Neurotoxicity and Intestinal Microbiota Dysbiosis in the Chinese Mitten Crab (Eriocheir sinensis) Induced by Anatoxin-a: A Microbiota–Intestine–Brain Axis Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Experimental Design
2.3. Biochemical Assay
2.4. Quantitative Real-Time PCR
2.5. Transcriptome Sequencing (RNA-Seq) Analysis
2.6. Microbiome Profiling
2.7. Integrative Analysis of Intestinal Microbiome and Metabolomics
2.8. Statistical Analyses
3. Results
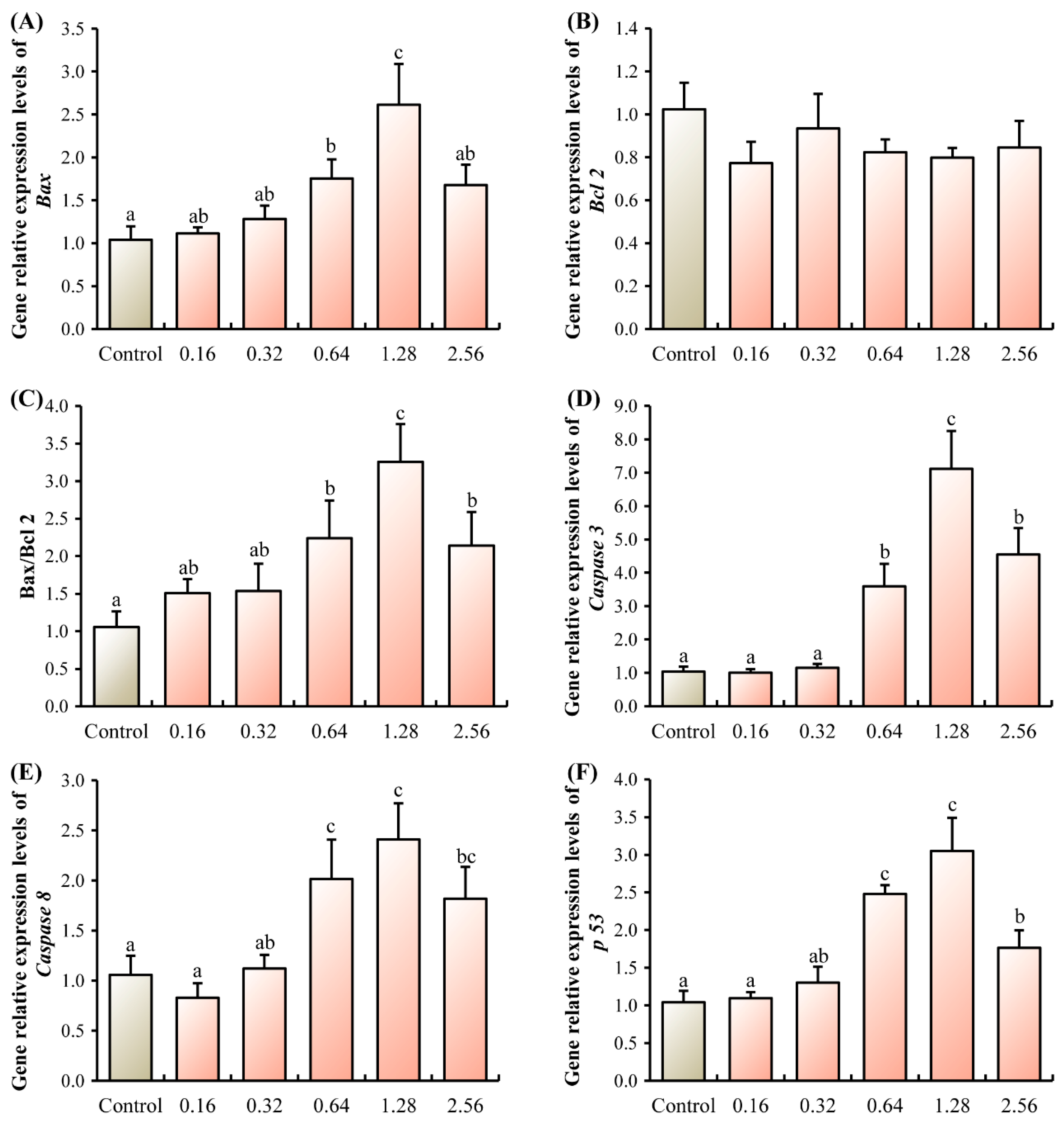
3.1. Apoptotic Analysis
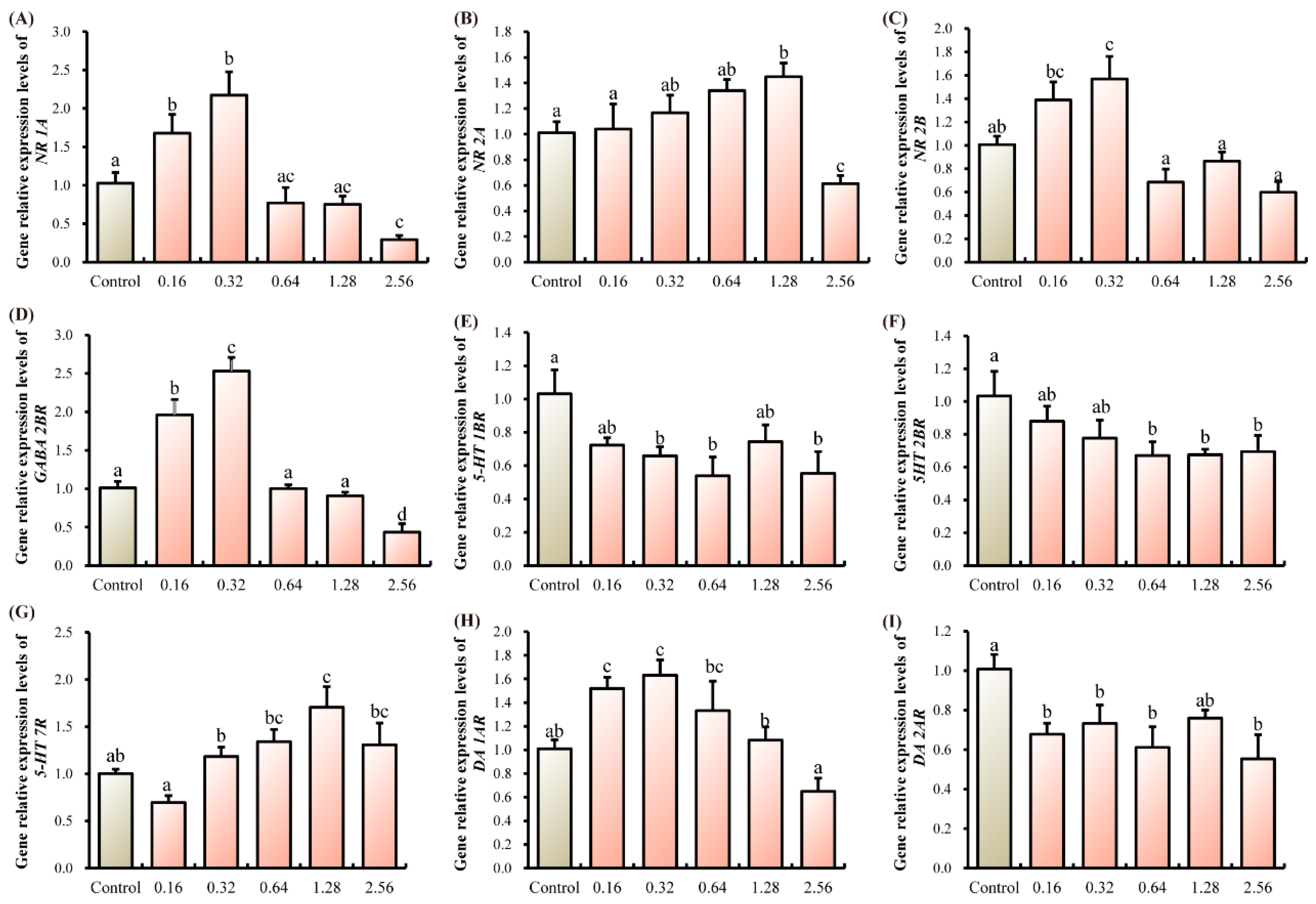
3.2. Neurotransmitter Homeostasis
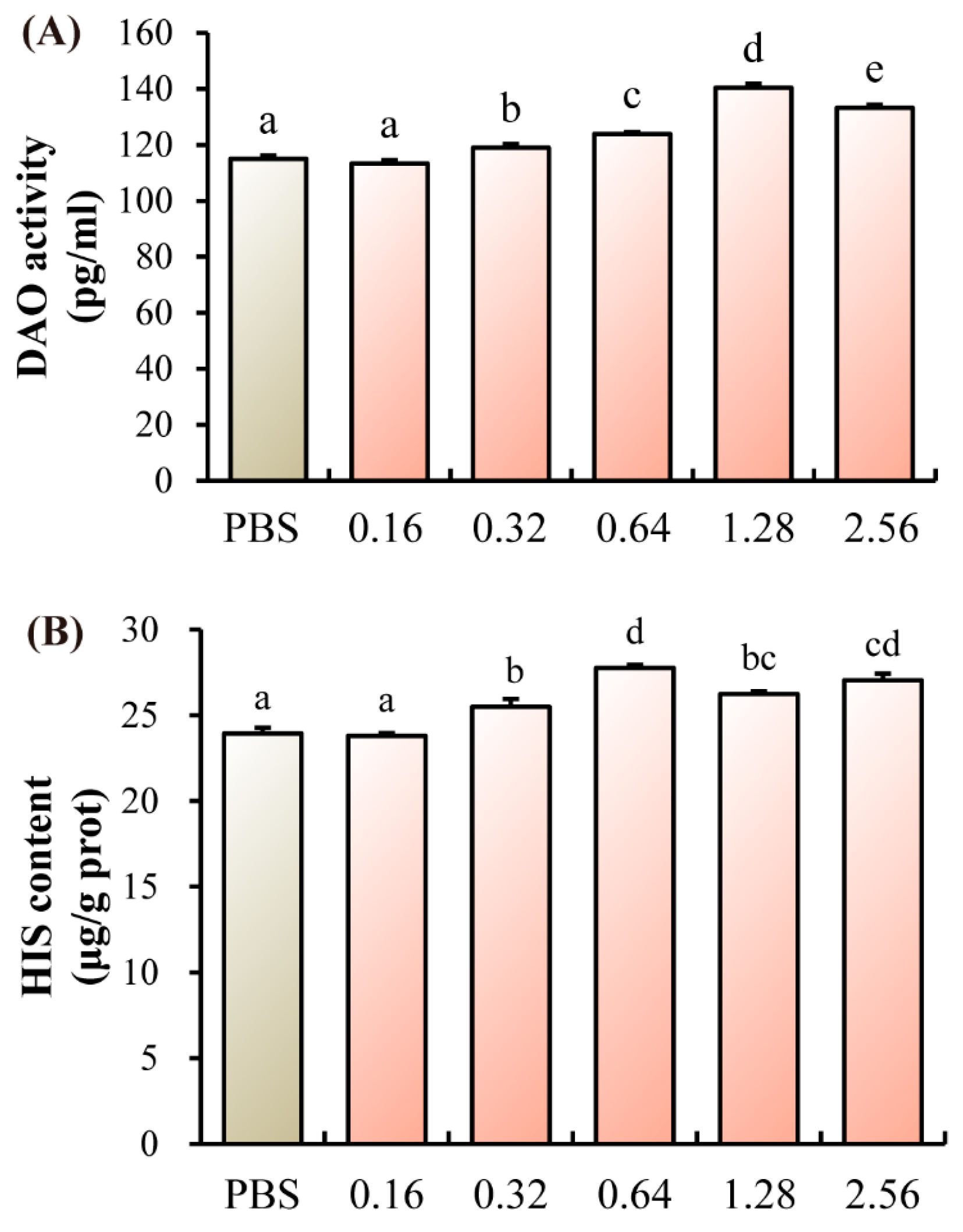
3.3. Intestinal Injury Indicators
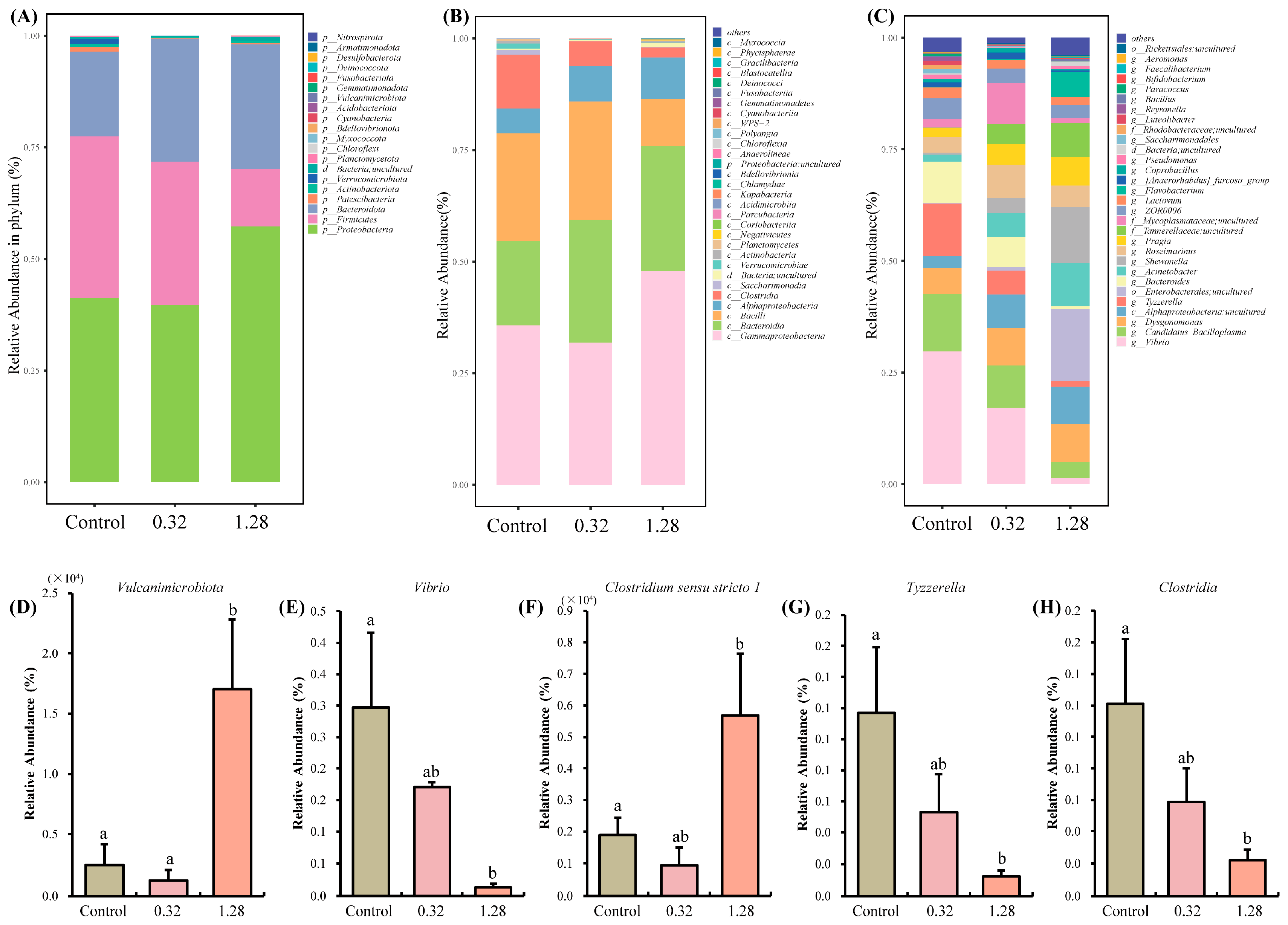
3.4. Intestinal Flora Analysis
3.4.1. Intestinal Microbiota Diversity Analysis
3.4.2. Microbial Species Analysis
3.5. Transcriptomic Alterations
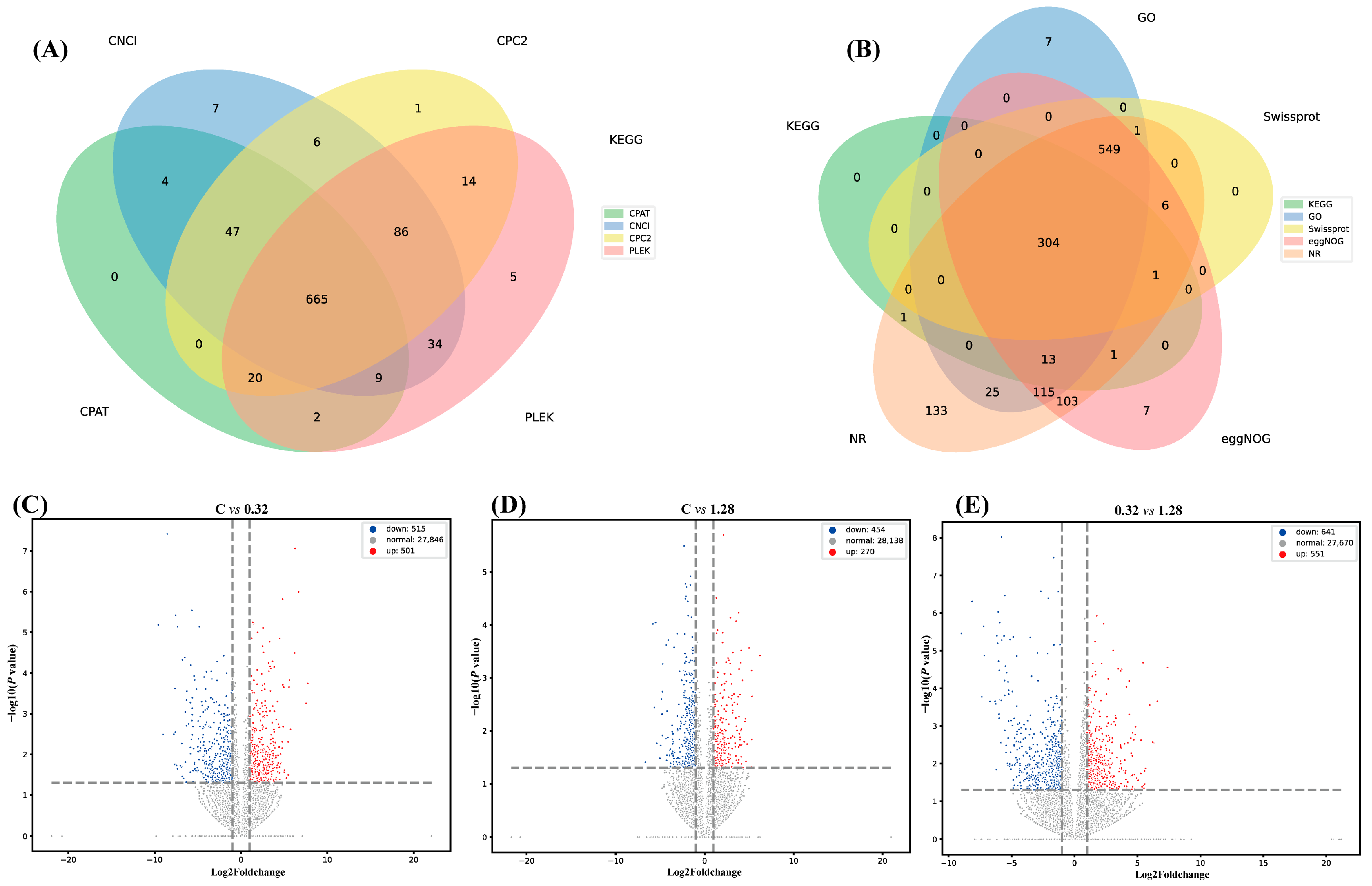
3.5.1. Transcriptome Sequence Evaluation and Annotation
3.5.2. Novel Gene Prediction
3.5.3. Differentially Expressed Gene Analysis
3.5.4. Functional Enrichment Analysis of Differentially Expressed Genes
3.5.5. RNA-Seq Validation by qRT-PCR
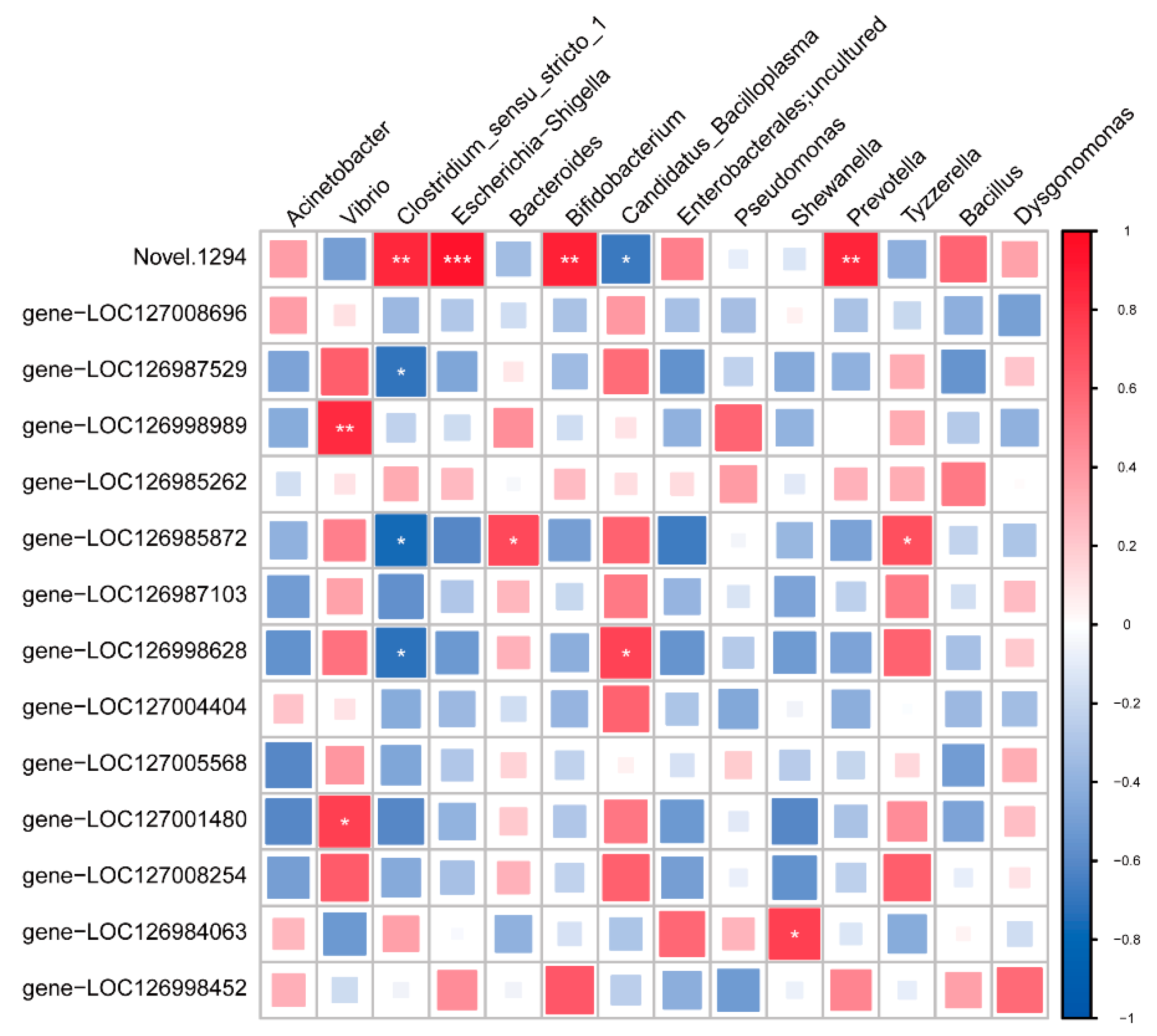
3.6. Comprehensive Flora and Transcriptome Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibelings, B.W.; Havens, K.E. Cyanobacterial toxins: A qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. Adv. Exp. Med. Biol. 2008, 619, 675–732. [Google Scholar]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Luo, C.; Wang, S.; Zuo, J.; Luo, Y.; Gan, N. Research and Prospects of Eco-toxicity of Anatoxins. Asian J. Ecotoxicol. 2022, 17, 217–225. [Google Scholar]
