Trends in the Antimicrobial Resistance Pattern of Bacterial Gram-Negative Pathogens in Elderly Patients Admitted to the Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
3. Results
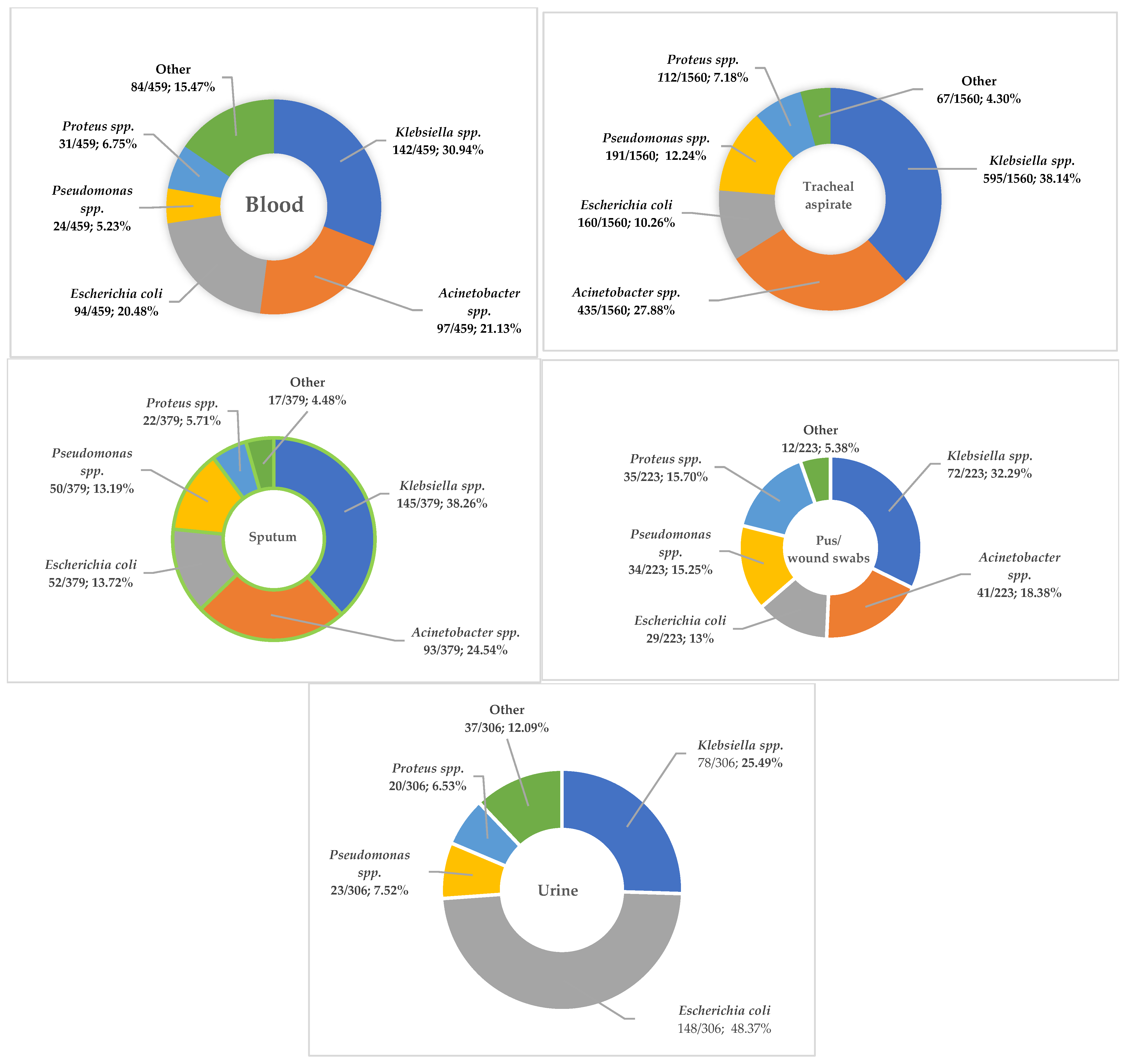
3.1. Distribution of the Main Isolates
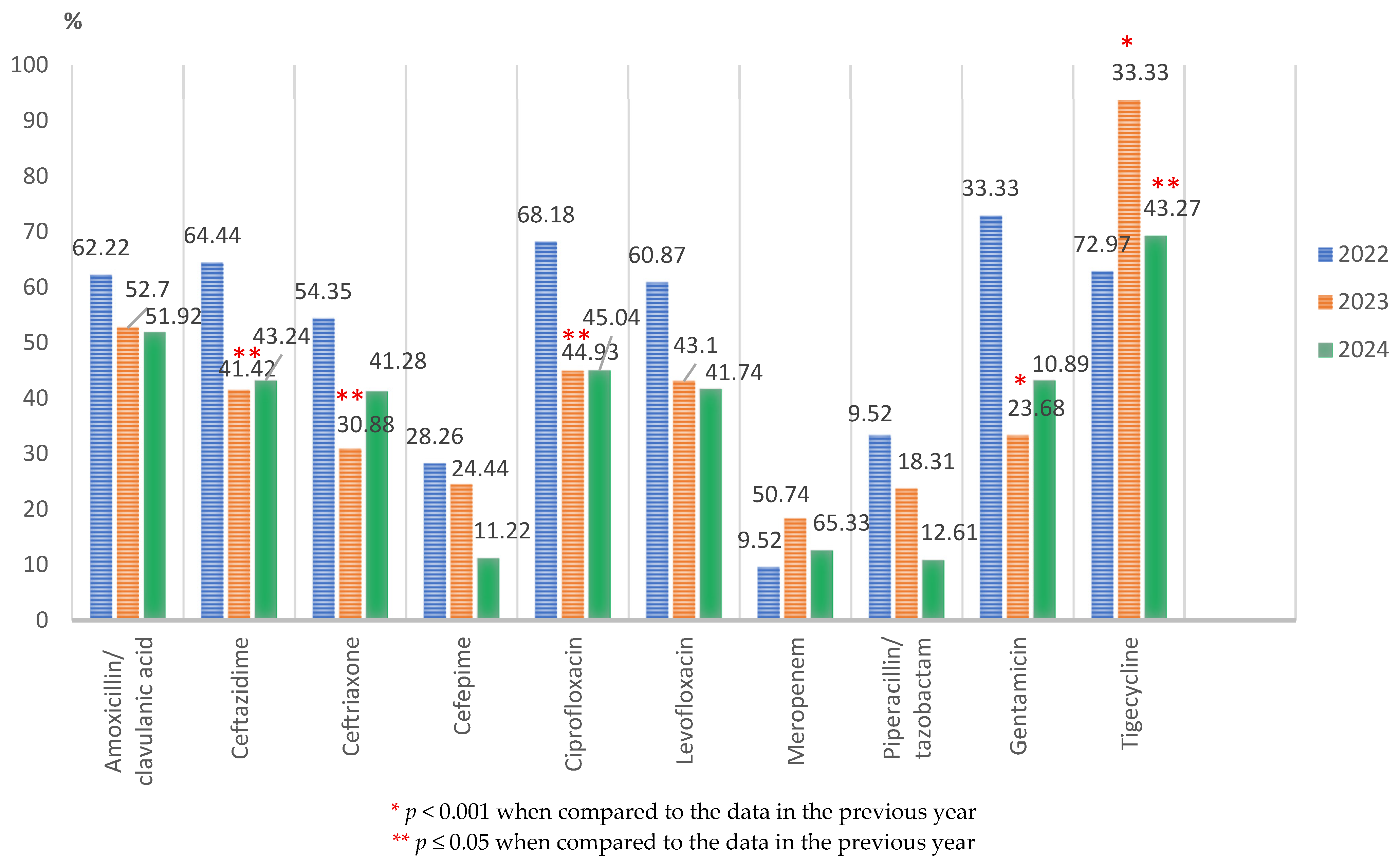
