. 2010, 11, 351–361. [Google Scholar] [CrossRef]
- Rather, I.A.; Kim, B.-C.; Lew, L.-C.; Cha, S.-K.; Lee, J.H.; Nam, G.-J.; Majumder, R.; Lim, J.; Lim, S.-K.; Seo, Y.-J.; et al. Oral Administration of Live and Dead Cells of Lactobacillus Sakei proBio65 Alleviated Atopic Dermatitis in Children and Adolescents: A Randomized, Double-Blind, and Placebo-Controlled Study. Probiotics Antimicro. Prot. 2021, 13, 315–326. [Google Scholar] [CrossRef]
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrión-Gutiérrez, M.; Horga De La Parte, J.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients With Moderate Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 37. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, M.; Jeon, S.A.; Kim, Y.; Lee, S. A Randomized Trial of Lactobacillus Rhamnosus IDCC 3201 Tyndallizate (RHT3201) for Treating Atopic Dermatitis. Pediatr. Allergy Immunol. 2020, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, A.; Cestone, E.; De Ponti, I.; Giardina, S.; Pisati, M.; Spartà, E.; Tursi, F. Efficacy of a Probiotic Supplement in Patients with Atopic Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Eur. J. Dermatol. 2021, 31, 225–232. [Google Scholar] [CrossRef]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus Rhamnosus and Lactobacillus Casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef]
- Eguren, C.; Navarro-Blasco, A.; Corral-Forteza, M.; Reolid-Pérez, A.; Setó-Torrent, N.; García-Navarro, A.; Prieto-Merino, D.; Núñez-Delegido, E.; Sánchez-Pellicer, P.; Navarro-López, V. A Randomized Clinical Trial to Evaluate the Efficacy of an Oral Probiotic in Acne Vulgaris. Acta Derm. Venereol. 2024, 104, adv33206. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Alirezaei, P.; Doosti-Irani, A.; Mehrpooya, M.; Nouri, F. The Efficacy of Lactocare® Synbiotic on the Clinical Symptoms in Patients with Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Dermatol. Res. Pract. 2022, 2022, 4549134. [Google Scholar] [CrossRef]
- Andrade, P.D.S.M.A.D.; Maria, E.; Silva, J.; Carregaro, V.; Sacramento, L.A.; Roberti, L.R.; Aragon, D.C.; Carmona, F.; Roxo-Junior, P. Efficacy of Probiotics in Children and Adolescents With Atopic Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2022, 8, 833666. [Google Scholar] [CrossRef]
- Carucci, L.; Nocerino, R.; Paparo, L.; De Filippis, F.; Coppola, S.; Giglio, V.; Cozzolino, T.; Valentino, V.; Sequino, G.; Bedogni, G.; et al. Therapeutic Effects Elicited by the Probiotic Lacticaseibacillus Rhamnosus GG in Children with Atopic Dermatitis. The Results of the ProPAD Trial. Pediatr. Allergy Immunol. 2022, 33, e13836. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Khedmatgozar, H.; Saiedi, S.; Razmi, H.; Alizadeh, M.; Ebrahimi, B. Probiotic Supplementation Improves Clinical Outcomes and Quality of Life Indicators in Patients with Plaque Psoriasis: A Randomized Double-Blind Clinical Trial. Clin. Nutr. ESPEN 2021, 46, 33–39. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Panelli, S.; Lunardon, L.; Pajoro, M.; Paradiso, L.; Beretta, S.; Loretelli, C.; Tosi, D.; Perini, M.; Bedogni, G.; et al. Rice Flour Fermented with Lactobacillus Paracasei CBA L74 in the Treatment of Atopic Dermatitis in Infants: A Randomized, Double- Blind, Placebo- Controlled Trial. Pharmacol. Res. 2021, 163, 105284. [Google Scholar] [CrossRef]
- Ahn, S.H.; Yoon, W.; Lee, S.Y.; Shin, H.S.; Lim, M.Y.; Nam, Y.-D.; Yoo, Y. Effects of Lactobacillus Pentosus in Children with Allergen-Sensitized Atopic Dermatitis. J. Korean Med. Sci. 2020, 35, e128. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; De Waard-van der Spek, F.B. Practical Issues on Interpretation of Scoring Atopic Dermatitis: The SCORAD Index, Objective SCORAD and the Three-item Severity Score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Schäbitz, A.; Eyerich, K.; Garzorz-Stark, N. So Close, and yet so Far Away: The Dichotomy of the Specific Immune Response and Inflammation in Psoriasis and Atopic Dermatitis. J. Intern. Med. 2021, 290, 27–39. [Google Scholar] [CrossRef]