- Carmichael, W.W.; Biggs, D.F.; Gorham, P.R. Toxicology and Pharmacological Action of Anabaena flos-aquae Toxin. Science 1975, 187, 542–544. [Google Scholar] [CrossRef]
- Thomas, P.; Stephens, M.; Wilkie, G.; Amar, M.; Lunt, G.G.; Whiting, P.; Gallagher, T.; Pereira, E.; Alkondon, M.; Albuquerque, E.X.; et al. (+)-Anatoxin-a is a potent agonist at neuronal nicotinic acetylcholine receptors. J. Neurochem. 1993, 60, 2308–2311. [Google Scholar] [CrossRef]
- Devlin, J.P.; Edwards, O.E.; Gorham, P.R.; Hunter, N.R.; Pike, R.K.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Zhong, Y.; Shen, L.; Ye, X.; Zhou, D.; He, Y.; Li, Y.; Ding, Y.; Zhu, W.; Ding, J.; Zhang, H. Neurotoxic Anatoxin-a Can Also Exert Immunotoxicity by the Induction of Apoptosis on Carassius auratus Lymphocytes in vitro When Exposed to Environmentally Relevant Concentrations. Front. Physiol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Bownik, A.; Rymuszka, A.; Sierosławska, A.; Skowroński, T. Anatoxin-a induces apoptosis of leukocytes and decreases the proliferative ability of lymphocytes of common carp (Cyprinus carpio L.) in vitro. Pol. J. Vet. Sci. 2012, 15, 531–535. [Google Scholar] [CrossRef]
- Biré, R.; Bertin, T.; Dom, I.; Hort, V.; Schmitt, C.; Diogène, J.; Lemée, R.; De Haro, L.; Nicolas, M. First Evidence of the Presence of Anatoxin-A in Sea Figs Associated with Human Food Poisonings in France. Mar. Drugs 2020, 18, 285. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Uptake and depuration of anatoxin-a by the mussel Mytilus galloprovincialis (Lamarck, 1819) under laboratory conditions. Chemosphere 2008, 72, 1235–1241. [Google Scholar] [CrossRef]