3.2. Antimicrobial Resistance in Main Bacterial Gram-Negative Species
3.2.1. Characteristics of the Main Gram-Negative Pathogens
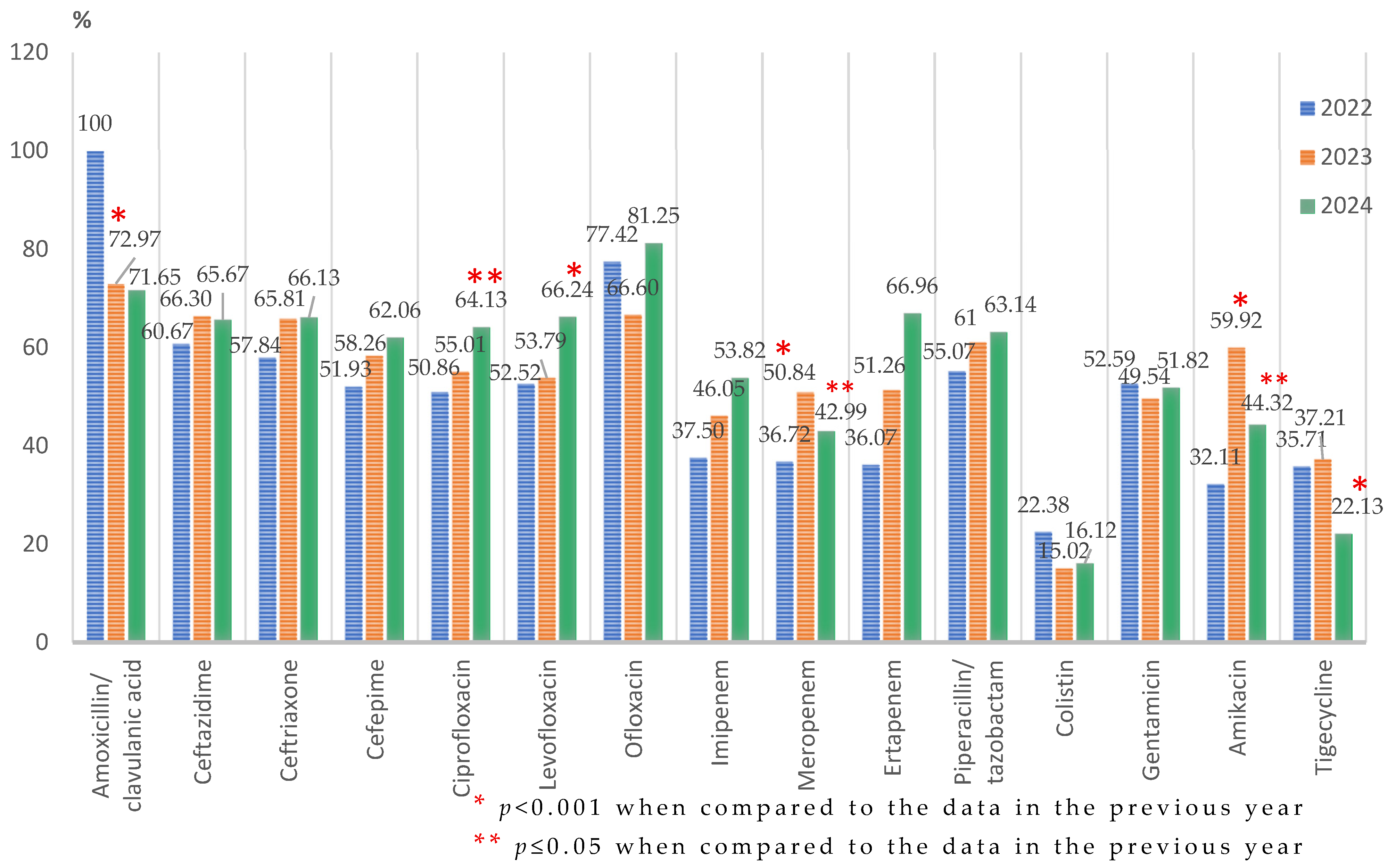
Klebsiella spp.
