1. Introduction
Infant skin is characterized by its soft and supple appearance, yet it is physiologically immature, delicate, and highly sensitive, requiring careful protection and support during the early years of life. Despite the critical role our skin plays in health and immunity from birth, there is sparce scientific literature on skin development, and even less is known about the contribution of the resident microbes. There is evidence that the infant skin microbiome may be impacted by the route of delivery, the retention of vernix, and caregivers’ habits and practices, including bathing frequency and skin care routines, which differ across the globe [
1,
2]. Dermatitis in the diapered area (DDA), commonly known as diaper rash or nappy rash, is the most prevalent inflammatory skin condition affecting non-toilet trained babies, with nearly every infant experiencing DDA at some point in their development [
1,
3,
4,
5]. This cutaneous inflammation arises from damage to the outermost layer of the epidermis, the stratum corneum, which is composed of corneocytes embedded in a lipid- and protein-rich matrix. Once this barrier is damaged, the breach allows for the entry of irritants from urine and feces to penetrate the skin and initiate an immune response visible as erythema (redness), papules and/or pustules. This condition not only causes discomfort for infants but also leads to sleep disturbances, increased crying and irritability, and significantly affects caregiver well-being contributing to parental stress [
3,
6,
7]. The causes of DDA are multifactorial and include disrupted skin ultrastructure due to prolonged exposure to moisture and skin-on-skin rubbing (intertrigo), which exacerbates frictional damage to skin. Additionally, microbial factors play a crucial role in its development, as the skin’s natural microbiota can become disrupted, leading to the opportunistic overgrowth of harmful bacteria or yeast in warm, moist environments [
8]. Skin contact with urine and stool are key drivers of rash, with the stool containing digestive enzymes (lipases and proteases) from the gut degrading and damaging skin. Prolonged exposure of skin to urine increases the risk of overhydration. Bacteria on the skin (either resident or from feces) contain enzymes (ureases) that convert the urea in urine to ammonia (pH = 11) that can increase skin pH, as well as activate several pH-dependent lipases and proteases that can damage skin lipids and protein, respectively. The elevation in skin pH can contribute to dysbiosis but also impairs normal skin function (e.g., desquamation) and slows skin healing. Some risk factors for DDA include prematurity, overhydrated skin, infrequent diaper changes, elevated skin pH, antibiotic use, and changes in the irritancy potential and frequency of stool (often diet related). Infants with certain medical conditions or those experiencing diarrhea are at higher risk [
1]. To prevent DDA, caregivers are instructed to use high-quality, absorbent diapers, conduct frequent diaper changes, apply topical products, and clean the skin with water or a low pH baby wipe.
Skin pH is emerging as a crucial marker of skin health. The physiological importance of maintaining an acidic superficial skin pH is evidenced by the existence of four unique pathways that functionally provide redundancy to ensure skin acidity. These include the following: (1) generation of free fatty acids by the hydrolysis of phospholipids by secretory phospholipases (sPLA2); (2) sodium–hydrogen antiporter (NHE1); (3) catabolism of filaggrin (FLG) to acidic components of the natural moisturizing factor (NMF); and (4) release of protons during melanin transfer from melanocytes to keratinocytes [
9]. The establishment of skin’s acidic nature begins at day 1, with near neutral skin pH at birth rapidly declining to values ranging from 4.5 to 5.5 within 2–3 weeks [
10], which coincides with the initial establishment of the skin’s microbiome. Thus, the connection between skin pH and microbiome are inherent from the very beginning of life. This low pH supports the skin barrier and inhibits the growth of pathogenic microorganisms [
11]. Lower skin pH levels are associated with reduced severity of DDA, highlighting the potential for pH management as a preventive strategy against DDA [
12,
13]. Moreover, skin pH can vary significantly across different populations due to factors such as diet, environmental conditions, and genetic predisposition.
Recent studies have revealed differences in skin pH among babies from diverse geographical backgrounds, including those from China, Germany, and the United States [
1]. Babies in certain regions may exhibit higher skin pH levels, which coincides with an increased risk of DDA. Understanding these variations is essential for developing targeted interventions and skincare products that address the specific needs of infants in different cultural contexts. A balanced microbiome can help prevent the overgrowth of pathogenic bacteria and fungi, thereby reducing the risk of inflammation and irritation. The skin microbiome in the diapered area of infants, while sharing some structural similarities with adult skin communities, is heavily influenced by the persistent presence of moisture and fecal material in the occluded environment [
14]. Unlike the adult skin microbiome, which is dominated by
Actinobacteria (e.g.,
Cutibacterium and
Corynebacterium),
Firmicutes (e.g.,
Staphylococcus,
Streptococcus, and
Lactobacillus),
Proteobacteria, and
Bacteroidetes, the infant diaper area microbiome is heavily influenced by gut-derived bacteria such as
Bifidobacteria,
Bacteroides,
Enterobacteria,
Eubacteria,
Clostridium, and
Lactobacillus [
14,
15]. This microbiome is highly heterogenous across four anatomical sites: genitals, intertriginous folds, buttocks, and perianal region, with buttocks samples often showing higher levels of skin-associated bacteria, while perianal samples are enriched with gut-associated species like
Veillonella and
Enterococcus [
16]. Typical microbial communities in the diaper area include purported beneficial bacteria such as
Bifidobacterium and
Lactobacillus, which may help maintain the skin’s protective barrier and support immune function [
16]. Conversely, pathogens like
Staphylococcus aureus and
Candida albicans can proliferate under disrupted alkaline conditions [
17], contributing to DDA [
16]. Additionally, increased levels of
Finegoldia and fecal coliforms have been associated with DDA. The interplay between the skin and gut microbiomes is especially important for infants, whose skin is more susceptible to environmental stressors and microbial imbalances, especially in the diaper area, which might impact the development of DDA. The skin–microbiome interaction should be considered bidirectional as it pertains to skin pH. Skin pH can play a crucial role in shaping the microbial environment on the skin, with lower (more acidic) pH generally promoting beneficial bacteria while inhibiting microbes considered to be pathogenic [
18]. Conversely, elevated skin pH can disrupt this balance, allowing harmful microorganisms to thrive and potentially worsen conditions like diaper rash. At the same time, microbes can produce chemicals that influence their surrounding environment and self-select for optimal growth conditions. By managing skin pH with appropriate skincare products, caregivers can enhance microbial balance in the diaper area, potentially reducing the incidence and severity of diaper rash in infants. Despite the growing research on the interplay between skin pH, microbiome composition, and DDA severity, significant knowledge gaps remain. There is a critical need for comprehensive studies that directly examine how variations in skin pH affect the composition and function of the microbiome in the diaper area.