- Colas, S.; Duval, C.; Marie, B. Toxicity, transfer and depuration of anatoxin-a (cyanobacterial neurotoxin) in medaka fish exposed by single-dose gavage. Aquat. Toxicol. 2020, 222, 105422. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; He, J.; Huang, Y.; Huang, Q.; Qin, C.; Qin, J.; Chen, L. Neural excitotoxicity and the toxic mechanism induced by acute hypoxia in Chinese mitten crab (Eriocheir sinensis). Aquat. Toxicol. 2022, 245, 106131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, Z.; Liu, X.; Song, Y.; Liu, C.X.; Zheng, J.; Zhao, Y. Effects of waterborne chronic copper exposure on hepatic lipid metabolism and metal-element composition in Synechogobius hasta. Arch. Environ. Contam. Toxicol. 2013, 64, 301–315. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656, Erratum in Cold Spring Harb Perspect Biol. 2015, 7, a026716. [Google Scholar] [CrossRef]
- Didonna, A.; Sussman, J.; Benetti, F.; Legname, G. The role of Bax and caspase-3 in doppel-induced apoptosis of cerebellar granule cells. Prion 2012, 6, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Tufi, S.; Leonards, P.; Lamoree, M.; de Boer, J.; Legler, J.; Legradi, J. Changes in Neurotransmitter Profiles during Early Zebrafish (Danio rerio) Development and after Pesticide Exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef]