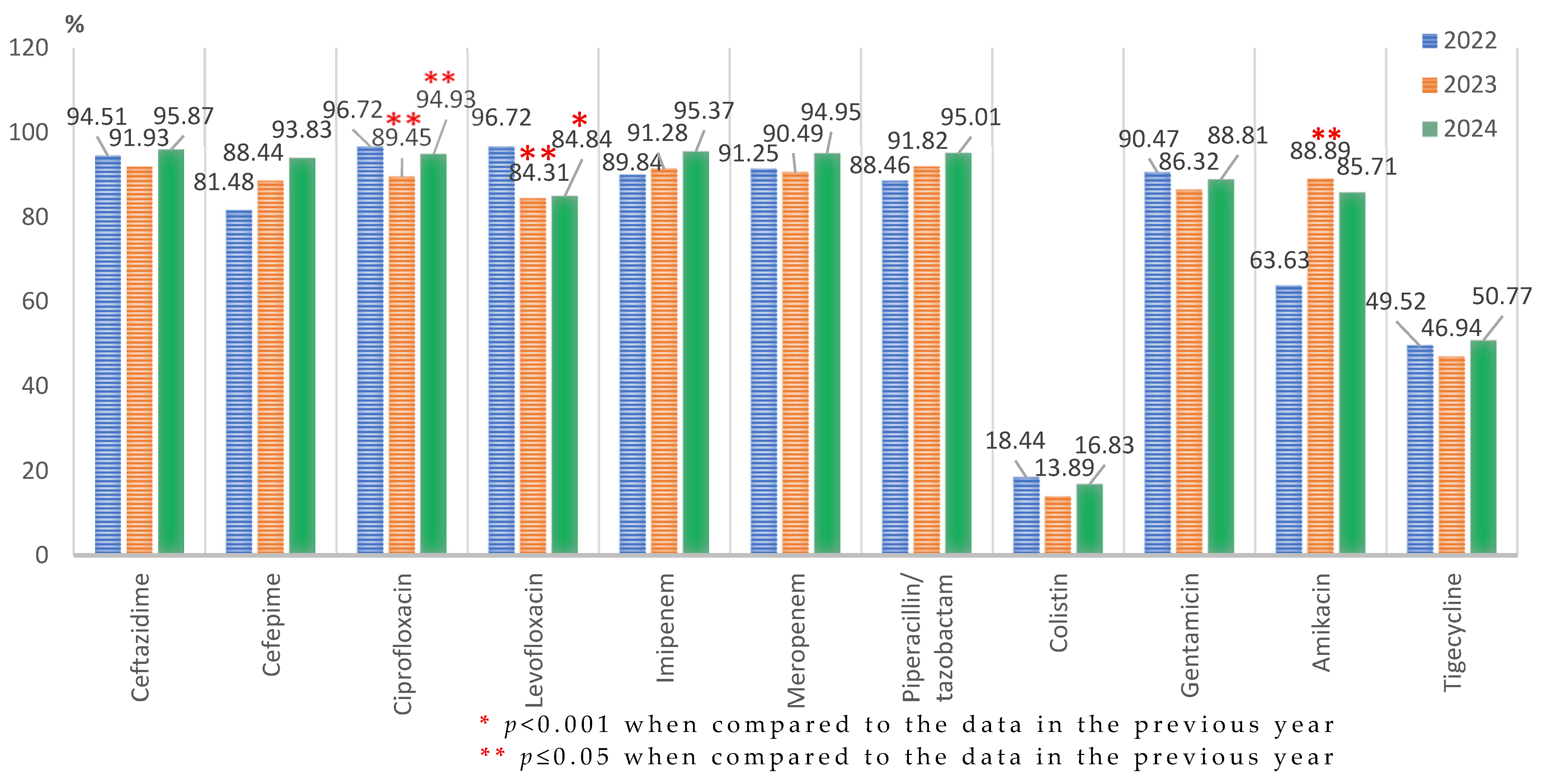
Acinetobacter spp.