In our previous study involving 1791 infants [
1], DDA was found to be most severe in Germany, followed by the USA, with the lowest rates in China. Chinese infants exhibited lower skin pH and improved skin barrier integrity, as indicated by reduced trans-epidermal water loss (TEWL). Additionally, Chinese caregivers reported more frequent diaper changes, higher use of topical products, more thorough cleaning routines after stooling, and less time in the overnight diaper, all of which may have contributed to lower DDA rates. The study also identified that higher humidity in the diaper area increased the risk of skin overhydration. The perianal region had the highest prevalence and severity of DDA, followed by the intertriginous (leg folds), genital, and buttock regions. These findings highlight the significance of geographic and body site differences in infant skin health, many of which are closely related to skin microbiome composition.
To deepen our understanding of the relationship between the skin microbiome and skin wellness in infants, we investigated a subset of 158 infants, focusing on three specific body sites: buttocks, perianal, and thighs. Our primary objective was to explore how microbial communities may influence or respond to the severity of diaper rash and inform better infant skincare practices. Specifically, we aimed to examine the relationships between skin pH, diaper rash severity, and microbiome composition across different geographical backgrounds. This study employs metagenomic analysis and statistical methods to thoroughly assess microbiome composition and its correlations with skin pH and rash severity. Previous research has indicated notable differences in microbial community composition at diaper sites, especially during instances of DDA, but these studies often relied on 16S rRNA sequencing, which limits species-level resolution and did not consider babies from different geographical locations. Thus, our study seeks to address these gaps using advanced technologies.
4. Discussion
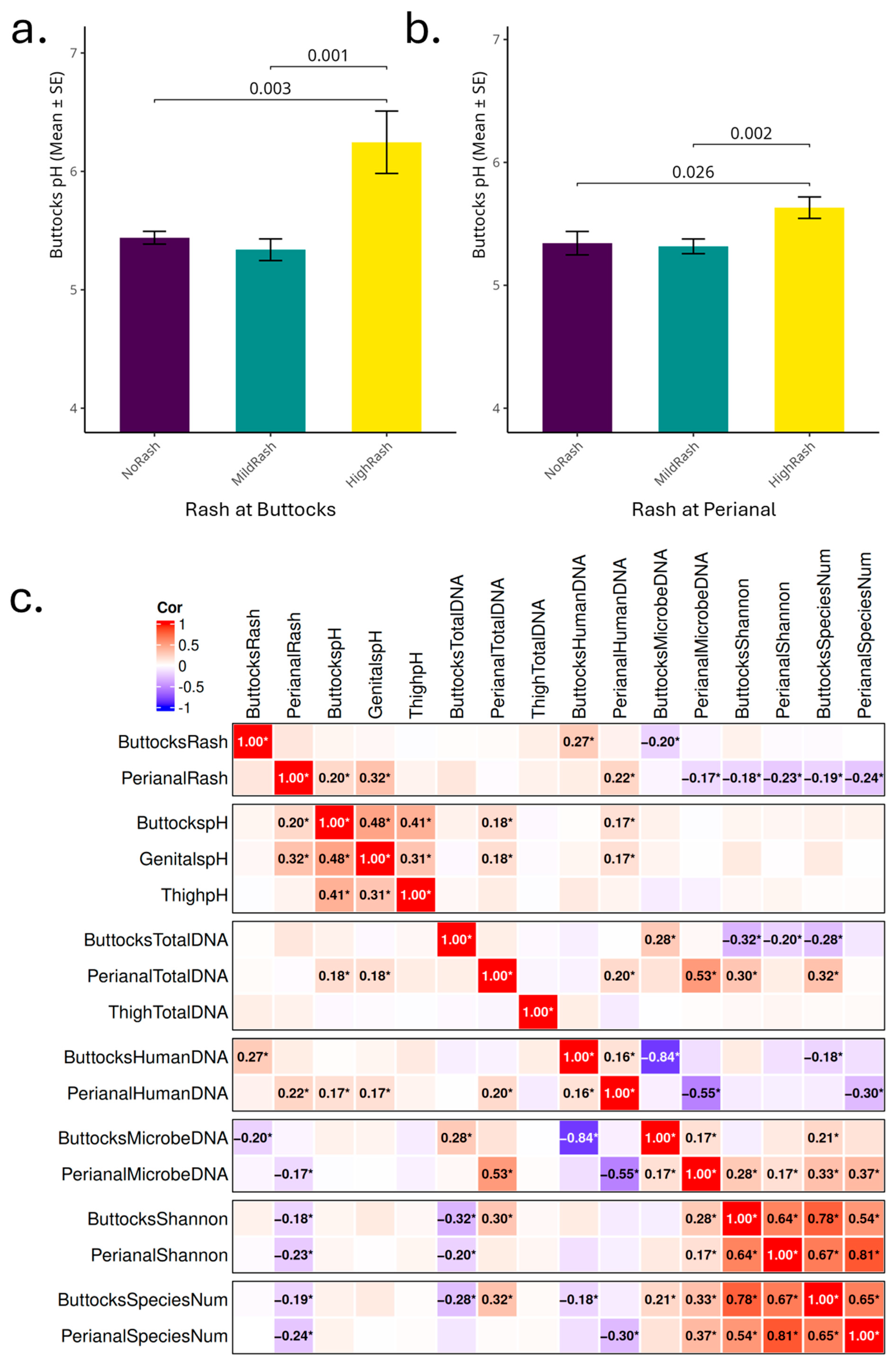
A significant association between elevated buttocks skin pH levels and increased severity of DDA was found in this study, reflecting the skin microbiome and its functional changes, consistent with previous findings linking lower skin pH and skin health [
1,
13]. Specifically, infants with buttocks pH levels above 5.5 are at a heightened risk for developing DDA, underscoring the importance of monitoring skin pH as a routine aspect of infant care. Additionally, our results suggest that in the perianal region, factors such as osmolarity and cleanliness may be important factors influencing DDA in addition to maintaining a mildly acidic pH.