- Aliakbarzadeh, F.; Rafiee, M.; Khodagholi, F.; Khorramizadeh, M.R.; Manouchehri, H.; Eslami, A.; Sayehmiri, F.; Mohseni-Bandpei, A. Adverse effects of polystyrene nanoplastic and its binary mixtures with nonylphenol on zebrafish nervous system: From oxidative stress to impaired neurotransmitter system. Environ. Pollut. 2023, 317, 120587. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, H.; Xia, Y.; Du, J. Development of light response and GABAergic excitation-to-inhibition switch in zebrafish retinal ganglion cells. J. Physiol. 2010, 588, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Jin, Q. Progress in research on the effects of dopamine and its receptor on the central nervous system. Med. J. Wuhan Univ. 2017, 38, 169–172. [Google Scholar]
- Cepeda, C.; Levine, M.S. Dopamine-NMDA receptor interactions: Twenty years later. Dev. Neurosci. 2012, 34, 2–4. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef]
- Fink, K.B.; Göthert, M. 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 2007, 59, 360–417. [Google Scholar] [CrossRef]
- Princivalle, A.P. GABAB Receptors in Neurodegeneration. Behav. Neurobiol. GABAB Recept. Funct. 2022, 52, 267–290. [Google Scholar]
- Yang, Y.; Yu, Q.; Zhang, C.; Wang, X.; He, L.; Huang, Y.; Li, E.; Qin, J.; Chen, L. Acute thiamethoxam exposure induces hepatotoxicity and neurotoxicity in juvenile Chinese mitten crab (Eriocheir sinensis). Ecotoxicol. Environ. Saf. 2023, 249, 114399. [Google Scholar] [CrossRef]
- Feng, H.; Deng, D.; Zhu, F.; Chen, S.; Geng, J.; Jiang, S.; Zhang, K.; Jiang, J.; Yin, S.; Zhang, C. Acute exposure to glufosinate-ammonium induces hepatopancreas toxicity in juvenile Chinese mitten crab (Eriocheir sinensis). J. Hazard. Mater. 2025, 488, 137487. [Google Scholar] [CrossRef]