- Xie, A.; Chen, A.; Chen, Y.; Luo, Z.; Jiang, S.; Chen, D.; Yu, R. Lactobacillus for the Treatment and Prevention of Atopic Dermatitis: Clinical and Experimental Evidence. Front. Cell. Infect. Microbiol. 2023, 13, 1137275. [Google Scholar] [CrossRef]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Liscano, Y.; Sanchez-Palacio, N. A Critical Look at Omega-3 Supplementation: A Thematic Review. Healthcare 2023, 11, 3065. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Demessant-Flavigny, A.; Connétable, S.; Kerob, D.; Moreau, M.; Aguilar, L.; Wollenberg, A. Skin Microbiome Dysbiosis and the Role of Staphylococcus Aureus in Atopic Dermatitis in Adults and Children: A Narrative Review. Acad. Dermatol. Venereol. 2023, 37, 3–17. [Google Scholar] [CrossRef]
- Edslev, S.; Agner, T.; Andersen, P. Skin Microbiome in Atopic Dermatitis. Acta Derm. Venereol. 2020, 100, adv00164. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for next-Generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, L. The Impact of Prebiotics, Probiotics and Synbiotics on the Prevention and Treatment of Atopic Dermatitis in Children: An Umbrella Meta-Analysis. Front. Pediatr. 2025, 13, 1498965. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, F.; Chen, H.; Zheng, Q. The Efficacy and Safety of Probiotics in the Adjuvant Treatment of Psoriasis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2024, 11, 1448626. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Bah, Y.-R.; Law, J.W.-F.; Tan, L.T.-H.; He, Y.-W.; Wong, S.-H.; Thurairajasingam, S.; Chan, K.-G.; Lee, L.-H.; Letchumanan, V. Gut–Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Steinhoff, M. Effects of Probiotic Supplementation in Adult with Atopic Dermatitis: A Systematic Review with Meta-Analysis. Clin. Exp. Dermatol. 2023, 49, 46–52. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, G.; Wu, Y.; Hao, W.; Chen, H. The Effectiveness and Safety of Probiotic Supplements for Psoriasis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Preclinical Trials. J. Immunol. Res. 2021, 2021, 7552546. [Google Scholar] [CrossRef]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; Chang, Y.; Florez, I.D.; Foroutan, F.; Shahid, S.; Zeraatkar, D. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-Analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, M.; Li, W.; Zhang, Y.; Zhao, T.; Song, Q.; Cong, J. Comparative Efficacy and Tolerability of Probiotic, Prebiotic, and Synbiotic Formulations for Adult Patients with Mild-Moderate Ulcerative Colitis in an Adjunctive Therapy: A Network Meta-Analysis. Clin. Nutr. 2023, 43, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Y.; Qin, L.; Yang, Z.; Wu, S.; Li, P.; Yi, P.; Chen, L.; Wenge, L.; Li, M. Lactobacillus GG and Other Probiotics in Pediatric Food Allergy Treatment: A Network Meta-Analysis. Front. Nutr. 2025, 12, 1565436. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multi-Strain Versus Single-Strain Probiotics: Current Status and Recommendations for the Future. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef]
- Mahajna, H.; Ben-Horin, S. Novel Bio-Genetic Predictors of Response to Biologic Treatment in Inflammatory Bowel Diseases. Curr. Opin. Pharmacol. 2020, 55, 132–140. [Google Scholar] [CrossRef]
- Meade, S.; Liu Chen Kiow, J.; Massaro, C.; Kaur, G.; Squirell, E.; Bressler, B.; Lunken, G. Gut Microbiome-Associated Predictors as Biomarkers of Response to Advanced Therapies in Inflammatory Bowel Disease: A Systematic Review. Gut Microbes 2023, 15, 2287073. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Levi, A.; Palladino, C.; Girolomoni, G. Enabling Precision Medicine with Biomarkers of Response to Treatment in Atopic Dermatitis: Where Are We Now? A Narrative Review. Front. Med. 2025, 12, 1574697. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Potočnjak, I.; Cindrić, T.; Ilić, I.; Lovrić, I.; Skalicki, L.; Bešlić, I.; Pondeljak, N. Atopic Dermatitis: Disease Features, Therapeutic Options, and a Multidisciplinary Approach. Life 2023, 13, 1419. [Google Scholar] [CrossRef]
