Hypericin-Mediated Photodynamic Inactivation Against the Plant Pathogen Clavibacter michiganensis: Preventative Seed Decontamination Enhanced by Potassium Iodide
Abstract
1. Introduction
2. Materials and Methods
2.1. HHL-PVP Stability Assays and ROS Production
2.2. Preparation of Stock Solutions for PDI
2.3. Cultivation of Clavibacter michiganensis
2.4. HHL-PVP-Mediated Photodynamic Inactivation
2.5. HHL-PVP-Mediated Seed Decontamination
3. Results
3.1. HHL-PVP Stability Assays
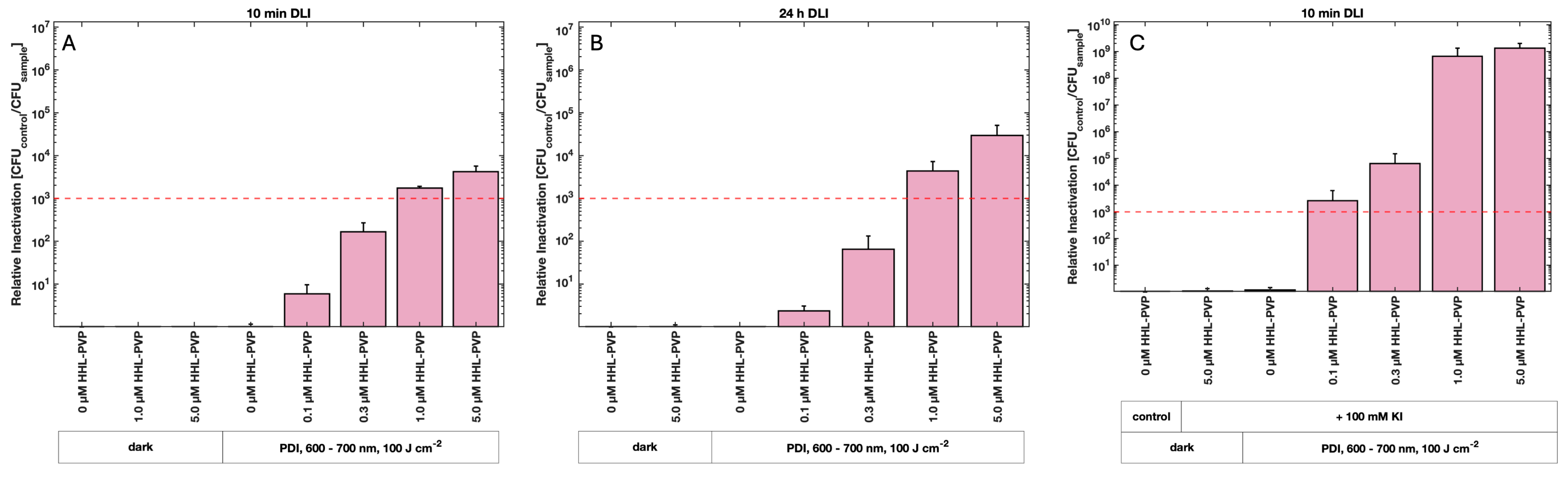
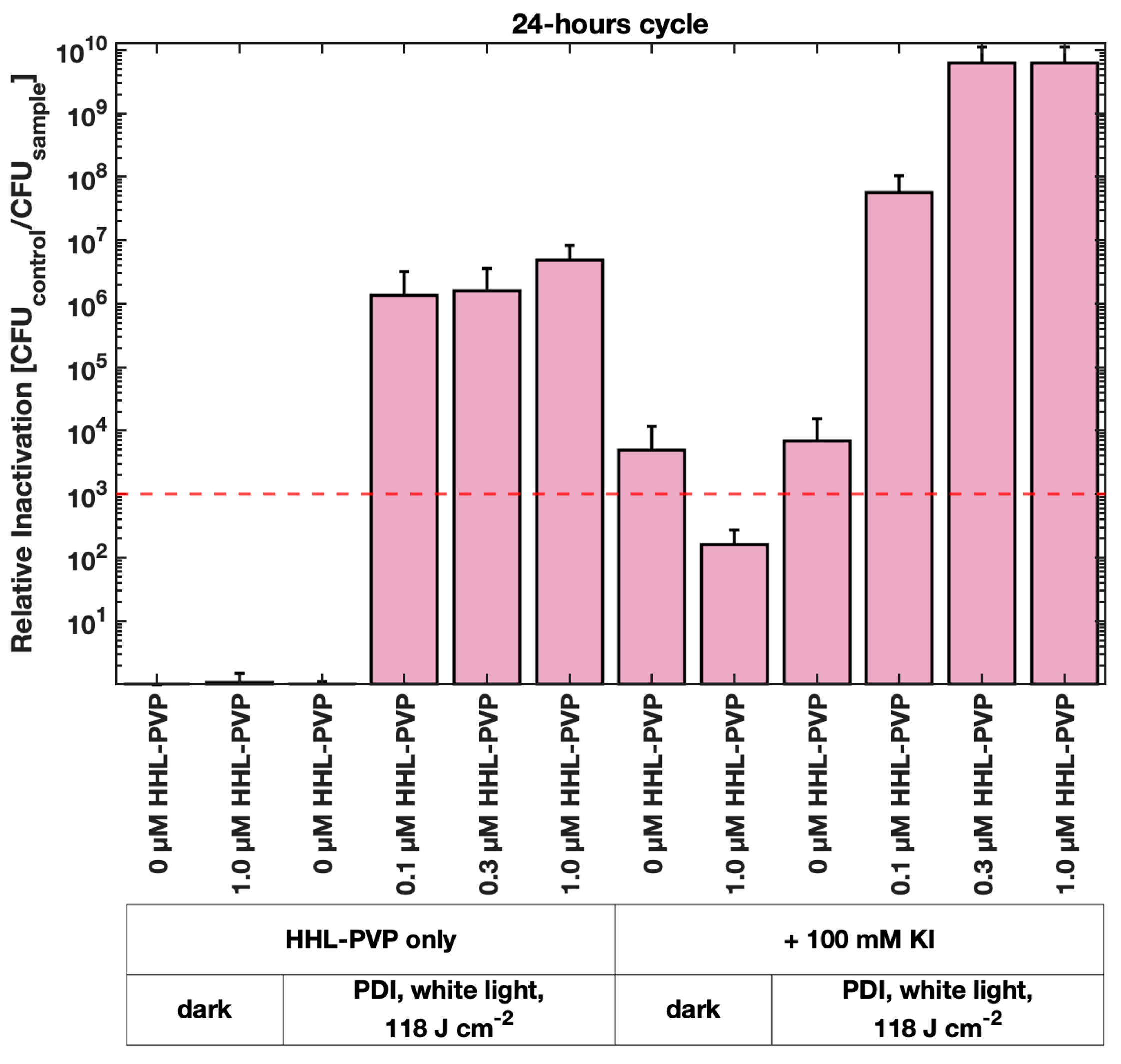
3.2. HHL-PVP-Mediated Photodynamic Inactivation
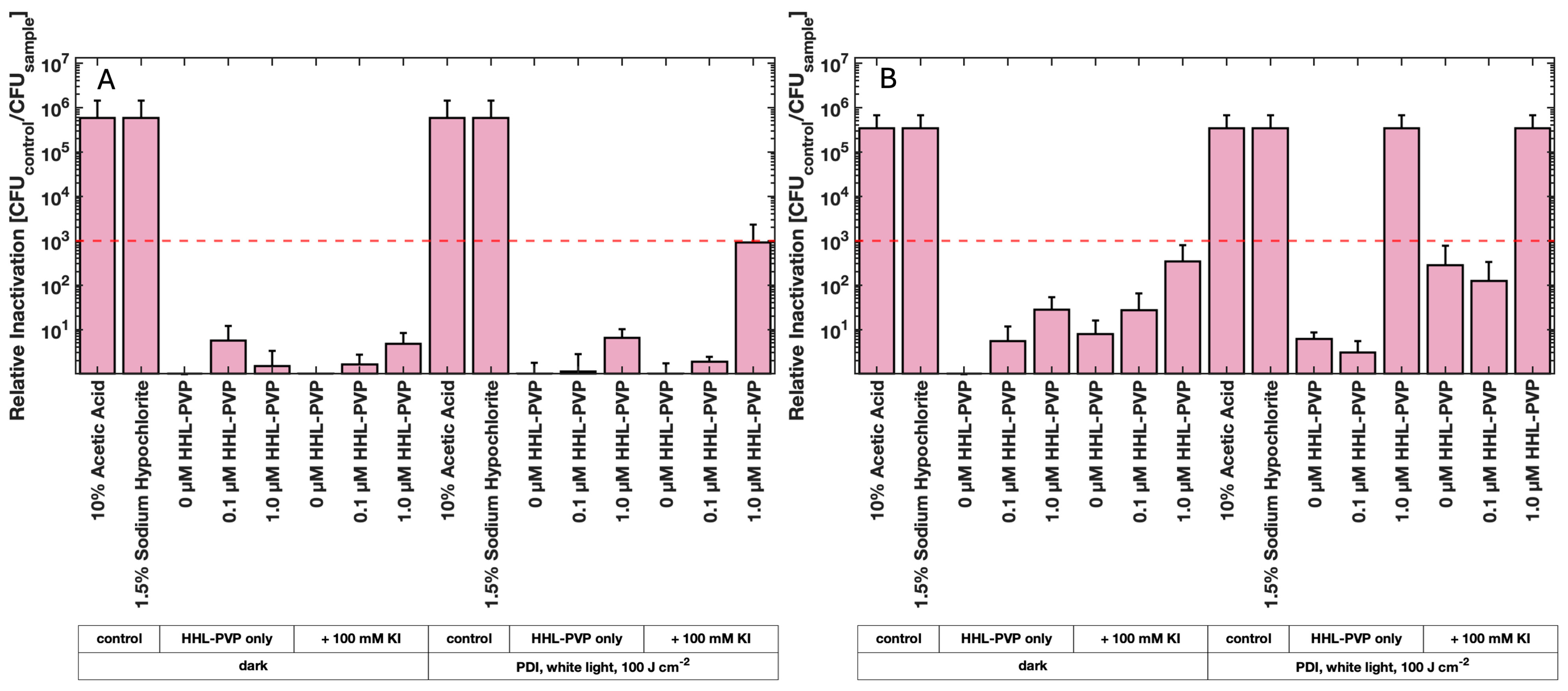
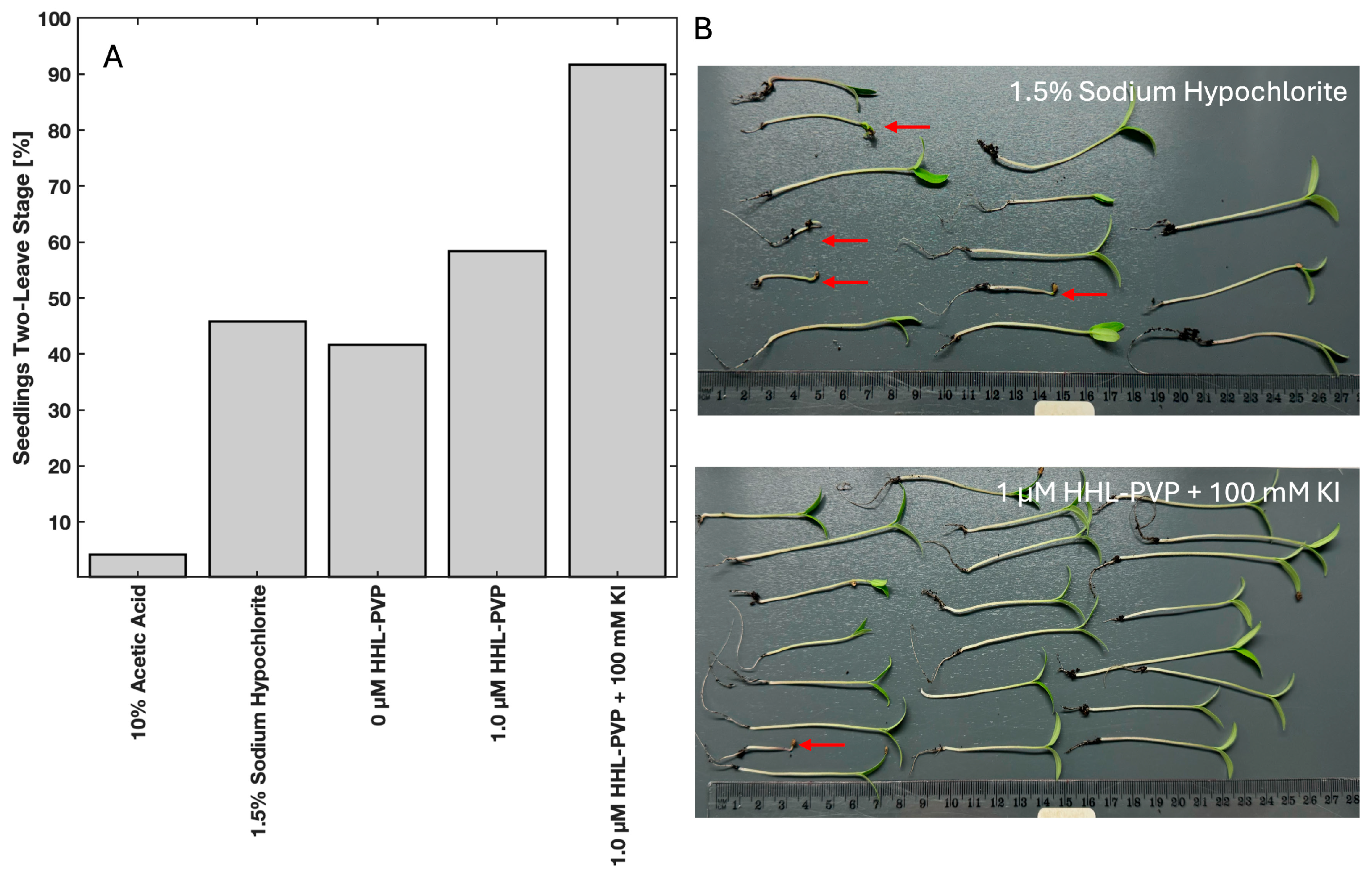
3.3. HHL-PVP-Mediated Seed Decontamination
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C. michiganensis | Clavibacter michiganenesis |
| DCFDA | 2′,7′-Dichlorofluorescin Diacetate |
| ddH2O | Double Distilled Water |
| DLI | Drug to Light Interval |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| HHL-PVP | High Hypericin-Loaded PVP |
| HPLC | High-Performance Liquid Chromatography |