- Chelyshev, Y.A.; Kabdesh, I.M.; Mukhamedshina, Y.O. Extracellular Matrix in Neural Plasticity and Regeneration. Cell. Mol. Neurobiol. 2022, 42, 647–664. [Google Scholar] [CrossRef]
- Ayub, M.; Jin, H.-K.; Bae, J.-S. Novelty of Sphingolipids in the Central Nervous System Physiology and Disease: Focusing on the Sphingolipid Hypothesis of Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 7353. [Google Scholar] [CrossRef]
- Liao, J.; Cao, Y.; Zhao, J.; Yu, B.; Wang, Y.; Li, W.; Li, H.; Lv, S.; Wen, W.; Cui, H.; et al. Aqueous extract of Polygala japonica Houtt. ameliorated nonalcoholic steatohepatitis in mice through restoring the gut microbiota disorders and affecting the metabolites in feces and liver. Phytomedicine 2023, 118, 154937. [Google Scholar] [CrossRef]
- Panther, E.J.; Dodd, W.; Clark, A.; Lucke-Wold, B. Gastrointestinal Microbiome and Neurologic Injury. Biomedicines 2022, 10, 500. [Google Scholar] [CrossRef]
- Huo, W.; Qiao, Y.; Li, E.; Li, M.; Che, L. Interplay between nutrition, microbiota, and immunity in rotavirus infection: Insights from human and animal models. Front. Vet. Sci. 2025, 12, 1680448. [Google Scholar] [CrossRef]
- Shao, R.; Tan, X.; Pan, M.; Huang, J.; Huang, L.; Bi, B.; Huang, X.; Wang, J.; Li, X. Inulin alters gut microbiota to alleviate post-stroke depressive-like behavior associated with the IGF-1-mediated MAPK signaling pathway. Brain Behav. 2024, 14, e3387. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Kulinich, A.; Wang, Q.; Duan, X.C.; Lyu, Y.M.; Zhang, X.Y.; Awad, F.N.; Liu, L.; Voglmeir, J. Biochemical characterization of the endo-α-N-acetylgalactosaminidase pool of the human gut symbiont Tyzzerella nexilis. Carbohydr. Res. 2020, 490, 107962. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Likhitweerawong, N.; Kunasol, C.; Thonusin, C.; Sriwichaiin, S.; Nawara, W.; Chattipakorn, N.; Chattipakorn, S.C. Impact of psychostimulants on microbiota and short-chain fatty acids alterations in children with attention-deficit/hyperactivity disorder. Sci. Rep. 2025, 15, 3034. [Google Scholar] [CrossRef] [PubMed]
- Takewaki, D.; Kiguchi, Y.; Masuoka, H.; Manu, M.S.; Raveney, B.J.E.; Narushima, S.; Kurokawa, R.; Ogata, Y.; Hattori, M.; Kimura, Y.; et al. Tyzzerella nexilis strains enriched in mobile genetic elements are involved in progressive multiple sclerosis. Cell Rep. 2025, 44, 114785. [Google Scholar] [CrossRef] [PubMed]
- Abu, Y.F.; Singh, S.; Tao, J.; Chupikova, I.; Singh, P.; Meng, J.; Roy, S. Opioid-induced dysbiosis of maternal gut microbiota during gestation alters offspring gut microbiota and pain sensitivity. Gut Microbes 2024, 16, 2292224. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Song, L.; Wu, Y.; Zhao, F.; Zhu, F.; Song, Z.; Zhang, K.; Jiang, J.; Cai, X.; Yin, S.; et al. Novel insight into the mechanisms of neurotoxicity induced by glufosinate-ammonium via the microbiota-intestine-brain axis in Chinese mitten crab (Eriocheir sinensis). Pestic. Biochem. Physiol. 2025, 211, 106426. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Gene Description | Pathway | Control vs. 0.32 |
|---|---|---|---|
| gene-LOC127004233 | cell adhesion molecule DSCAML1-like | ECM-receptor interaction | up |