- Amerio, P.; Ferrucci, S.M.; Galluzzo, M.; Napolitano, M.; Narcisi, A.; Levi, A.; Di Fino, S.; Palladino, C.; Patruno, C.; Rossi, M. A Multidisciplinary Approach Is Beneficial in Atopic Dermatitis. Dermatol. Ther. 2024, 14, 1443–1455. [Google Scholar] [CrossRef]
- Tier, H.L.; Balogh, E.A.; Bashyam, A.M.; Fleischer, A.B.; Spergel, J.M.; Masicampo, E.J.; Kammrath, L.K.; Strowd, L.C.; Feldman, S.R. Tolerability of and Adherence to Topical Treatments in Atopic Dermatitis: A Narrative Review. Dermatol. Ther. 2021, 11, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Eicher, L.; Knop, M.; Aszodi, N.; Senner, S.; French, L.E.; Wollenberg, A. A Systematic Review of Factors Influencing Treatment Adherence in Chronic Inflammatory Skin Disease—Strategies for Optimizing Treatment Outcome. Acad. Dermatol. Venereol. 2019, 33, 2253–2263. [Google Scholar] [CrossRef] [PubMed]



| Study Reference | Type of Probiotic, Prebiotic, or Synbiotic | Pharmaceutical Form | Dosage | Duration | Comparison Group |
|---|---|---|---|---|---|
| Gerasimov et al., 2010 [28] | L. acidophilus DDS-1, B. lactis UABLA-12 with FOS (Synbiotic) | Oral powder | 10 × 109 CFU/day | 8 weeks | Placebo (rice maltodextrin) |
| Rather et al., 2021 [29] | L. sakei proBio65 (Live and Dead cells) | Oral powder (sachet) | 1 × 1010 cells/day | 12 weeks | Placebo (microcrystalline cellulose) and dead cell group |
| Navarro et al., 2017 [30] | B. lactis CECT 8145, B. longum CECT 7347, L. casei CECT 9104 | Oral capsule | 1 × 109 CFU/day | 12 weeks | Placebo (maltodextrin) |
| Wu et al., 2015 [24] | L. rhamnosus (MP108) | Oral capsule | 350 mg/day | 8 weeks | Placebo (maltodextrin) |
| Jeong et al., 2020 [31] | L. rhamnosus IDCC 3201 tyndallizate (Postbiotic) | Oral capsule | 1.0 × 1010 CPU/day | 12 weeks | Placebo |
| Michelotti et al., 2021 [32] | L. plantarum PBS067, L. reuteri PBS072, L. rhamnosus LRH020 | Oral capsule | 3 × 109 CFU/day (total) | 56 days | Placebo (corn starch) |
| Umborowati et al., 2024 [25] | L. plantarum IS-10506 | Oral capsule | 2 × 1010 CFU/day | 12 weeks | Placebo (both groups with topical standard treatment) |
| Cukrowska et al., 2021 [33] | L. rhamnosus ŁOCK 0900, L. rhamnosus ŁOCK 0908, L. casei ŁOCK 0918 | Oral powder (sachet) | 1 × 109 CFU/day (total) | 3 months | Placebo (maltodextrin) |
| Eguren et al., 2024 [34] | L. rhamnosus CECT 30031 and Arthrospira platensis | Oral capsule | 1 × 109 CFU/day | 12 weeks | Placebo (maltodextrin) |
| Akbarzadeh et al., 2022 [35] | Lactocare® (8-strain mix + FOS) (Synbiotic) | Oral capsule | Not Specified | 3 months | Placebo |
| Albuquerque et al., 2022 [36] | L. rhamnosus, L. acidophilus, L. paracasei, B. lactis | Oral powder (sachet) | 1 g/day (4 × 109 CFU total) | 6 months | Placebo (maltodextrin) |
| Carucci et al., 2022 [37] | L. rhamnosus GG (LGG) | Oral capsule | 1 × 1010 CFU/day | 12 weeks | Placebo |
| Moludi et al., 2021 [38] | L. acidophilus, B. bifidum, B. lactis, B. longum | Oral drink | 1.8 × 109 CFU/day (total) | 8 weeks | Placebo |
| D’Auria et al., 2021 [39] | L. paracasei CBA L74 (Heat-killed, Postbiotic) | Fermented rice flour powder | 8 g/day | 12 weeks | Placebo (rice powder) |
| Ahn et al., 2020 [40] | L. pentosus | Oral | 1.0 × 1010 CFU/day | 12 weeks | Placebo |
| Gilli et al., 2023 [26] | L. rhamnosus Lr-G14 | Oral capsule | 5 × 109 CFU/day | 60 days | Placebo |
| Jacobson et al., 2024 [23] | FB-401 (3 strains of Roseomonas mucosa) (Live Biotherapeutic) | Topical spray | 1x 107 CFU/mL | 16 weeks | Placebo (sucrose solution) |
| Prakoeswa et al., 2020 [22] | L. plantarum IS-10506 | Oral capsule (microencapsulated) | 2 × 1010 CFU/day | 8 weeks | Placebo (skim milk-Avicel) |
| Suriano et al., 2023 [27] | L. rhamnosus ATCC 7469 | Oral liquid (whey formula) | 6 × 105 bacteria/mL | 6 months | Placebo (whey formula) |
| Study Reference | Primary Results | Secondary Results | Comparative Effects (vs. Control) | Adherence | Side Effects | Author’s Conclusions | Study Limitations | |