| KI | Potassium Iodide |
| PDI | Photodynamic Inactivation |
| PS | Photosensitizer |
| PVP | Polyvinylpyrrolidone |
| ROS | Reactive Oxygen Species |
| SOSG | Singlet Oxygen Sensor Green |
References
- United Nations Population Division. World Population Prospects 2022: Summary of Results. Available online: https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022 (accessed on 30 June 2025).
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Newbery, F.; Qi, A.; Fitt, B.D. Modelling Impacts of Climate Change on Arable Crop Diseases: Progress, Challenges and Applications. Curr. Opin. Plant Biol. 2016, 32, 101–109. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. Chapter Two—The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: New York, NY, USA, 2015; Volume 90, pp. 29–92. [Google Scholar]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- Yan, Z.; Xiong, C.; Liu, H.; Singh, B.K. Sustainable Agricultural Practices Contribute Significantly to One Health. J. Sustain. Agric. Environ. 2022, 1, 165–176. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) Health Components: From the Seed to the Consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Bacterial Canker of Tomato. Available online: https://www.ages.at/en/plant/plant-health/pests-from-a-to-z/bacterial-canker-of-tomato (accessed on 30 June 2025).
- de León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Clavibacter Michiganesis Subsp. Michiganensis, a Seedborne Tomato Pathogen: Healthy Seeds Are Still the Goal. Plant Dis. 2011, 95, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Peritore-Galve, F.C.; Tancos, M.A.; Smart, C.D. Bacterial Canker of Tomato: Revisiting a Global and Economically Damaging Seedborne Pathogen. Plant Dis. 2021, 105, 1581–1595. [Google Scholar] [CrossRef]
- Basim, H.; Basim, E.; Tombuloglu, H.; Unver, T. Comparative Transcriptome Analysis of Resistant and Cultivated Tomato Lines in Response to Clavibacter michiganensis Subsp. michiganensis. Genomics 2021, 113, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- do Prado-Silva, L.; Brancini, G.T.P.; Braga, G.