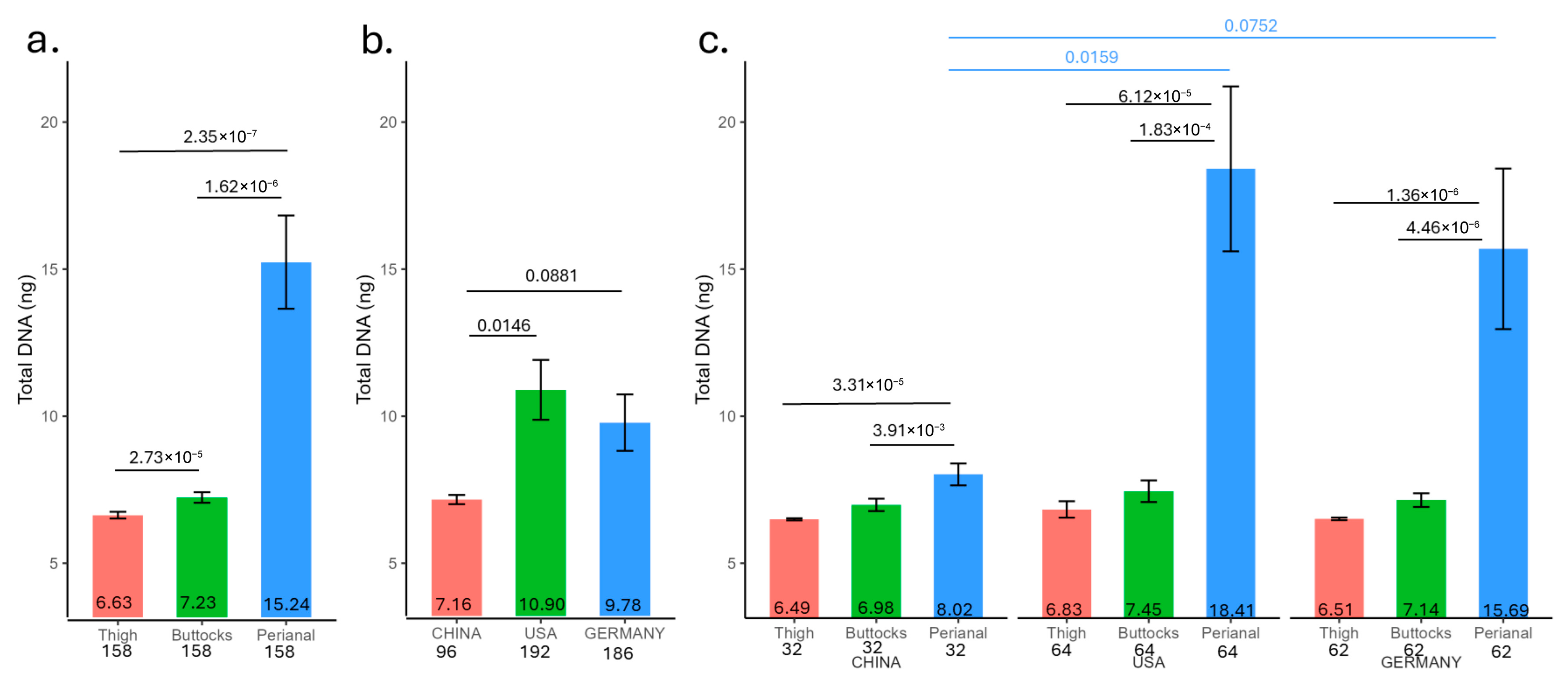
The total DNA yield from skin tape samples corresponds to the rash severity (
Figure 1). The human DNA extracted from these tapes shows a positive correlation with rash severity, while the microbe DNA exhibits a negative correlation (
Figure 3c). When the skin barrier is compromised due to rash conditions, the integrity of the stratum corneum is disrupted and cell turnover accelerated, facilitating easier detachment of skin cells by tape strips and increased exposure of smaller living cells. This might explain the resulting higher quantity of extracted human DNA for high-rash infants. Since all tape strips are of the same size and adhesive strength, this might also explain how a greater attachment of human cells could reduce the capacity for microbial cells to adhere to a greater contact area and cause deeper penetration of the microbes below the stratum corneum surface, leading to the observed strong negative correlation between microbial and human DNA yields. Elevated skin pH in the perianal region, accompanied by higher DNA yield, further underscores the relationship between pH and rash.