Escherichia coli
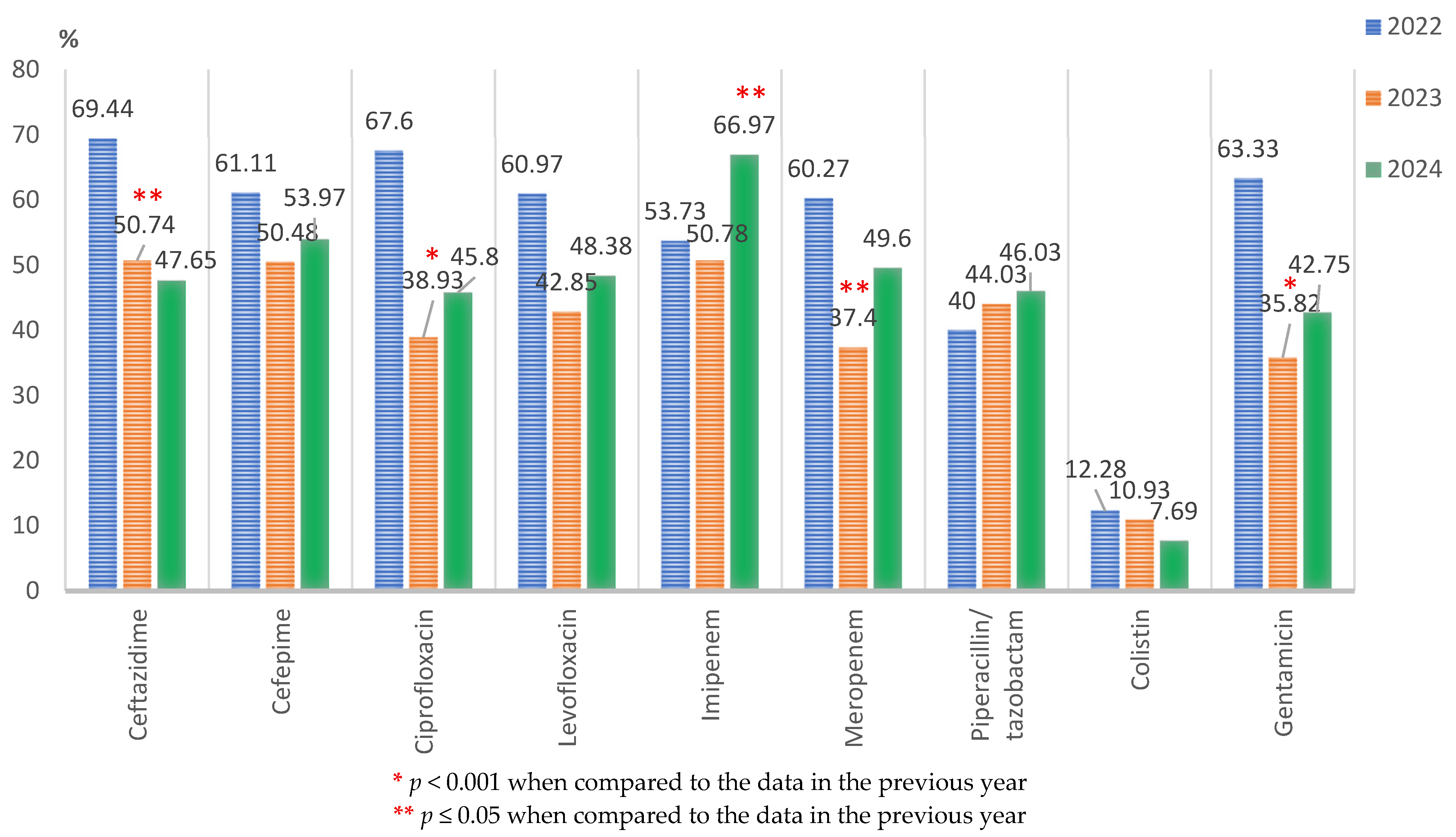
Pseudomonas spp.
Proteus spp.