Ú.L.; Liao, X.; Ding, T.; Sant’Ana, A.S. Antimicrobial Photodynamic Treatment (aPDT) as an Innovative Technology to Control Spoilage and Pathogenic Microorganisms in Agri-Food Products: An Updated Review. Food Control 2022, 132, 108527. [Google Scholar] [CrossRef]
- Glueck, M.; Hamminger, C.; Fefer, M.; Liu, J.; Plaetzer, K. Save the Crop: Photodynamic Inactivation of Plant Pathogens I: Bacteria. Photochem. Photobiol. Sci. 2019, 18, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Hamminger, C.; Glueck, M.; Fefer, M.; Ckurshumova, W.; Liu, J.; Tenhaken, R.; Plaetzer, K. Photodynamic Inactivation of Plant Pathogens Part II: Fungi. Photochem. Photobiol. Sci. 2022, 21, 195–207. [Google Scholar] [CrossRef]
- Jernej, L.; Liu, J.; Fefer, M.; Plaetzer, K. Chlorophyllin and Sunlight against Penicillium Digitatum: Exploring Photodynamic Inactivation as a Green Postharvest Technology in Citriculture. Photochem. Photobiol. Sci. 2025, 24, 555–568. [Google Scholar] [CrossRef]
- Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Available online: https://www.mdpi.com/2227-9059/9/6/584 (accessed on 7 October 2025).
