ROS-Mediated Necroptosis Promotes Coxsackievirus B3 Replication and Myocardial Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell and Virus
2.3. TCID50 Assay
2.4. Drug Treatment
2.5. Blood Tests
2.6. Western Blot
2.7. RT-qPCR
2.8. Immunofluorescence Staining
2.9. Histopathology
2.10. Cell Counting
2.11. PI Staining
2.12. Detection of ROS
2.13. Statistical Analyses
3. Results
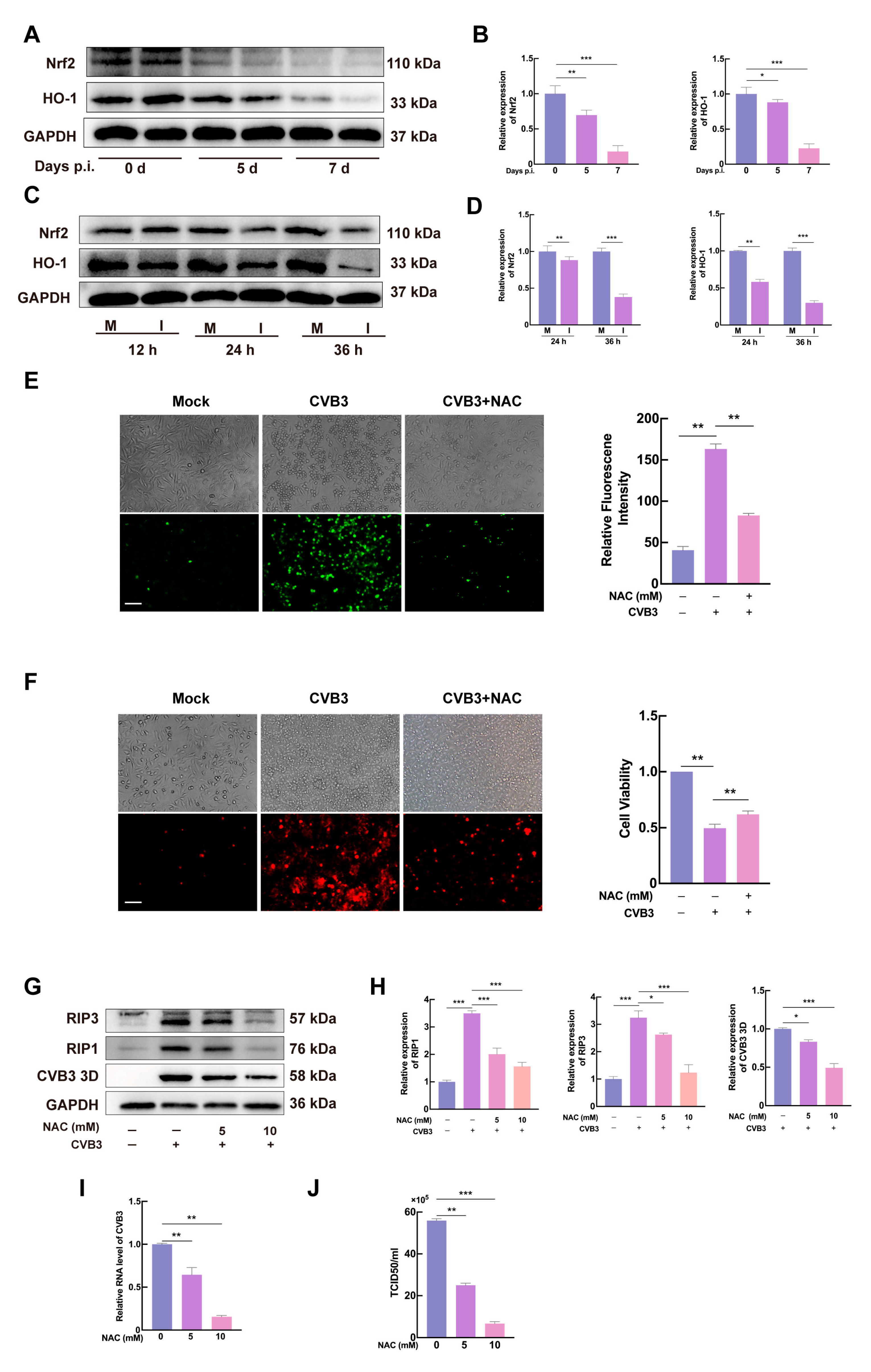
3.1. CVB3 Infection Induces Necroptosis in HeLa Cells
3.2. CVB3 Induces Necroptosis via the RIP1/RIP3 Pathway
3.3. Necroptosis Promotes CVB3 Replication In Vitro
3.4. Inhibition of CVB3-Induced Necroptosis Reduces Viral Replication and Ameliorates Myocardial Injury In Vivo
3.5. CVB3 Induces Necroptosis via ROS Production in HeLa Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tschöpe, C.; Cooper, L.T.; Torre-Amione, G.; Van Linthout, S. Management of Myocarditis-Related Cardiomyopathy in Adults. Circ. Res. 2019, 124, 1568–1583. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Yu, M.; Long, Q.; Li, H.H.; Liang, W.; Liao, Y.H.; Yuan, J.; Cheng, X. IL-9 Inhibits Viral Replication in Coxsackievirus B3-Induced Myocarditis. Front. Immunol. 2016, 7, 409. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; You, B.; Zhou, C. Advances in cell death mechanisms involved in viral myocarditis. Front. Cardiovasc. Med. 2022, 9, 968752. [Google Scholar] [CrossRef] [PubMed]
- Garmaroudi, F.S.; Marchant, D.; Hendry, R.; Luo, H.; Yang, D.; Ye, X.; Shi, J.; McManus, B.M. Coxsackievirus B3 replication and pathogenesis. Future Microbiol. 2015, 10, 629–653. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Yue, Y.; Dong, N.; Xiong, S. Endoplasmic Reticulum Stress Aggravates Viral Myocarditis by Raising Inflammation Through the IRE1-Associated NF-κB Pathway. Can. J. Cardiol. 2015, 31, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, E.; Niehusmann, P.; Fogdell-Hahn, A. The effect of human herpesvirus 6B infection on the MAPK pathway. Virus Res. 2018, 256, 134–141. [Google Scholar] [CrossRef]
- Adamson, A.L.; Jeffus, D.; Davis, A.; Greengrove, E. Epstein-Barr virus lytic replication activates and is dependent upon MAPK-interacting kinase 1/2 in a cell-type dependent manner. Virology 2022, 572, 72–85. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Wang, Y.; Yu, C.; Wang, Z.; Liu, H.; Wei, H.; Han, L.; Cheng, J.; Wang, F.; et al. Mechanisms and Therapeutic Strategies of Viral Myocarditis Targeting Autophagy. Front. Pharmacol. 2022, 13, 843103. [Google Scholar] [CrossRef]
- Chang, H.; Li, X.; Cai, Q.; Li, C.; Tian, L.; Chen, J.; Xing, X.; Gan, Y.; Ouyang, W.; Yang, Z. The PI3K/Akt/mTOR pathway is involved in CVB3-induced autophagy of HeLa cells. Int. J. Mol. Med. 2017, 40, 182–192. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Nie, W.; Jiang, J.; Li, S.; Li, Z.; Tian, L.; Ma, X. Exosomes derived from cardiac progenitor cells attenuate CVB3-induced apoptosis via abrogating the proliferation of CVB3 and modulating the mTOR signaling pathways. Cell Death Dis. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Hu, Y.; Wu, Z.; Li, Y.; Kong, M.; Kang, Z.; Zuoyuan, B.; Yang, Z. TFRC upregulation promotes ferroptosis in CVB3 infection via nucleus recruitment of Sp1. Cell Death Dis. 2022, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jiang, X.; Teng, L.; Yang, J.; Ding, J.; He, C. Necroptosis may be a novel mechanism for cardiomyocyte death in acute myocarditis. Mol. Cell Biochem. 2018, 442, 11–18. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Kroemer, G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 2012, 31, 1055–1057. [Google Scholar] [CrossRef]
- Wen, C.; Yu, Y.; Gao, C.; Qi, X.; Cardona, C.J.; Xing, Z. RIPK3-Dependent Necroptosis Is Induced and Restricts Viral Replication in Human Astrocytes Infected With Zika Virus. Front. Cell Infect. Microbiol. 2021, 11, 637710. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; McQuade, T.; Zhang, H.; Zhang, J.; Chan, F.K. RIP1-dependent and independent effects of necrostatin-1 in necrosis and T cell activation. PLoS ONE 2011, 6, e23209. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Liu, N.; Huang, Y.; Jin, Z.; Zhang, S.; Ming, Z.; Chen, H. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020, 11, 134. [Google Scholar] [CrossRef]
- Sugaya, T.; Kanno, H.; Matsuda, M.; Handa, K.; Tateda, S.; Murakami, T.; Ozawa, H.; Itoi, E. B-RAF(V600E) Inhibitor Dabrafenib Attenuates RIPK3-Mediated Necroptosis and Promotes Functional Recovery after Spinal Cord Injury. Cells 2019, 8, 1582. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12, Erratum in Mol. Cell Biochem. 2020, 467, 13. [Google Scholar] [CrossRef]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postep. Hig. Med. Dosw. (Online) 2016, 70, 1150–1165. [Google Scholar] [CrossRef]
- Bedient, L.; Pokharel, S.M.; Chiok, K.R.; Mohanty, I.; Beach, S.S.; Miura, T.A.; Bose, S. Lytic Cell Death Mechanisms in Human Respiratory Syncytial Virus-Infected Macrophages: Roles of Pyroptosis and Necroptosis. Viruses 2020, 12, 932. [Google Scholar] [CrossRef]
- Yang, P.; Xu, B.; Zhu, R.; Zhang, T.; Wang, Z.; Lin, Q.; Yan, M.; Yu, Z.; Mao, H.; Zhang, Y. ROS-mediated mitophagy and necroptosis regulate osteocytes death caused by TCP particles in MLO-Y4 cells. Toxicology 2023, 496, 153627. [Google Scholar] [CrossRef]
- Shang, L.; Ding, W.; Li, N.; Liao, L.; Chen, D.; Huang, J.; Xiong, K. The effects and regulatory mechanism of RIP3 on RGC-5 necroptosis following elevated hydrostatic pressure. Acta Biochim. Biophys. Sin. 2017, 49, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Zhang, Y.; He, X.; Zhong, C.Q.; Ni, H.; Chen, X.; Liang, Y.; Wu, J.; Zhao, S.; et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 2018, 20, 186–197, Erratum in Nat. Cell Biol. 2024, 26, 1225. [Google Scholar] [CrossRef]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef]
- Jin, M.; Wei, Y.; Yu, H.; Ma, X.; Yan, S.; Zhao, L.; Ding, L.; Cheng, J.; Feng, H. Erythritol Improves Nonalcoholic Fatty Liver Disease by Activating Nrf2 Antioxidant Capacity. J. Agric. Food Chem. 2021, 69, 13080–13092. [Google Scholar] [CrossRef]
