The Influence of Seasonal Variations in Clinical Trials Based on Gut Microbiota Studies
Abstract
1. Introduction
2. Search Strategies
3. Mechanisms and Evidence of Gut Microbiota Seasonality
Seasonal Food Availability and Dietary Influence
4. Environmental and Behavioral Factors
4.1. Effect of Sunlight Exposure and Vitamin D Production on Gut Microbiota
4.2. Thermogenic Adaptation and Climate-Related Effects on Gut Microbiota
4.3. Effect of Seasonal Infections and Immune System Interactions on Gut Microbiota
4.4. Seasonal-Dependent Physical Activity Impact on Gut Microbiota
4.5. Circadian Rhythms and Light Exposure Effects on Gut Microbiota
5. Implications for Non-Communicable Chronic Diseases
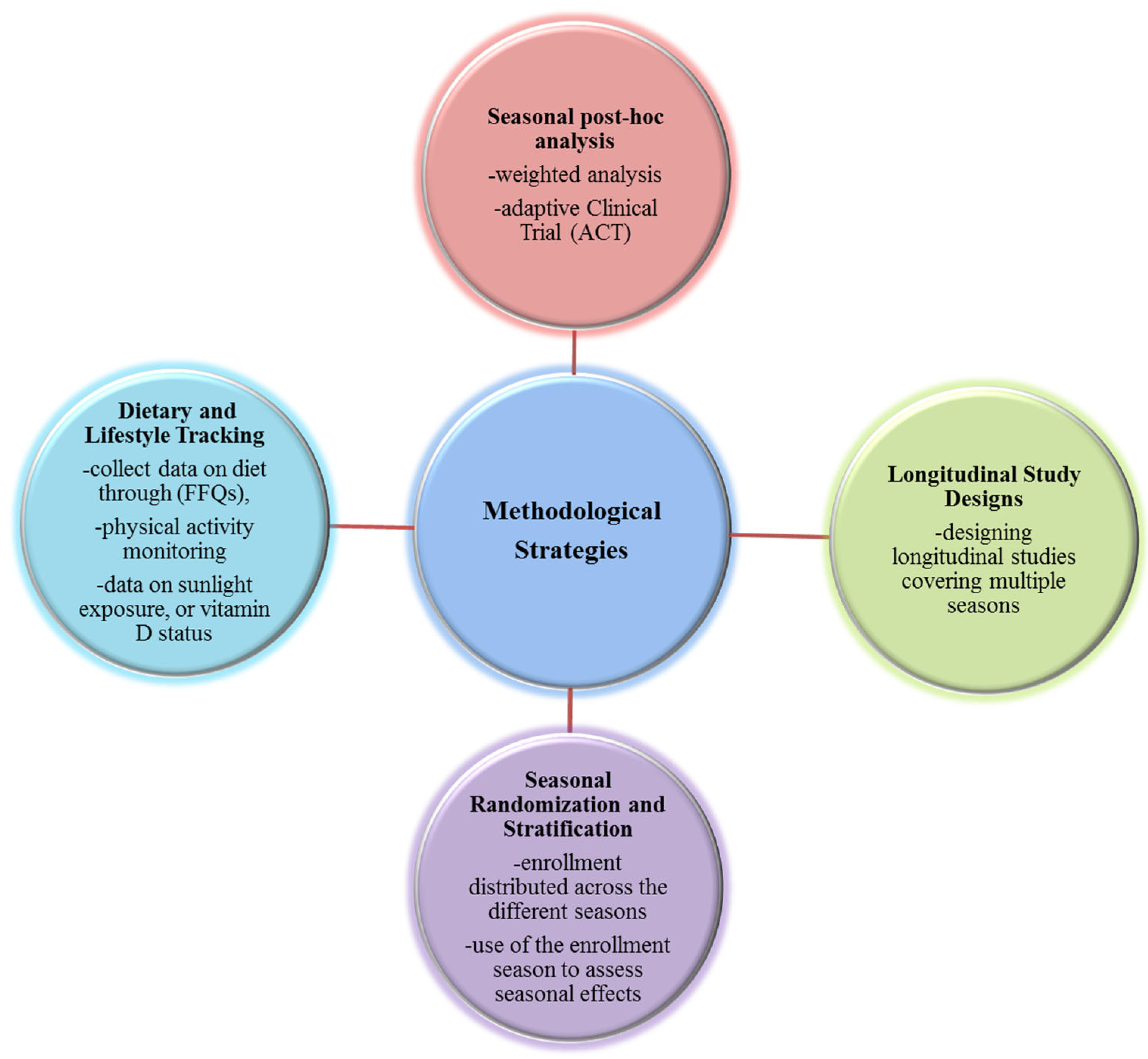
6. Methodological Strategies for Clinical Trials
6.1. Longitudinal Study Designs
6.2. Seasonal Randomization and Stratification
6.3. Dietary and Lifestyle Tracking
6.4. Seasonal Post Hoc Analysis
- the sample size (sample size re-estimation);
- the randomization (response-adaptive randomization);
- the duration of follow-up;
- even the early closure of an arm.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Ahn, J.; Hayes, R.B. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu. Rev. Public Health 2021, 42, 277–292. [Google Scholar] [CrossRef]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef]
- Davenport, E.R.; Mizrahi-Man, O.; Michelini, K.; Barreiro, L.B.; Ober, C.; Gilad, Y. Seasonal variation in human gut microbiome composition. PLoS ONE 2014, 9, e90731. [Google Scholar] [CrossRef]
- Ding, T.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.T.; Hennessy, E.A. Systematic reviews and meta-analyses in the health sciences: Best practice methods for research syntheses. Soc. Sci. Med. 2019, 233, 237–251. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Z.; Lim, A.A.; Zheng, Y.; Koh, E.Y.; Ho, D.; Qiao, J.; Huo, D.; Hou, Q.; Huang, W.; et al. Mongolians core gut microbiota and its correlation with seasonal dietary changes. Sci. Rep. 2014, 4, 5001. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wu, C.; Cai, S.; Huang, W.; Chen, J.; Xi, X.; Liang, Z.; Hou, Q.; Zhou, B.; et al. Unique Features of Ethnic Mongolian Gut Microbiome revealed by metagenomic analysis. Sci. Rep. 2017, 7, 39576, Erratum in Sci. Rep. 2016, 6, 34826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Piven, L.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Seasonal variation in gut microbiota composition: Cross-sectional evidence from Ukrainian population. BMC Microbiol. 2020, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wu, Q.; Shi, F.; Niu, J.; Zhang, T.; Degen, A.A.; Fang, Q.; Ding, L.; Shang, Z.; Zhang, Z.; et al. Seasonal dynamics of diet-gut microbiota interaction in adaptation of yaks to life at high altitude. npj Biofilms Microbiomes 2021, 7, 38. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 5, 1469–1476. [Google Scholar] [CrossRef]