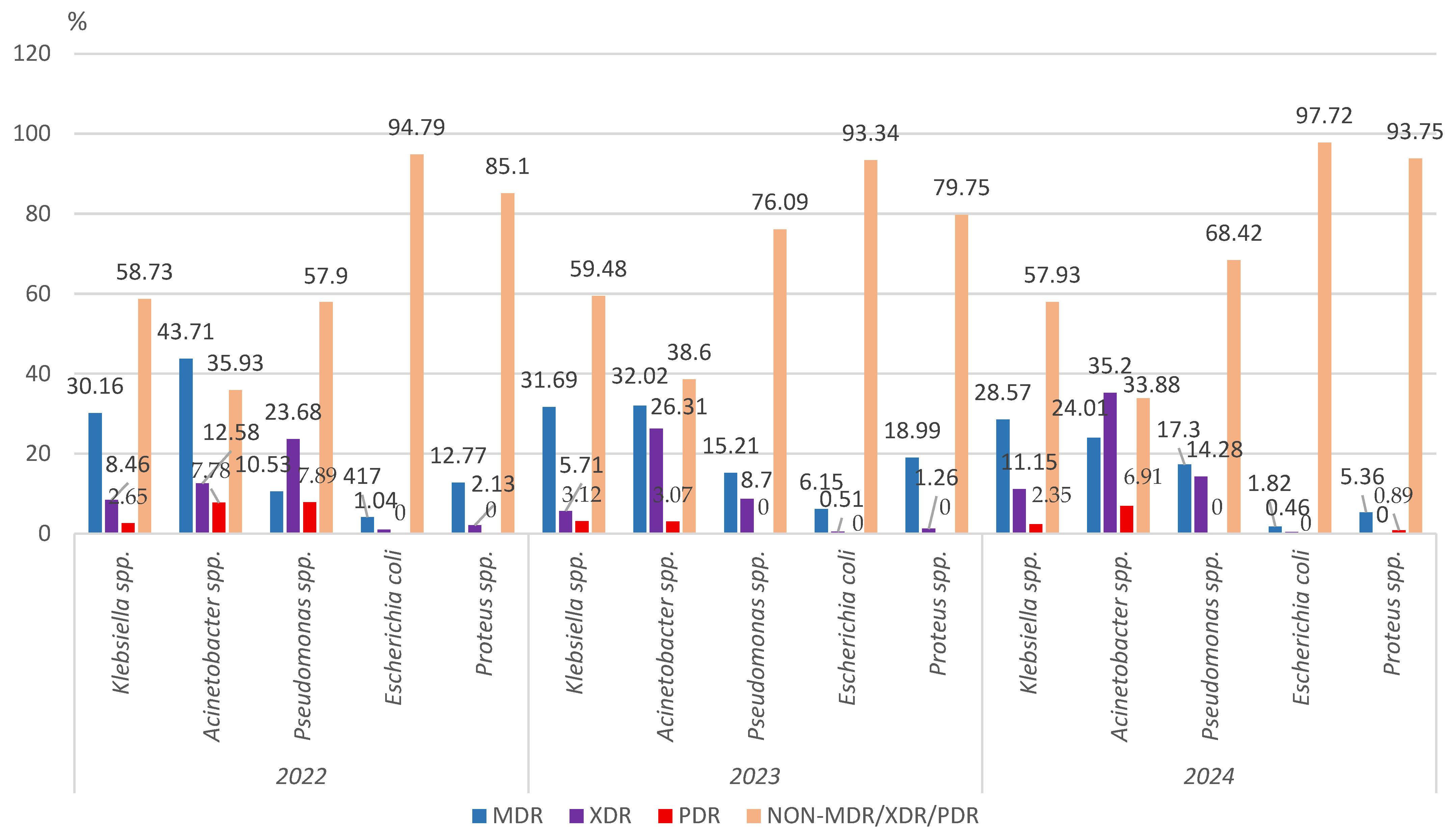
3.2.2. Multidrug Resistance of the Main Gram-Negative Pathogens
3.2.3. Carbapenem Non-Susceptibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ECDC; WHO. Antimicrobial Resistance Surveillance in Europe 2023: 2021 Data; Surveillance Report; ECDC: Stockholm, Sweden, 2023. [Google Scholar]
- European Commission. 2022. Available online: https://health.ec.europa.eu/document/download/18c127ce-da4b-4e4e-a27c-f7b93efb2980_en?filename=hera_factsheet_health-threat_mcm.pdf (accessed on 15 January 2024).