|---|---|---|---|---|---|---|---|---|
| Gerasimov et al., 2010 [28] | SCORAD reduction of 33.7% | IDQOL and DFI scores improved; reduced topical steroid use; CD4/CD25 decreased, CD8 increased | Probiotic > placebo (p = 0.001) | >90% in both groups | Similar frequency of AEs in both groups | Probiotic mix was associated with significant clinical improvement and corresponding lymphocyte changes | Absence of pretrial run-in period | |
| Rather et al., 2021 [29] | SCORAD score decreased in live (p = 0.0015) and dead cell (p = 0.0017) groups | Skin sebum content increased; IGA score decreased; eosinophil count decreased in live cell group | Live and dead probiotic > placebo for SCORAD (p < 0.05) | Not specified | No serious reactions reported | Both live and dead cells of | L. sakei proBio65 clinically improved AD symptoms | Short duration (12 weeks), small number of subjects per arm, limited biomarkers studied |
| Navarro et al., 2017 [30] | SCORAD reduction was 19.2 points greater in probiotic group | Significant reduction in topical steroid use in probiotic group | Probiotic > placebo (p < 0.001) | Not specified | No relevant adverse events reported | The probiotic mixture was effective in reducing SCORAD index and the use of topical steroids | Short follow-up, single center, not applicable to other populations/diets | |
| Wu et al., 2015 [24] | SCORAD change from baseline was −21.69 in probiotic group vs. −12.35 in placebo | No significant difference in topical corticosteroid use or symptom-free duration | Probiotic > placebo (p = 0.014) | >80% for per-protocol analysis | Similar AE rates in both groups | L. rhamnosus was effective in decreasing symptoms of AD after 8 weeks | Lack of long-term follow-up; lack of laboratory data (e.g., IgE, IL-4) | |
| Jeong et al., 2020 [31] | SCORAD change was greater in probiotic group (−13.89 vs. −8.37) | ECP and IL-31 levels tended to decrease (significant in subgroup) | Probiotic > placebo (p = 0.0283) | Adherence rate was ~91% in both groups | No significant differences in safety parameters | Oral administration of RHT3201 showed therapeutic effect on AD | Could not completely stop TCS use; could not measure IFN-γ | |
| Michelotti et al., 2021 [32] | Significant decrease in SCORAD index in probiotic group | Improved skin smoothness, moisturization, and decreased inflammatory markers (TNF-alpha, TARC, TSLP) | Probiotic > placebo (p < 0.001) for SCORAD | No drop-outs reported | Well tolerated, no adverse events reported | Administration of selected probiotic strains resulted in a fast and sustained improvement in AD-related symptoms | Not specified by authors | |
| Umborowati et al., 2024 [25] | PASI score significantly reduced at week 6 and 12 | DLQI scores were lower; IL-17 decreased, IL-10 and Foxp3 increased; lower probability of flares at 6 months | Probiotic > placebo for PASI (p = 0.049 at 12 wks) | 47/49 completed the trial | Mild changes in defecation frequency and mild nausea | L. plantarum IS-10506 might effectively improve clinical outcomes and immune biomarkers in psoriasis | Small sample size, single-center study | |
| Cukrowska et al., 2021 [33] | SCORAD decreased in both groups, but proportion of children with >30% improvement was higher in probiotic group | No significant difference in total IgE levels | Probiotic > placebo for proportion improved (OR 2.56, p = 0.012) | 134/151 completed the 3-month intervention | Well tolerated, sporadic changes in stool consistency | The probiotic mixture offers benefits for children with AD and CMP allergy, especially in sensitized patients | Microbiome analysis not performed; adherence not systematically verified | |
| Eguren et al., 2024 [34] | 50% of probiotic group improved in AGSS vs. 29.4% in placebo group | Significant reduction in non-inflammatory lesions; GAGS improvement was higher in probiotic group | Probiotic > placebo for AGSS improvement (p = 0.03) | 74/81 completed the study | Number of AEs was similar in both groups | The probiotic used was effective and well tolerated for acne vulgaris patients | Diet not analyzed as a variable; results not applicable to populations outside 12–30 years | |