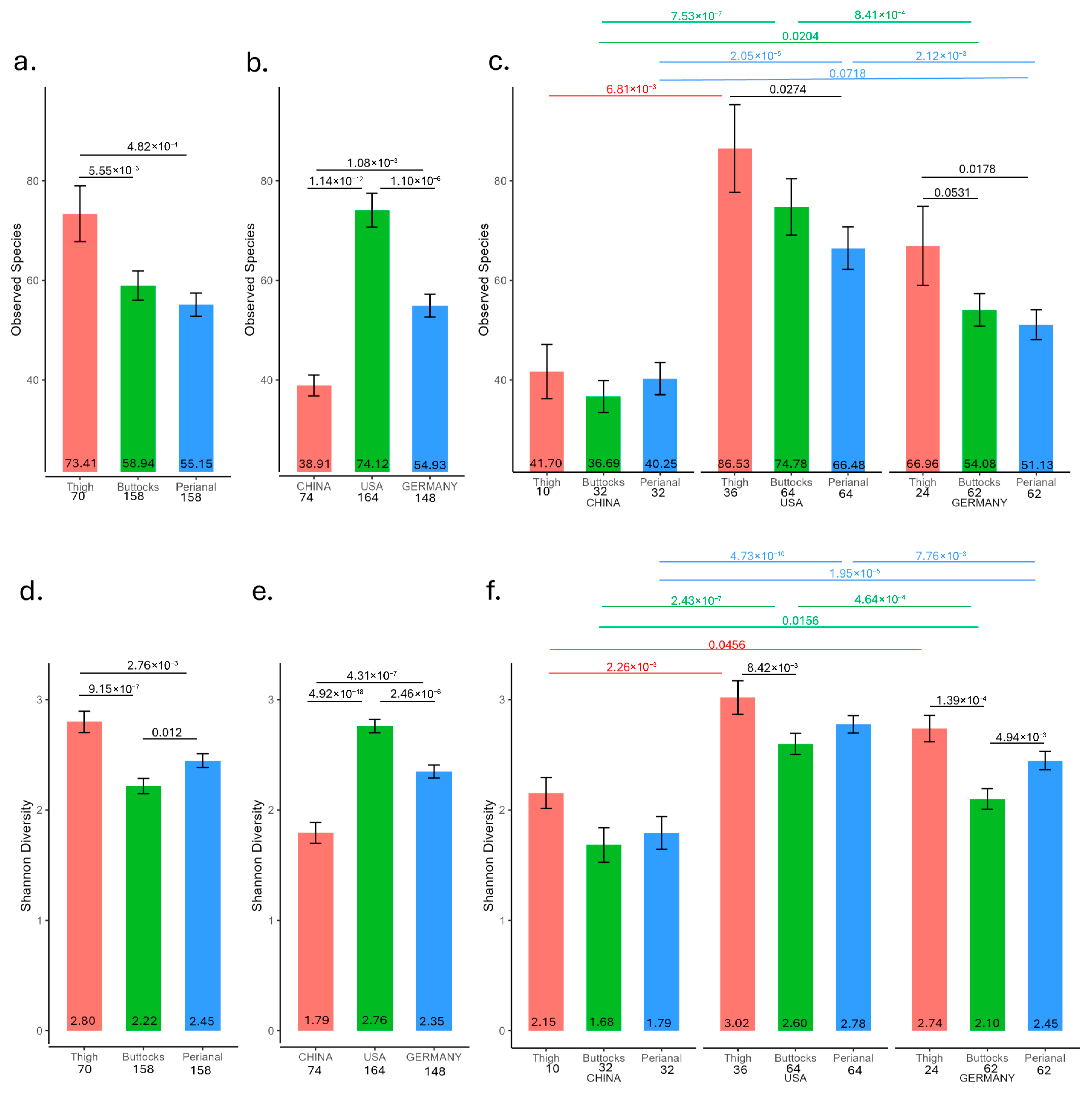
A diverse skin microbiome is widely recognized as essential for maintaining healthy skin. High microbial diversity supports skin barrier function, immune regulation, and protection against pathogens, while reduced diversity (dysbiosis) is linked to inflammatory skin conditions such as atopic dermatitis, acne, and psoriasis [
29,
30,
31]. The observation that the thigh microbiome has the highest microbial diversity, followed by the buttocks and that the lowest diversity is in the perianal microbiome, agrees with this hypothesis (
Figure 2a). Additionally, perianal microbial diversity is significantly higher in subjects without rashes compared to those with mild or severe rashes. (
Figure S3).
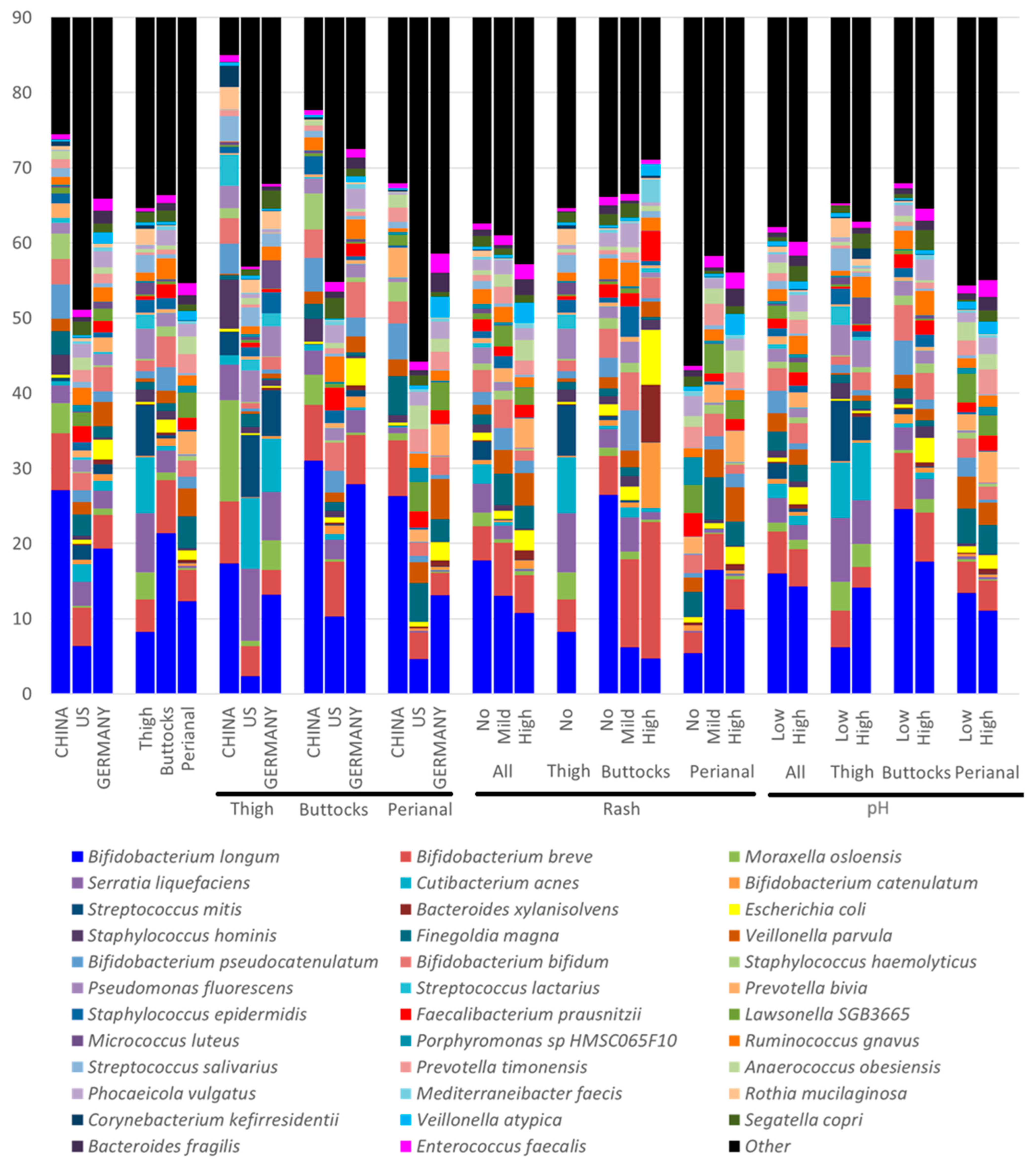
While microbial diversity is important, microbial composition and function also play key roles in infant skin health. For example, Chinese infants exhibit lower microbial diversity than German and US infants (
Figure 2b,e and
Figure 4); however, German infants in this study have higher overall rash scores. A closer examination of the microbiota reveals that German samples have the greatest abundance of
E. coli (China: 0.36%, Germany: 2.57%, and US: 0.58%),
Enterococcus faecalis (China: 0.65%, Germany: 1.55%, and US: 0.98%), and
Veillonella atypica (China: 0.29%, Germany: 1.52%, and US: 0.33%), all of which are strongly associated with increased rash severity in our findings. Many of those species are key fecal coliforms. In contrast, Chinese samples have the highest abundance of
Staphylococcus hominis (China: 2.66%, Germany: 0.33%, and US: 0.57%) and
Bifidobacterium longum (China: 27.14%, Germany: 19.33%, and US: 6.75%), which showed a negative impact on rash severity. These geographic differences align with our previous publication [