- Delsaux, P. Preparing Europe for future health threats and crises—the European Health Emergency and Preparedness Authority; improving EU preparedness and re-sponse in the area of medical countermeasures. Eurosurveillance 2022, 27, 2200893. [Google Scholar] [CrossRef]
- Hetta, H.F.A.; Alanazi, F.E.; Ali, M.A.S.; Alatawi, A.D.; Aljohani, H.M.; Ahmed, R.; Alansari, N.A.; Alkhathami, F.M.; Albogmi, A.; Alharbi, B.M.; et al. Hypervirulent Klebsiella pneumoniae: Insights into Virulence, Antibiotic Resistance, and Fight Strategies Against a Superbug. Pharmaceuticals 2025, 18, 724. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic anal-ysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Alatawi, A.D.; Hetta, H.F.; Ali, M.A.S.; Ramadan, Y.N.; Alaqyli, A.B.; Alansari, W.K.; Aldhaheri, N.H.; Bin Selim, T.A.; Merdad, S.A.; Alharbi, M.O.; et al. Diagnostic Innovations to Combat Antibiotic Resistance in Critical Care: Tools for Targeted Therapy and Stewardship. Diagnostics 2025, 15, 2244. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT. Population Structure and Ageing. In Statistics Explained; EUROSTAT: Luxembourg, 2025. [Google Scholar]
- Tang, B.; Li, Z.; Hu, S.; Xiong, J. Economic Implications of Health Care Burden for Elderly Population. J. Health Care Organ. Provis. Financ. 2022, 59, 00469580221121511. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- INSP-CNSCBT. Sentinel Surveillance Methodology for Healthcare Associated Infections and Antimicrobial Resistance; INSP: Indian Land, SC, USA, 2018. [Google Scholar]
- Zlatian, O.; Balasoiu, A.T.; Balasoiu, M.; Cristea, O.; Docea, A.O.; Mitrut, R.; Spandidos, D.A.; Tsatsakis, A.M.; Bancescu, A.; Calina, D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp. Ther. Med. 2018, 16, 4499–4510. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement, M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Poulakou, G.; Papatheodoridi, M.; Lagou, S.; Papadatos, S.; Anagnostopoulos, I.; Dimopoulos, G. Infections in elderly intensive care unit patients. J. Emerg. Crit. Care Med. 2019, 3, 44. [Google Scholar] [CrossRef]
- Marchaim, D.; Katz, D.E.; Munoz-Price, L.S. Emergence and Control of Antibiotic-resistant Gram-negative Bacilli in Older Adults. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 113–124. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Boufeas, I.Z.; Sakagianni, A.; Paxinou, E.; Verykios, V.S.; et al. Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions. Microorganisms 2024, 12, 1978. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E. High antimicrobial resistant rates among Gram-negative pathogens in intensive care units. A retrospective study at a tertiary care hospital in Southwest Saudi Arabia. Saudi Med. J. 2018, 39, 1035–1043. [Google Scholar] [CrossRef]
- Martins, A.P.S.; da Mata, C.P.S.M.; Dos Santos, U.R.; de Araújo, C.A.; Leite, E.M.M.; de Carvalho, L.D.; Vidigal, P.G.; Vieira, C.D.; Dos Santos-Key, S.G. Association between multidrug-resistant bacteria and outcomes in intensive care unit patients: A non-interventional study. Front. Public Health 2024, 8, 1297350. [Google Scholar] [CrossRef] [PubMed]
- Ulu Kılıç, A.; Kalın Ünüvar, G.; Cevahir, F.; Alp, E. Economic Burden of Multidrug-Resistant Gram-Negative Infections in a Developing Country. Erciyes Med. J. 2019, 41, 312–315. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Streit, J.M.; Carvalhaes, C.G.; Castanheira, M. Antimicrobial susceptibility of Gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018–2020). Diagn. Microbiol. Infect. Dis. 2022, 102, 115557. [Google Scholar] [CrossRef] [PubMed]
- Brusaferro, S.; Regattin, L.; Silvestro, A.; Vidotto, L. Incidence of hospital-acquired infections in Italian long term-care facilities: A prospective six-month surveillance. J. Hosp. Infect. 2006, 63, 211–215. [Google Scholar] [CrossRef]
- Narayanan, N.; Lin, T.; Vinarov, D.; Bucek, T.; Johnson, L.; Mathew, C.; Chaudhry, S.; Brunetti, L. Relationship Between Multidrug-Resistant Enterobacterales and Obesity in Older Adults. Infect. Drug Resist. 2021, 14, 2527–2532. [Google Scholar] [CrossRef]