- Carey, H.V.; Walters, W.A.; Knight, R. Seasonal restructuring of the ground squirrel gut microbiota over the annual hibernation cycle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R33–R42. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiota alterations in high-fat-diet-fed mice are associated with antibiotic use and vitamin D deficiency. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef]
- Soltys, K.; Stuchlikova, M.; Hlavaty, T.; Gaalova, B.; Budis, J.; Gazdarica, J.; Krajcovicova, A.; Zelinkova, Z.; Szemes, T.; Kuba, D.; et al. Seasonal changes of circulating 25-hydroxyvitamin D correlate with the lower gut microbiome composition in inflammatory bowel disease patients. Sci. Rep. 2020, 10, 6024. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, H.; Herlemann, D.P.; Klinitzke, P.; Berlin, P.; Kreikemeyer, B.; Jaster, R.; Lamprecht, G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 2018, 19, 225–234. [Google Scholar] [CrossRef]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Ullah, H. Gut-vitamin D interplay: Key to mitigating immunosenescence and promoting healthy ageing. Immun. Ageing 2025, 22, 20. [Google Scholar] [CrossRef]
- Krutzik, S.R.; Hewison, M.; Liu, P.T.; Robles, J.A.; Stenger, S.; Adams, J.S.; Modlin, R.L. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 2008, 181, 7115–7120. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Tsounis, E.P.; Mouzaki, A.; Triantos, C. Exploring the Role of Vitamin D and the Vitamin D Receptor in the Composition of the Gut Microbiota. Front. Biosci. (Landmark Ed.) 2023, 28, 116. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, W.B.; Abadi, M.N.P.; Fadhillah, F.S.; Nurkolis, F.; Pramono, A. The Interlink between Climate Changes, Gut Microbiota, and Aging Processes. Hum. Nutr. Metab. 2023, 32, 200193. [Google Scholar] [CrossRef]
- Ottman, N.; Ruokolainen, L.; Suomalainen, A.; Sinkko, H.; Karisola, P.; Lehtimäki, J.; Lehto, M.; Hanski, I.; Alenius, H.; Fyhrquist, N. Soil Exposure Modifies the Gut Microbiota and Supports Immune Tolerance in a Mouse Model. J. Allergy Clin. Immunol. 2019, 143, 1198–1206.e12. [Google Scholar] [CrossRef]
- Hylander, B.L.; Repasky, E.A. Temperature as a modulator of the gut microbiome: What are the implications and opportunities for thermal medicine? Int. J. Hyperth. 2019, 36 (Suppl. 1), 83–89. [Google Scholar] [CrossRef]
- Liu, S.; Wen, D.; Feng, C.; Yu, C.; Gu, Z.; Wang, L.; Zhang, Z.; Li, W.; Wu, S.; Liu, Y.; et al. Alteration of gut microbiota after heat acclimation may reduce organ damage by regulating immune factors during heat stress. Front. Microbiol. 2023, 14, 1114233. [Google Scholar] [CrossRef]
- Albiero, M.; Migliozzi, L.; Boscaro, C.; Rodella, A.; Ciciliot, S.; Ivan Amendolagine, F.; Scattolini, V.; Treu, L.; Cappellari, R.; Lanuti, P.; et al. Padi4-dependent NETosis enables diet-induced gut hyperpermeability, translating dysbiosis into systemic inflammation and dysmetabolism. Diabetes 2025, 74, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Van de Wiele, T.; Verstraete, W.; Possemiers, S. The host selects mucosal and luminal associations of coevolved gut microorganisms: A novel concept. FEMS Microbiol. Rev. 2011, 35, 681–1385. [Google Scholar] [CrossRef]