| gene-LOC127005077 | agrin-like | ECM-receptor interaction | up |
| gene-LOC127006442 | integrin alpha-PS2-like | ECM-receptor interaction | up |
| gene-LOC126981348 | oplophorus-luciferin 2-monooxygenase non-catalytic subunit-like | ECM-receptor interaction | up |
| gene-LOC126988411 | integrin alpha-PS3-like | ECM-receptor interaction | up |
| gene-LOC127009668 | sphingomyelin phosphodiesterase-like | Sphingolipid metabolism | up |
| gene-LOC126997840 | putative glucosylceramidase 4 | Sphingolipid metabolism | up |
| gene-LOC126983484 | arylsulfatase A-like | Sphingolipid metabolism | up |
| gene-LOC126985871 | sphingosine-1-phosphate phosphatase 2-like | Sphingolipid metabolism | up |
| Gene ID | Gene Description | Pathway | Control vs. 1.28 |
|---|---|---|---|
| gene-LOC127006442 | integrin alpha-PS2-like | ECM-receptor interaction | up |
| gene-LOC126988417 | integrin alpha-PS3-like | ECM-receptor interaction | up |
| gene-LOC127000170 | ficolin-1-like | ECM-receptor interaction | down |
| gene-LOC127008206 | glutamate receptor ionotropic, kainate 4-like | Neuroactive ligand-receptor interaction | up |
| gene-LOC127005040 | 5-hydroxytryptamine receptor-like | Neuroactive ligand-receptor interaction | down |
| gene-LOC126981054 | diuretic hormone receptor-like | Neuroactive ligand-receptor interaction | down |
| gene-LOC126983114 | muscarinic acetylcholine receptor DM1-like | Neuroactive ligand-receptor interaction | down |
| gene-LOC126983773 | galanin receptor 2a-like | Neuroactive ligand-receptor interaction | down |