- Kashef, N.; Hamblin, M.R. Can Microbial Cells Develop Resistance to Oxidative Stress in Antimicrobial Photodynamic Inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef]
- Braga, G.Ú.L.; Silva-Junior, G.J.; Brancini, G.T.P.; Hallsworth, J.E.; Wainwright, M. Photoantimicrobials in Agriculture. J. Photochem. Photobiol. B Biol. 2022, 235, 112548. [Google Scholar] [CrossRef]
- Islam, M.T.; Ng, K.; Fefer, M.; Liu, J.; Uddin, W.; Ckurshumova, W.; Rosa, C. Photosensitizer to the Rescue: In Planta and Field Application of Photodynamic Inactivation Against Plant-Pathogenic Bacteria. Plant Dis. 2023, 107, 870–878. [Google Scholar] [CrossRef]
- Kubin, A.; Wierrani, F.; Burner, U.; Alth, G.; Grunberger, W. Hypericin—The Facts About a Controversial Agent. Curr. Pharm. Des. 2005, 11, 233–253. [Google Scholar] [CrossRef]
- Siewert, B. Does the Chemistry of Fungal Pigments Demand the Existence of Photoactivated Defense Strategies in Basidiomycetes? Photochem. Photobiol. Sci. 2021, 20, 475–488. [Google Scholar] [CrossRef]
- Yow, C.M.N.; Tang, H.M.; Chu, E.S.M.; Huang, Z. Hypericin-Mediated Photodynamic Antimicrobial Effect on Clinically Isolated Pathogens. Photochem. Photobiol. 2012, 88, 626–632. [Google Scholar] [CrossRef]
- Kiesslich, T.; Krammer, B.; Plaetzer, K. Cellular Mechanisms and Prospective Applications of Hypericin in Photodynamic Therapy. Curr. Med. Chem. 2006, 13, 2189–2204. [Google Scholar] [CrossRef]
- Kubin, A. Hypericin-pvp Komplex Mit Hohem Hypericinanteil. European Patent EP3820524B1, 6 September 2023. [Google Scholar]
- Vejzovic, D.; Kubin, A.; Fechter, K.; Karner, C.; Hartmann, J.; Ackerbauer, T.; Radović, B.; Ritter, G.; Üçal, M.; Ropele, S.; et al. Glioblastoma Targeting by Water-Soluble Hypericin Derivate HHL-PVP and Photodynamic Tumour Killing. Biomed. Pharmacother. 2025, 186, 118041. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Sain, M.; Stark, C.; Fefer, M.; Liu, J.; Hoare, T.; Ckurshumova, W.; Rosa, C. Overview of Methods and Considerations for the Photodynamic Inactivation of Microorganisms for Agricultural Applications. Photochem. Photobiol. Sci. 2023, 22, 2675–2686. [Google Scholar] [CrossRef]
- Engelhardt, V.; Krammer, B.; Plaetzer, K. Antibacterial Photodynamic Therapy Using Water-Soluble Formulations of Hypericin or mTHPC Is Effective in Inactivation of Staphylococcus aureus. Photochem. Photobiol. Sci. 2010, 9, 365–369. [Google Scholar] [CrossRef]
- Fellner, A.; Hamminger, C.; Fefer, M.; Liu, J.; Plaetzer, K. Towards Microbial Food Safety of Sprouts: Photodynamic Decontamination of Seeds. Photonics 2023, 10, 239. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Lichtveld, M.; Mazet, J.A.K.; Togami, E.; Miller, S.A. Plant Health and Its Effects on Food Safety and Security in a One Health Framework: Four Case Studies. One Health Outlook 2021, 3, 6. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.; Wang, L.; Sun, J. Photodynamic Inactivation and Its Application in Food Preservation. Crit. Rev. Food Sci. Nutr. 2023, 63, 2042–2056. [Google Scholar] [CrossRef] [PubMed]
- Darmanyan, A.P.; Burel, L.; Eloy, D.; Jardon, P. Singlet Oxygen Production by Hypericin in Various Solvents. J. Chim. Phys. 1994, 91, 1774–1785. [Google Scholar] [CrossRef]
- Nair, B. Final Report On the Safety Assessment of Polyvinylpyrrolidone (PVP). Int. J. Toxicol. 1998, 17, 95–130. [Google Scholar] [CrossRef]