| Akbarzadeh et al., 2022 [35] | Significant decrease in PASI, VAS, and DLQI scores at week 12 | 100% of treatment group showed some PASI reduction vs. 7.4% of control group | Probiotic > placebo for PASI, VAS, DLQI (p < 0.05) at 12 wks | 52 patients completed the study | Mild GI symptoms in a minority of patients | Oral administration of Lactocare® resulted in the improvement in PASI, DLQI, and VAS scores | Small sample size due to COVID-19 pandemic dropouts | |
| Albuquerque et al., 2022 [36] | SCORAD decreased significantly more in the probiotic group | Probiotic group required topical immunosuppressant less frequently; no significant changes in IgE, SPT, or cytokines | Probiotic > placebo (p < 0.05) for SCORAD change | 40/60 completed the study | Nausea, abdominal pain, pruritic episodes (more frequent in placebo group) | The probiotic mixture promoted a significant clinical response in children and adolescents with AD | Treatment duration may not have been optimal; small number of completers | |
| Carucci et al., 2022 [37] | Rate of subjects achieving MCID for SCORAD was higher in LGG group | IDQOL improved; beneficial modulation of gut (increased butyrate) and skin microbiome | Probiotic > placebo for achieving MCID (p < 0.05) | >80% consumption for per-protocol analysis | No adverse events reported | The probiotic LGG could be useful as adjunctive therapy in pediatric AD | Not specified by authors | |
| Moludi et al., 2021 [38] | Significant reduction in BDI, DLQI, PASI, and PSS scores | Significant increase in TAC and decrease in hs-CRP, IL-6, and MDA levels | Probiotic > placebo for all clinical scores and biomarkers (p < 0.05) | >90% compliance | Mild GI symptoms (12% placebo, 8% probiotic) | Probiotics improve patients’ quality of life and inflammatory biomarkers in psoriatic patients | Small sample size, short intervention period | |
| D’Auria et al., 2021 [39] | No significant difference in SCORAD change between groups | Significant steroid-sparing effect in the experimental group; no significant differences in cytokines or gut microbiota | No significant difference for SCORAD (p = 0.223) | 53/58 completed the trial | No serious adverse events reported | Heat-killed | L. paracasei showed a steroid sparing effect, but was not effective in reducing AD severity | Not specified by authors |
| Ahn et al., 2020 [40] | No significant difference in SCORAD change between groups | Subjective SCORAD scores were significantly lower in the probiotic group for IgE sensitized AD subgroup (p = 0.019) | No overall difference vs. placebo. Significant for subjective score in sensitized subgroup | 82/95 completed the study | Not specified | Could not find additional effects of | L. pentosus in AD, but may be effective in allergen-sensitized AD | Could not exclude intake of other fermented foods; baseline SCORAD difference; small subgroups |
| Gilli et al., 2023 [26] | Significant improvement in DLQI change (p = 0.035); reduction in PASI and BSA | No reduction in IL-17 and IL-23 levels | Probiotic > placebo for DLQI change. Numerical improvement in PASI/BSA | Adherence confirmed at day 30 and 60 | More GI side effects in placebo group (64.3% vs. 35.7%) | Positive correlation between probiotic use and improvement in clinical aspects and scores | Limited number of patients, short follow-up period | |
| Jacobson et al., 2024 [23] | No significant difference in proportion of subjects achieving EASI-50 | No significant differences in any secondary outcomes (EASI-75/90, IGA, BSA, Pruritus) | No difference vs. placebo (p = 0.7657) | ~96% in both groups | Similar number of AEs; 1 unrelated SAE in probiotic group | FB-401 failed to prove superior to placebo but showed an acceptable safety profile | Inability to correlate with microbiome changes; restricted to mild–moderate population | |