1], suggesting that cultural practices and dietary habits can significantly influence the DDA outcomes. The higher microbial diversity observed in German samples may be attributed to residual fecal material and associated microbes. In contrast, the lower DNA yield and diversity in Chinese samples likely reflect more stringent cleaning practices by caregivers (
Figure 4,
Table S3). Additionally, Chinese infants tend to spend less time in overnight diapers [
1], which may further contribute to these differences, linking to Chinese samples with the highest amount of commensal skin microbes such as
Moraxella osloensis (China: 3.98%, Germany: 0.85%, and US: 0.27%) and
Corynebacterium amycolatum (China: 1.38%, Germany: 0.01%, and US: 0.03%). These observations, alongside variations in skin pH and the prevalence of diaper dermatitis among populations in China, Germany, and the US, suggest that cultural, dietary, and environmental factors play significant roles.
These observations, alongside variations in skin pH and the prevalence of diaper dermatitis among populations in China, Germany, and US, suggest that cultural, dietary, and environmental factors play significant roles. Differences in diapering routines, skin care practices, and available diapering products may also drive these variations. Thus, it is essential to tailor infant care approaches to different cultural contexts, particularly regarding practices like air exposure to diapered skin and cleaning protocols, to better prevent perianal rashes. Ultimately, these insights may inform pediatric skin care guidelines aimed at reducing or preventing diaper dermatitis globally.