- Burnett, C.L. PVP (Polyvinylpyrrolidone). Int. J. Toxicol. 2017, 36, 50S–51S. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the Safety of Polyvinylpyrrolidone-Vinyl Acetate Copolymer for the Proposed Uses as a Food Additive. EFSA J. 2010, 8, 1948. [Google Scholar] [CrossRef]
- Morenko, I.; Isaeva, I.; Ostaeva, G. Environmental Aspects of the Use of Water-Soluble Polymers as Stabilizers for Metal Nanoparticles. E3S Web Conf. 2025, 614, 4018. [Google Scholar] [CrossRef]
- Mennini, T.; Gobbi, M. The Antidepressant Mechanism of Hypericum perforatum. Life Sci. 2004, 75, 1021–1027. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-Mediated Photodynamic Therapy for the Treatment of Cancer: A Review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef]
- Couldwell, W.T.; Surnock, A.A.; Tobia, A.J.; Cabana, B.E.; Stillerman, C.B.; Forsyth, P.A.; Appley, A.J.; Spence, A.M.; Hinton, D.R.; Chen, T.C. A Phase 1/2 Study of Orally Administered Synthetic Hypericin for Treatment of Recurrent Malignant Gliomas. Cancer 2011, 117, 4905–4915. [Google Scholar] [CrossRef]
- Limantara, L.; Koehler, P.; Wilhelm, B.; Porra, R.J.; Scheer, H. Photostability of Bacteriochlorophyll a and Derivatives: Potential Sensitizers for Photodynamic Tumor Therapy. Photochem. Photobiol. 2006, 82, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and Photochemical Properties of Curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mikulich, A.V.; Plavskii, V.Y.; Tretyakova, A.I.; Nahorny, R.K.; Sobchuk, A.N.; Dudchik, N.V.; Emeliyanova, O.A.; Zhabrouskaya, A.I.; Plavskaya, L.G.; Ananich, T.S.; et al. Potential of Using Medicinal Plant Extracts as Photosensitizers for Antimicrobial Photodynamic Therapy. Photochem. Photobiol. 2024, 100, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Zheng, J.; Chen, Y.; Liu, Z.; Xie, Q.; Li, D.; Xi, L.; Zheng, J.; Liu, H. Potassium Iodide Enhances the Killing Effect of Methylene Blue Mediated Photodynamic Therapy against F. Monophora. Photodiagnosis Photodyn. Ther. 2024, 48, 104255. [Google Scholar] [CrossRef]
- Reynoso, E.; Quiroga, E.D.; Agazzi, M.L.; Ballatore, M.B.; Bertolotti, S.G.; Durantini, E.N. Photodynamic Inactivation of Microorganisms Sensitized by Cationic BODIPY Derivatives Potentiated by Potassium Iodide. Photochem. Photobiol. Sci. 2017, 16, 1524–1536. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Huang, S.; Wang, S.; Wang, Y.; Lei, L.; Zhang, C.; Huang, X. Antimicrobial Photodynamic Effect of Cross-Kingdom Microorganisms with Toluidine Blue O and Potassium Iodide. Int. J. Mol. Sci. 2022, 23, 11373. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Brancini, G.T.P.; Costa, L.D.; Biazzotto, J.C.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S.; Almeida, A.; Hamblin, M.R.; da Silva, R.S.; et al. Efficient Photodynamic Inactivation of Candida Albicans by Porphyrin and Potassium Iodide Co-Encapsulation in Micelles. Photochem. Photobiol. Sci. 2020, 19, 1063–1071. [Google Scholar] [CrossRef]
- Wei, D.; Hamblin, M.R.; Wang, H.; Fekrazad, R.; Wang, C.; Wen, X. Rose Bengal Diacetate-Mediated Antimicrobial Photodynamic Inactivation: Potentiation by Potassium Iodide and Acceleration of Wound Healing in MRSA-Infected Diabetic Mice. BMC Microbiol. 2024, 24, 246. [Google Scholar] [CrossRef]