- Kurtz, C.C.; Carey, H.V. Seasonal changes in the intestinal immune system of hibernating ground squirrels. Dev. Comp. Immunol. 2007, 31, 415–428. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, F.; Wei, H.; Lian, Z.X.; Sun, R.; Tian, Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014, 211, 2397–2410, Erratum in J. Exp. Med. 2014, 211, 2683; Erratum in J. Exp. Med. 2014, 211, 2396–2397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Wang, F.; Liu, Y.; Gu, F. Intestinal microbiota dysbiosis in children with recurrent respiratory tract infections. Microb. Pathog. 2019, 136, 103709. [Google Scholar] [CrossRef]
- Chunxi, L.; Haiyue, L.; Yanxia, L.; Jianbing, P.; Jin, S. The Gut Microbiota and Respiratory Diseases: New Evidence. J. Immunol. Res. 2020, 2020, 2340670. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Di Carlo, P.; Serra, N.; Sergi, C.M.; Toia, F.; Battaglia, E.; Fasciana, T.M.A.; Rodolico, V.; Giammanco, A.; Salamone, G.; Cordova, A.; et al. Seasonal Change in Microbial Diversity: Bile Microbiota and Antibiotics Resistance in Patients with Bilio-Pancreatic Tumors: A Retrospective Monocentric Study (2010–2020). Antibiotics 2025, 14, 283. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’SUllivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’REilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Karahan, F. Environmental Factors Affecting the Gut Microbiota and Their Consequences. Nat. Cell Sci. 2024, 2, 133–140. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pé-rez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Yoshimoto, S.; Yoshida, K.; Mitsuyama, E.; Iwabuchi, N.; Hosomi, K.; Sanada, T.J.; Tanaka, M.; Nanri, H.; Kunisawa, J.; et al. An Exploratory Study on Seasonal Variation in the Gut Microbiota of Athletes: Insights from Japanese Handball Players. Microorganisms 2024, 12, 781. [Google Scholar] [CrossRef]
- Hu, G.X.; Chen, G.R.; Xu, H.; Ge, R.S.; Lin, J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses 2010, 74, 123–126. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 1. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Lee, R.P.; Lu, Q.Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.H.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, S.; Zou, D.; Dong, D.; He, X.; Liu, N.; Liu, W.; Huang, L. Metabolic shifts and structural changes in the gut microbiota upon branched-chain amino acid supplementation in middle-aged mice. Amino Acids 2016, 48, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef]
- Honma, K.; Honma, S.; Kohsaka, M.; Fukuda, N. Seasonal variation in the human circadian rhythm: Dissociation between sleep and temperature rhythm. Am. J. Physiol. 1992, 262 Pt 2, R885–R891. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, K.M.; Stensrud, S.; Asnicar, F.; Valdes, A.M.; Franks, P.W.; Wolf, J.; Hadjigeorgiou, G.; Davies, R.; Spector, T.D.; Segata, N.; et al. Exploring the relationship between social jetlag with gut microbial composition, diet and cardiometabolic health, in the ZOE PREDICT 1 cohort. Eur. J. Nutr. 2023, 62, 3135–3147. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Frazier, K.; Chang, E.B. Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol. Metab. 2020, 31, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Lotti, S.; Dinu, M.; Colombini, B.; Amedei, A.; Sofi, F. Circadian rhythms, gut microbiota, and diet: Possible implications for health. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1490–1500. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Allaband, C.; Lingaraju, A.; Flores Ramos, S.; Kumar, T.; Javaheri, H.; Tiu, M.D.; Dantas Machado, A.C.; Richter, R.A.; Elijah, E.; Haddad, G.G.; et al. Time of sample collection is critical for the replicability of microbiome analyses. Nat. Metab. 2024, 6, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- Berglund, L.; Berne, C.; Svärdsudd, K.; Garmo, H.; Melhus, H.; Zethelius, B. Seasonal variations of insulin sensitivity from a euglycemic insulin clamp in elderly men. Upsala J. Med. Sci. 2012, 117, 35–40. [Google Scholar] [CrossRef]
- Moon, S.J.; Lee, Y.C.; Kim, T.J.; Kim, K.; Son, H.J. Effects of temperature, weather, seasons, atmosphere, and climate on the exacerbation of inflammatory bowel diseases: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0279277. [Google Scholar] [CrossRef]
- Tani, M.; Shinzaki, S.; Asakura, A.; Tashiro, T.; Amano, T.; Otake-Kasamoto, Y.; Yoshihara, T.; Yoshii, S.; Tsujii, Y.; Hayashi, Y.; et al. Seasonal variations in gut microbiota and disease course in patients with inflammatory bowel disease. PLoS ONE 2023, 18, e0283880. [Google Scholar] [CrossRef]
- Schlesinger, N.; Brunetti, L.; Androulakis, I.P. Does seasonality of the microbiota contribute to the seasonality of acute gout flare? Clin. Exp. Rheumatol. 2022, 40, 1793–1800. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef]
- McCauley, K.E.; Flynn, K.; Calatroni, A.; DiMassa, V.; LaMere, B.; Fadrosh, D.W.; Lynch, K.V.; Gill, M.A.; Pongracic, J.A.; Khurana Hershey, G.K.; et al. Seasonal airway microbiome and transcriptome interactions promote childhood asthma exacerbations. J. Allergy Clin. Immunol. 2022, 150, 204–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Shen, J.; Galloway-Peña, J.; Shelburne, S.; Wang, L.; Hu, L. Inverse Probability Weighting-based Mediation Analysis for Microbiome Data. arxiv 2021, arXiv:2110.02440. [Google Scholar]
- Berry, D.A. Adaptive clinical trials: The promise and the caution. J. Clin. Oncol. 2011, 29, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Lelwala, E.I.; Seamasinghe, W.M.; Gunarathna, K.M.L.M. Nonparametric Approach to Detecting Seasonality in Time Series: Application of the Kruskal-Wallis (KW) Test on Tourist Arrivals to Sri Lanka. South Asian J. Bus. Insights 2024, 4, 3–19. [Google Scholar] [CrossRef]

| Seasonal Factor | Population | Method | Gut Bacteria Over-Represented | Gut-Bacteria Under-Represented | Ref. |
| High-fat, high-protein diets | Hutterites | 16S rRNA gene sequencing | Actinobacteria | Bacteroidetes | [7] |