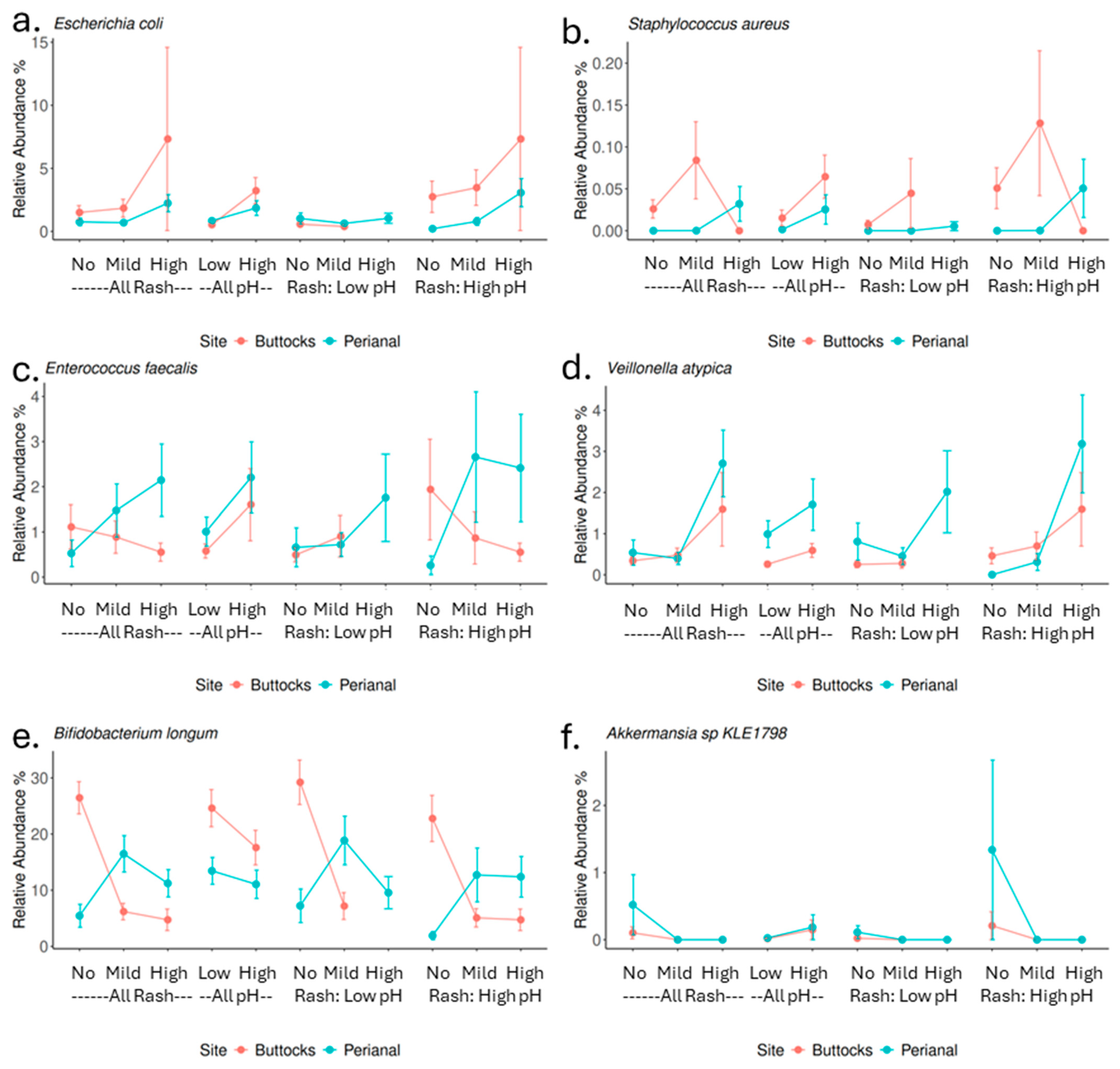
The findings of this study mostly align with and expand upon previous research regarding the role of specific microbial species in DDA. Consistent with earlier findings, our study confirms that
Bifidobacterium longum is negatively correlated with rash severity, emphasizing its role in supporting the skin barrier and immune function. Furthermore,
Finegoldia and
Staphylococcus species has been previously linked to increased rash severity [
14,
16], our results showed that high levels of
Staphylococcus aureus observed in perianal high rash samples and samples with elevated skin pH (
Figure 5d), while
Finegoldia magna only showed a significant correlation with buttock rash (Spearman correlation rho = 0.17,
p value = 0.03), not a link to perianal rash or skin pH (
Table S5). Additionally, the positive correlations of
Veillonella species, particularly
Veillonella atypica and
Enterococcus faecalis, with perianal rash scores and pH resonate with earlier studies that reported the association of gut-associated bacteria or fecal coliform bacteria with DDA (
Figure 5b,c). Another important fecal coliform
Escherichia coli is also enriched (but not significant) in rash samples while associated positively with higher skin pH. Although
Candida albicans is recognized as an important pathogen for DDA, we did not observe that in this population. In fact,
Candida albicans was only detected in one sample out of these 158 infants’ 386 samples.
There are significant differences in microbial composition between buttocks and perianal samples, with gut-associated bacteria predominating in perianal samples. This shift in microbial profile has important implications for DDA severity. In perianal rash samples, we observed a higher abundance of gut microbes typically linked to digestive issues, while beneficial microbes that support immunity and gut barrier function were less abundant. The more severe rashes observed in the perianal area, compared to the buttocks, may result from fecal and urine accumulation, friction from skin-to-skin contact (intertrigo), as well as heat generation in this more occluded region, further contributing to the distinct microbial profiles. Notably,
Akkermansia sp.
KLE1798, a commensal, is found only in higher abundance in groups without rashes (
Figure 5f).
Akkermansia species are known for their mucin-degrading abilities, which nourish colon cells and support gut barrier integrity [
32,
33,
34]. Certain
Akkermansia species can metabolize Human Milk Oligosaccharides (HMOs) resulting in the production of organic acids which would lower or maintain skin pH in the healthy, acidic range, suggesting a role in infant health [
35].
Alterations in skin pH can significantly influence microbial diversity and composition, potentially fostering an environment that promotes the development of diaper rash. There exists a bidirectional relationship between skin pH and microbial communities, suggesting that interventions aimed at maintaining lower pH levels may help mitigate the severity of DDA. Notably, in the buttock region,
Bifidobacterium longum shows a negative correlation with both pH and rash severity (
Figure 5e), indicating its potential as a target for probiotic-based interventions. Conversely, the perianal area appears to also be affected by factors such as osmolarity, heat, and degradative enzymes. Therefore, enhanced cleaning and protective measures in this region are crucial for preventing DDA.