| gene-LOC126986214 | glutamate receptor ionotropic, NMDA 2B-like | Neuroactive ligand-receptor interaction | down |
| gene-LOC127003406 | histidine decarboxylase-like | Histidine metabolism | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Hu, S.; Song, Z.; Lin, Z.; Zhang, K.; Ning, X.; Zhang, C.; Yin, S. Neurotoxicity and Intestinal Microbiota Dysbiosis in the Chinese Mitten Crab (Eriocheir sinensis) Induced by Anatoxin-a: A Microbiota–Intestine–Brain Axis Perspective. Microorganisms 2025, 13, 2380. https://doi.org/10.3390/microorganisms13102380
Feng H, Hu S, Song Z, Lin Z, Zhang K, Ning X, Zhang C, Yin S. Neurotoxicity and Intestinal Microbiota Dysbiosis in the Chinese Mitten Crab (Eriocheir sinensis) Induced by Anatoxin-a: A Microbiota–Intestine–Brain Axis Perspective. Microorganisms. 2025; 13(10):2380. https://doi.org/10.3390/microorganisms13102380
Chicago/Turabian StyleFeng, Huixia, Shengyu Hu, Zihao Song, Ziqi Lin, Kai Zhang, Xianhui Ning, Cong Zhang, and Shaowu Yin. 2025. "Neurotoxicity and Intestinal Microbiota Dysbiosis in the Chinese Mitten Crab (Eriocheir sinensis) Induced by Anatoxin-a: A Microbiota–Intestine–Brain Axis Perspective" Microorganisms 13, no. 10: 2380. https://doi.org/10.3390/microorganisms13102380
APA StyleFeng, H., Hu, S., Song, Z., Lin, Z., Zhang, K., Ning, X., Zhang, C., & Yin, S. (2025). Neurotoxicity and Intestinal Microbiota Dysbiosis in the Chinese Mitten Crab (Eriocheir sinensis) Induced by Anatoxin-a: A Microbiota–Intestine–Brain Axis Perspective. Microorganisms, 13(10), 2380. https://doi.org/10.3390/microorganisms13102380