| High fiber and low protein diets | Hutterites | 16S rRNA gene sequencing | Bacteroidetes | Actinobacteria, Firmicutes | [7] |
| Sunlight and vitamin D | Slovaks | 16S rRNA gene sequencing 16S rRNA gene sequencing | Enterobacteriaceae, Roseburia, | Eggerthella lenta, Helicobacter, | [21,23] |
| German | Alistipes, Faecalibacterium | Fusobacterium, Faecalibacterium prausnitzii | |||
| Physical Activity | Spanish | 16S rRNA gene sequencing | Faecalibacterium prausnitzii, Roseburia hominis, Akkermansia muciniphila | _ | [50] |
| Japanese | 16S rRNA gene sequencing | Faecalibacterium, Streptococcus | [51] | ||
| Circadian Rhythms, active phase | murine models | 16S rRNA gene sequencing | Firmicutes | Bacteroidetes | [64] |
| Circadian Rhythms, resting phase | murine models | 16S rRNA gene sequencing | Bacteroidetes | Firmicutes | [64] |
| Heat Temperature | Chinese | 16S rRNA gene sequencing | Escherichia, Shigella | Dorea, Blautia, Lactobacillus, Subdoligranulum | [33] |
| Cold Temperature | murine models | 16S rRNA gene sequencing | Firmicutes/Bacteroidetes ratio | Akkermansia muciniphila | [35] |
| Gout exacerbation in spring | Chinese | 16S rRNA gene sequencing | Bacteroides caccae, Bacteroides xylanisolvens | SCFA-producing bacteria | [72] |
| IBD in summer/autumn | Slovaks | 16S rRNA gene sequencing | Pediococcus, Clostridium, Escherichia/Shigella | Eggerthella lenta, Helicobacter, Fusobacterium, Faecalibacterium prausnitzii | [21] |
| Respiratory tract infections | Chinese | 16S rRNA gene sequencing | Enterobacteriaceae | Lactobacillus | [41] |
| Chinese | 16S rRNA gene sequencing | Enterococcus | Bifidobacterium | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocomazzi, G.; Panebianco, C.; Vallelunga, A.; De Ruvo, D.; Del Pup, L.; Smeazzetto, S.; Antinori, M.; Chimienti, V.; Maggio, G.; Finocchiaro, C.; et al. The Influence of Seasonal Variations in Clinical Trials Based on Gut Microbiota Studies. Microorganisms 2025, 13, 2386. https://doi.org/10.3390/microorganisms13102386
Cocomazzi G, Panebianco C, Vallelunga A, De Ruvo D, Del Pup L, Smeazzetto S, Antinori M, Chimienti V, Maggio G, Finocchiaro C, et al. The Influence of Seasonal Variations in Clinical Trials Based on Gut Microbiota Studies. Microorganisms. 2025; 13(10):2386. https://doi.org/10.3390/microorganisms13102386
Chicago/Turabian StyleCocomazzi, Giovanna, Concetta Panebianco, Annamaria Vallelunga, Daniele De Ruvo, Lino Del Pup, Serena Smeazzetto, Monica Antinori, Valeria Chimienti, Gabriele Maggio, Concetta Finocchiaro, and et al. 2025. "The Influence of Seasonal Variations in Clinical Trials Based on Gut Microbiota Studies" Microorganisms 13, no. 10: 2386. https://doi.org/10.3390/microorganisms13102386
APA StyleCocomazzi, G., Panebianco, C., Vallelunga, A., De Ruvo, D., Del Pup, L., Smeazzetto, S., Antinori, M., Chimienti, V., Maggio, G., Finocchiaro, C., Contu, V., & Pazienza, V. (2025). The Influence of Seasonal Variations in Clinical Trials Based on Gut Microbiota Studies. Microorganisms, 13(10), 2386. https://doi.org/10.3390/microorganisms13102386