- Ikram, N.A.; Abdalla, M.A.; Mühling, K.H. Developing Iron and Iodine Enrichment in Tomato Fruits to Meet Human Nutritional Needs. Plants 2024, 13, 3438. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Gonzali, S.; Holwerda, H.T.; Real Ibaceta, R.; Perata, P. Tomato Fruits: A Good Target for Iodine Biofortification. Front. Plant Sci. 2013, 4, 47070. [Google Scholar] [CrossRef] [PubMed]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New Horizons in Microbiological Food Safety: Photodynamic Decontamination Based on a Curcumin Derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef]
- Žudyté, B.; Lukšiené, Ž. Toward Better Microbial Safety of Wheat Sprouts: Chlorophyllin-Based Photosensitization of Seeds. Photochem. Photobiol. Sci. 2019, 18, 2521–2530. [Google Scholar] [CrossRef]
- Gilbert, G.S.; Diaz, A.; Bregoff, H.A. Seed Disinfestation Practices to Control Seed-Borne Fungi and Bacteria in Home Production of Sprouts. Foods 2023, 12, 747. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Quispe, A.P.V.; de Morais, E.G.; Benevenute, P.A.N.; Lima, J.d.S.; dos Santos, L.C.; Silva, M.A.; Chalfun-Júnior, A.; Marchiori, P.E.R.; Guilherme, L.R.G. Priming Effect with Selenium and Iodine on Broccoli Seedlings: Activation of Biochemical Mechanisms to Mitigate Cold Damages. Plant Physiol. Biochem. 2025, 223, 109876. [Google Scholar] [CrossRef]
- Mejía-Ramírez, F.; Benavides-Mendoza, A.; González-Morales, S.; Juárez-Maldonado, A.; Lara-Viveros, F.M.; Morales-Díaz, A.B.; Morelos-Moreno, Á. Seed Priming Based on Iodine and Selenium Influences the Nutraceutical Compounds in Tomato (Solanum lycopersicum L.) Crop. Antioxidants 2023, 12, 1265. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different Modes of Hydrogen Peroxide Action During Seed Germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of Reactive Oxygen Species in the Regulation of Arabidopsis Seed Dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-F.; Wang, Z.-H.; Chen, S.-L. Hypericin: Chemical Synthesis and Biosynthesis. Chin. J. Nat. Med. 2014, 12, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kraus, G.A.; Pratt, D.; Tossberg, J.; Carpenter, S. Antiretroviral Activity of Synthetic Hypericin and Related Analogs. Biochem. Biophys. Res. Commun. 1990, 172, 149–153. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jernej, L.; Gschwendtner, S.; Kubin, A.; Wightman, L.; Plaetzer, K. Hypericin-Mediated Photodynamic Inactivation Against the Plant Pathogen Clavibacter michiganensis: Preventative Seed Decontamination Enhanced by Potassium Iodide. Microorganisms 2025, 13, 2360. https://doi.org/10.3390/microorganisms13102360
Jernej L, Gschwendtner S, Kubin A, Wightman L, Plaetzer K. Hypericin-Mediated Photodynamic Inactivation Against the Plant Pathogen Clavibacter michiganensis: Preventative Seed Decontamination Enhanced by Potassium Iodide. Microorganisms. 2025; 13(10):2360. https://doi.org/10.3390/microorganisms13102360
Chicago/Turabian StyleJernej, Linda, Sonja Gschwendtner, Andreas Kubin, Lionel Wightman, and Kristjan Plaetzer. 2025. "Hypericin-Mediated Photodynamic Inactivation Against the Plant Pathogen Clavibacter michiganensis: Preventative Seed Decontamination Enhanced by Potassium Iodide" Microorganisms 13, no. 10: 2360. https://doi.org/10.3390/microorganisms13102360
APA StyleJernej, L., Gschwendtner, S., Kubin, A., Wightman, L., & Plaetzer, K. (2025). Hypericin-Mediated Photodynamic Inactivation Against the Plant Pathogen Clavibacter michiganensis: Preventative Seed Decontamination Enhanced by Potassium Iodide. Microorganisms, 13(10), 2360. https://doi.org/10.3390/microorganisms13102360