| Prakoeswa et al., 2020 [22] | SCORAD score was significantly lower in the probiotic group at week 8 | IL-4 and IL-17 decreased; IFN-γ and Foxp3+ increased; no significant change in IgE | Probiotic > placebo for SCORAD and immune markers (p < 0.05) | All 30 patients completed the study | Not specified | L. plantarum IS-10506 is effective for alleviating AD symptoms in adults owing to its immunomodulatory effects | Not specified by authors | |
| Suriano et al., 2023 [27] | No significant change in PASI for experimental group (p = 0.105). Placebo group improved significantly (p = 0.019) | No significant change in DLQI for experimental group. Placebo group improved significantly (p = 0.031) | No significant difference between groups (p = 0.620) | Not specified | 1 AE in experimental group vs. 4 in control group | Findings do not support the hypothesis that | L. rhamnosus produces clinical improvement in psoriasis | Single-center, hospital-based study; potential for placebo effect |
| Author (Year) | Randomized Study? (1 Point) | Appropriate Randomization Method? (1 Point) | Double-Blind Study? (1 Point) | Appropriate Blinding Method? (1 Point) | Description of Withdrawals/Dropouts? (1 Point) | Total Jadad Score | Quality |
|---|---|---|---|---|---|---|---|
| Gerasimov (2010) [28] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Jeong (2020) [31] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Michelotti (2021) [32] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Navarro (2017) [30] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Rather (2021) [29] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Umborowati (2024) [25] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Wu (2015) [24] | Yes | NR | Yes | Yes | Yes | 4 | High |
| Ahn (2020) [40] | Yes | NR | Yes | Yes | Yes | 4 | High |
| Akbarzadeh (2022) [35] | Yes | Yes | Yes | NR | Yes | 4 | High |
| Albuquerque (2022) [36] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Carucci (2022) [37] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Cukrowska (2021) [33] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| D’Auria (2021) [39] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Eguren (2024) [34] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Gilli (2023) [26] | Yes | NR | Yes | Yes | NR | 3 | High |
| Jacobson (2024) [23] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Prakoeswa (2020) [22] | Yes | NR | Yes | Yes | NR | 3 | High |
| Suriano (2023) [27] | Yes | Yes | Yes | Yes | Yes | 5 | High |
| Moludi (2021) [38] | Yes | Yes | Yes | Yes | Yes | 5 | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liscano, Y.; Muñoz Morales, D.; Suarez Daza, F.; Vidal Cañas, S.; Martinez Guevara, D.; Artunduaga Cañas, E. Microbial Interventions for Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis of Atopic Dermatitis and Psoriasis. Microorganisms 2025, 13, 2416. https://doi.org/10.3390/microorganisms13112416
Liscano Y, Muñoz Morales D, Suarez Daza F, Vidal Cañas S, Martinez Guevara D, Artunduaga Cañas E. Microbial Interventions for Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis of Atopic Dermatitis and Psoriasis. Microorganisms. 2025; 13(11):2416. https://doi.org/10.3390/microorganisms13112416
Chicago/Turabian StyleLiscano, Yamil, Daniel Muñoz Morales, Fernanda Suarez Daza, Sinthia Vidal Cañas, Darly Martinez Guevara, and Esteban Artunduaga Cañas. 2025. "Microbial Interventions for Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis of Atopic Dermatitis and Psoriasis" Microorganisms 13, no. 11: 2416. https://doi.org/10.3390/microorganisms13112416
APA StyleLiscano, Y., Muñoz Morales, D., Suarez Daza, F., Vidal Cañas, S., Martinez Guevara, D., & Artunduaga Cañas, E. (2025). Microbial Interventions for Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis of Atopic Dermatitis and Psoriasis. Microorganisms, 13(11), 2416. https://doi.org/10.3390/microorganisms13112416