- Hua, W.; Jiang, J.B.; Rong, X.; Wu, R.Z.; Qiu, H.X.; Zhang, Y.H.; Chen, Q. Expression of cystathionine-γ-lyase/hydrogen sulfide pathway in CVB3-induced myocarditis in mice. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2010, 12, 744–748. [Google Scholar]
- Tong, L.; Lin, L.; Zhao, W.; Wang, B.; Wu, S.; Liu, H.; Zhong, X.; Cui, Y.; Gu, H.; Zhang, F.; et al. Destabilization of coxsackievirus b3 genome integrated with enhanced green fluorescent protein gene. Intervirology 2011, 54, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Vlok, M.; Solis, N.; Sadasivan, J.; Mohamud, Y.; Warsaba, R.; Kizhakkedathu, J.; Luo, H.; Overall, C.M.; Jan, E. Identification of the proteolytic signature in CVB3-infected cells. J. Virol. 2024, 98, e0049824. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, H.; Zou, Z.; Gong, J.; Zhou, J.; Peng, N.; Su, L.; Maegele, M.; Cai, D.; Gu, Z. Preventing necroptosis by scavenging ROS production alleviates heat stress-induced intestinal injury. Int. J. Hyperth. 2020, 37, 517–530. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Kishimoto, C.; Takada, H.; Kurokawa, M.; Ochiai, H.; Shiraki, K.; Sasayama, S. Role of oxygen derived free radicals in the pathogenesis of coxsackievirus B3 myocarditis in mice. Cardiovasc. Res. 1993, 27, 957–961. [Google Scholar] [CrossRef]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef]
- Ammirati, E.; Moslehi, J.J. Diagnosis and Treatment of Acute Myocarditis: A Review. JAMA 2023, 329, 1098–1113. [Google Scholar] [CrossRef]
- Koehler, H.; Cotsmire, S.; Zhang, T.; Balachandran, S.; Upton, J.W.; Langland, J.; Kalman, D.; Jacobs, B.L.; Mocarski, E.S. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe 2021, 29, 1266–1276.e1265. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. DAI/ZBP1/DLM-1 Complexes with RIP3 to Mediate Virus-Induced Programmed Necrosis that Is Targeted by Murine Cytomegalovirus vIRA. Cell Host Microbe 2019, 26, 564. [Google Scholar] [CrossRef]
- Omoto, S.; Guo, H.; Talekar, G.R.; Roback, L.; Kaiser, W.J.; Mocarski, E.S. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J. Biol. Chem. 2015, 290, 11635–11648. [Google Scholar] [CrossRef] [PubMed]
- Dufour, F.; Sasseville, A.M.; Chabaud, S.; Massie, B.; Siegel, R.M.; Langelier, Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFα- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis 2011, 16, 256–271. [Google Scholar] [CrossRef]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Guan, Z.; Li, H.; Ye, M.; Chen, X.; Shen, J.; Zhou, Y.; Shi, Z.L.; Zhou, P.; et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020, 5, 235. [Google Scholar] [CrossRef]
- Ino, S.; Yano, T.; Kuno, A.; Tanno, M.; Kouzu, H.; Sato, T.; Yamashita, T.; Ohwada, W.; Osanami, A.; Ogawa, T.; et al. Nuclear translocation of MLKL enhances necroptosis by a RIP1/RIP3-independent mechanism in H9c2 cardiomyoblasts. J. Pharmacol. Sci. 2023, 151, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yin, C.; Boyd, D.F.; Quarato, G.; Ingram, J.P.; Shubina, M.; Ragan, K.B.; Ishizuka, T.; Crawford, J.C.; Tummers, B.; et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell 2020, 180, 1115–1129.e1113. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.K.; Danthi, P. Reovirus activates a caspase-independent cell death pathway. mBio 2013, 4, e00178-13. [Google Scholar] [CrossRef]