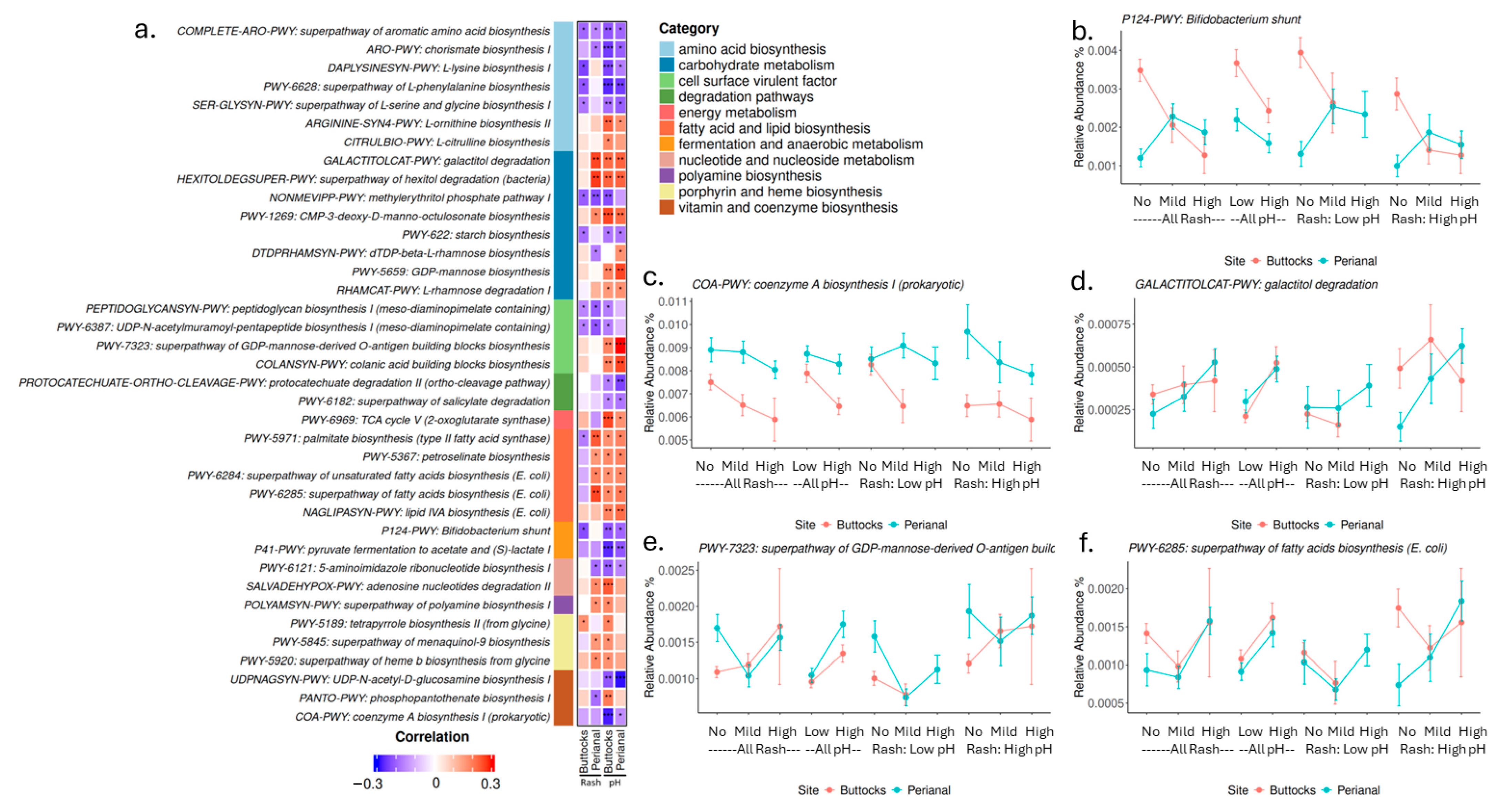
In addition to microbial composition, we examined microbial biological pathways and gene functions associated with skin pH and diaper rash severity. Several pathways for amino acid, vitamin, and cofactor biosynthesis exhibited negative correlations with skin pH and DDA severity. This suggests that microbiota may play a role in supplying micronutrients that nourish the skin. On the other hand, the biosynthesis of L-ornithine and L-citrulline, along with polyamine production, is elevated in samples with high pH and high rash severity. Arginine, L-ornithine, L-citrulline, and polyamines (such as putrescine, spermidine, and spermine) are interconnected in metabolic pathways and serve critical roles in various physiological processes [
36]. These metabolites are essential for wound healing, modulating immune responses, and regulating inflammation, all of which are crucial for skin health and recovery. Elevated levels of these microbial pathways in rash-affected areas may indicate an active metabolic response to tissue damage or inflammation, suggesting that the microbiota’s metabolic profile could significantly influence the skin’s ability to heal and maintain its barrier function. Pathways related to lipid and fatty acid biosynthesis were positively associated with perianal rash severity and skin pH but showed an inverse trend in buttocks rash (
Figure 6). Given that lipids are essential for maintaining skin barrier function and integrity, and fatty acids can influence skin pH, these data suggest that microbial metabolites may play a key role in regulating the skin environment. Subsequently, dysregulated metabolic processes may contribute to the pathophysiology of DDA. Several cell surface virulence factors, such as lipid A and O-antigen biosynthesis (both associated with Gram-negative bacteria), correlated positively with DDA severity, while peptidoglycan biosynthesis (linked to Gram-positive bacteria) correlated negatively. This aligns with our earlier finding that higher levels of fecal coliforms, predominantly Gram-negative bacteria, on infant skin may correlate with an increased risk of DDA.
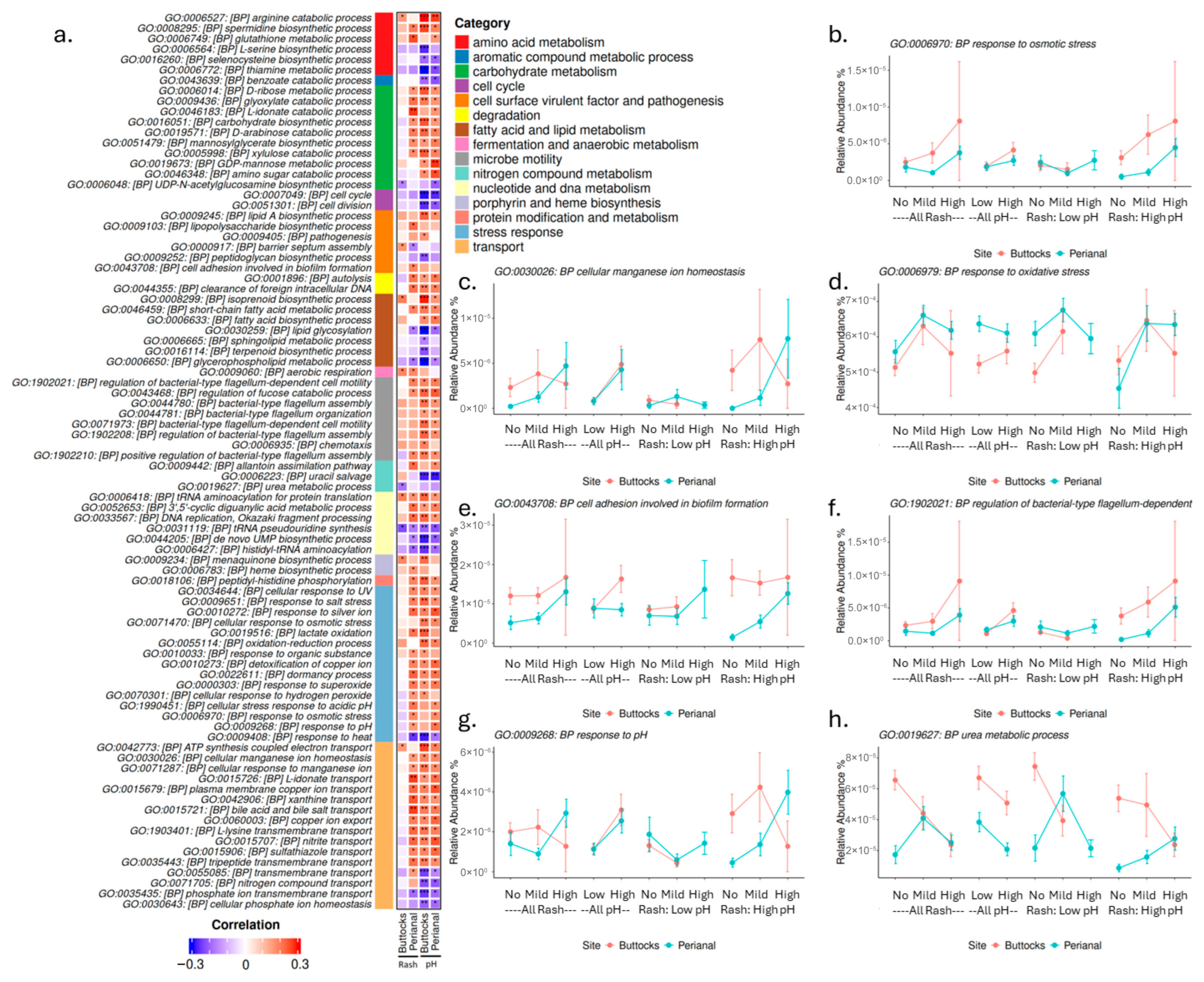
Moreover, many stress response genes were elevated in rash samples, particularly those related to oxidative response and osmolarity, while responses to heat showed a negative correlation with both pH and rash severity (
Figure 7). A potential link exists between osmolarity stress and homeostatic transport functions, which were elevated in cases of higher skin pH and perianal rash but did not correlate strongly with buttocks rash. This supports our hypothesis that perianal rashes may be more significantly affected by residual urine and fecal matter, leading to increased stress for microbes and irritation for the infant’s skin. As osmolarity and oxidative stress rise, microbes may activate detoxification pathways or biosynthesize substances like rhamnose to manage these stressors.
This manuscript presents a comprehensive analysis that integrates the contributions of skin pH and site-specific microbiome compositions (buttocks and perianal) across diverse infant populations to investigate the causes of DDA, a prevalent health concern for caregivers that remains underexplored, as evidenced by its high prevalence across all three geographies studied. By identifying specific microbial species correlated with DDA severity and skin pH, our study provides valuable insights into the microbial dynamics associated with DDA, paving the way for potential probiotic or microbiome-targeted interventions. Furthermore, these data suggest a necessity for the development of diapering interventions which support a healthy microbiome in the diapered area to mitigate the dysbiosis observed in DDA. The inclusion of infants from multiple countries allows for an examination of geographical variability in skin pH and microbiome composition, enhancing our understanding of how cultural and environmental factors impact infant skin health. The current findings across all three geographies underscore the importance of maintaining a slightly acidic skin pH on both non-diapered and diapered areas to reduce the incidence and severity of DDA. Caregivers should be aware of how products and behaviors can influence skin pH. When feasible, especially for patients with frequent or severe DDA, integrating skin pH monitoring into routine pediatric care is recommended. Our analysis of metabolic pathways and gene functions related to skin pH and diaper rash severity further enriches the understanding of the biological mechanisms underlying DDA. Understanding the factors influencing DDA has broader public health implications for infant care practices and recommendations. These findings can guide caregivers and healthcare professionals in implementing effective strategies to prevent and manage DDA, ultimately improving infant health outcomes. Insights from this study will also inform future research directions, paving the way for further exploration of the intricate dynamics between skin pH, microbiome health, and DDA.
The study is not without limitations, including the lack of longitudinal data, potential limitation for the classification of microbes through current available libraries, the use of tape strips for microbiome sampling (which may capture more background microbes than swabs), and the use of buttock pH due to inability to obtain perianal pH. Future research should focus on longitudinal studies to track changes in skin measurements, such as pH stratum corneum hydration, trans-epidermal water loss (TEWL), and microbiome composition related to DDA development over time, particularly addressing microbial functional changes to enhance understanding of effective prevention strategies.