- Thapa, R.J.; Nogusa, S.; Balachandran, S. Analysis of Cytokine- and Influenza A Virus-Driven RIPK3 Necrosome Formation. Methods Mol. Biol. 2018, 1857, 93–99. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, X.; Meng, X.; Huo, W.; Su, Y.; Liu, J.; Liu, Y.; Zhang, J.; Wang, S.; Yu, J. Coxsackievirus A6 Induces Necroptosis for Viral Production. Front. Microbiol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, L.; Ma, X.; Wang, J.; Yang, J.; Zhou, X.; Yang, Y.; Liu, H. RIP1/RIP3/MLKL-mediated necroptosis contributes to vinblastine-induced myocardial damage. Mol. Cell Biochem. 2021, 476, 1233–1243. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Kim, D.H.; Chung, H.Y. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. 2021, 44, 102022. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Chen, Z.; Yu, Y.; Shi, H.; Yu, Y.; Wang, Y.; Chen, R.; Ge, J. Mitochondrial calpain-1 activates NLRP3 inflammasome by cleaving ATP5A1 and inducing mitochondrial ROS in CVB3-induced myocarditis. Basic Res. Cardiol. 2022, 117, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.Q.; Chen, X.; Cai, Q.; Yang, Z.H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, J.; Yu, Z.; Qu, X.; Guan, S. 1,3-Dichloro-2-propanol-Induced Renal Tubular Cell Necroptosis through the ROS/RIPK3/MLKL Pathway. J. Agric. Food Chem. 2022, 70, 10847–10857. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Qin, B.; Gu, Y.; Zhou, L.; Chen, S.; Zhang, S.; Zhang, S.; Han, Q.; Liu, Y.; Wu, X. ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxid. Med. Cell Longev. 2020, 2020, 1295382. [Google Scholar] [CrossRef] [PubMed]
- Weindel, C.G.; Martinez, E.L.; Zhao, X.; Mabry, C.J.; Bell, S.L.; Vail, K.J.; Coleman, A.K.; VanPortfliet, J.J.; Zhao, B.; Wagner, A.R.; et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 2022, 185, 3214–3231.e3223. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Di, Y.; Song, Q.; Cheng, Z.; Wu, H.; Wu, M.; He, M.; Zhang, G.; Wang, F.; Tong, L. ROS-Mediated Necroptosis Promotes Coxsackievirus B3 Replication and Myocardial Injury. Microorganisms 2025, 13, 2389. https://doi.org/10.3390/microorganisms13102389
Huang J, Di Y, Song Q, Cheng Z, Wu H, Wu M, He M, Zhang G, Wang F, Tong L. ROS-Mediated Necroptosis Promotes Coxsackievirus B3 Replication and Myocardial Injury. Microorganisms. 2025; 13(10):2389. https://doi.org/10.3390/microorganisms13102389
Chicago/Turabian StyleHuang, Junbo, Yanjun Di, Qing Song, Zhiyun Cheng, Hao Wu, Mei Wu, Minjian He, Genrui Zhang, Fucai Wang, and Lei Tong. 2025. "ROS-Mediated Necroptosis Promotes Coxsackievirus B3 Replication and Myocardial Injury" Microorganisms 13, no. 10: 2389. https://doi.org/10.3390/microorganisms13102389
APA StyleHuang, J., Di, Y., Song, Q., Cheng, Z., Wu, H., Wu, M., He, M., Zhang, G., Wang, F., & Tong, L. (2025). ROS-Mediated Necroptosis Promotes Coxsackievirus B3 Replication and Myocardial Injury. Microorganisms, 13(10), 2389. https://doi.org/10.3390/microorganisms13102389