- Zetu, C.; Popa, S.; Golli, A.L.; Condurache, A.; Munteanu, R. Long-term improvement of dyslipidaemia, hyperuricemia and metabolic syndrome in patients undergoing laparoscopic sleeve gastrectomy. Arch. Endocrinol. Metab. 2021, 64, 704–709. [Google Scholar] [CrossRef]
- INSP. CARMIAAM-ROM 2020 (Consumul de Antibiotice, Rezistența Microbiană și Infecții Asociate Asistenței Medicale în România-2020); INSP: Bucuresti, Romania, 2020. [Google Scholar]
- Ghenea, A.E.; Cioboată, R.; Drocaş, A.I.; Țieranu, E.N.; Vasile, C.M.; Moroşanu, A.; Țieranu, C.G.; Salan, A.-I.; Popescu, M.; Turculeanu, A.; et al. Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania. Antibiotics 2021, 10, 868. [Google Scholar] [CrossRef]
- Golli, A.L.; Nitu, F.M.; Balasoiu, M.; Nemes, R.M.; Tudorache, S.I.; Mahler Boca, B.; Olteanu, M. Bacterial Isolates from Endotracheal Aspirates and their Antimicrobial Resistance Pattern in Patients from Intensive Care Unit. Rev. Chim. 2019, 70, 3299–3304. [Google Scholar] [CrossRef]
- Golli, A.L.; Nitu, F.M.; Balasoiu, M.; Lungu, M.A.; Dragomirescu, C.C.; Olteanu, M.; Nemes, R.; Tantu, M.M.; Olteanu, M. The Characterization of Antibiotic Resistance of Bacterial Isolates from Intensive Care Unit Patient Samples in a University Affiliated Hospital in Romania. Rev. Chim. 2019, 70, 1778–1783. [Google Scholar] [CrossRef]
- Balkhy, H.H.; El-Saed, A.; Alshamrani, M.M.; Alsaedi, A.; Al Nasser, W.; El Gammal, A.; Aljohany, S.M.; Arabi, Y.; Alqahtani, S.; Bonnie, H.B.; et al. High Burden of Resistant Gram Negative Pathogens Causing Device-associated Healthcare Infections in a Tertiary Care Setting in Saudi Arabia, 2008–2016. J. Glob. Antimicrob. Resist. 2020, 23, 26–32. [Google Scholar] [CrossRef]
- Kilinc, M. Antibiotic Resistance and Mortality in ICU Patients: A Retrospective Analy-sis of First Culture Growth Results. Antibiotics 2025, 14, 290. [Google Scholar] [CrossRef]
- Golli, A.L.; Popa, S.G.; Ghenea, A.E.; Turcu, F.L. The Impact of the COVID-19 Pandemic on the Antibiotic Resistance of Gram-Negative Pathogens Causing Bloodstream Infections in an Intensive Care Unit. Biomedicines 2025, 13, 379. [Google Scholar] [CrossRef]
- Strategies and Action Plans on Antimicrobial Resistance (europa.eu) Council Recommendation on Stepping Up EU Actions to Combat Antimicrobial Resistance in a One Health Approach (2023/C 220/01). 2023. Available online: https://data.consilium.europa.eu/doc/document/ST-9581-2023-INIT/en/pdf (accessed on 2 September 2024).
- Bandić Pavlović, D.; Pospišil, M.; Nađ, M.; Vrbanović Mijatović, V.; Luxner, J.; Zarfel, G.; Grisold, A.; Tonković, D.; Dobrić, M.; Bedenić, B. Multidrug-Resistant Bacteria in Surgical Intensive Care Units: Antibiotic Susceptibility and β-Lactamase Characterization. Pathogens 2024, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Brink, A.J. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr. Opin. Infect. Dis. 2019, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- WHO. Critically Important Antimicrobials for Human Medicine: 6th Revision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [PubMed]
- Popescu, G.A.; Codiţă, I.; Szekely, E.; Şerban, R.; Ruja, G.; Tălăpan, D. Ghid Privind Enterobacteriaceae Producătoare de Carbapenemaze: Diagnosticul, Prevenirea Transmiterii Interumane și Tratamentul Infecțiilor Produse. 2016. Available online: www.cnscbt.ro/index.php/ghiduri-si-protocoale/ghiduri/522-ghid-privind-enterobacteriaceae-producatoare-de-carbapenemaze-diagnosticul-prevenirea-transmiterii-interumane-si-tratamentul-infectiilor-produse/file (accessed on 20 August 2022).
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef]
- Esme, M.; Topeli, A.; Yavuz, B.B.; Akova, M. Infections in the Elderly Critically-Ill Patients. Front. Med. 2019, 6, 118. [Google Scholar] [CrossRef]
- Madrazo, M.; Esparcia, A.; López-Cruz, I.; Alberola, J.; Piles, L.; Viana, A.; Eiros, J.M.; Artero, A. Clinical impact of multidrug-resistant bacteria in older hospitalized patients with communi-ty-acquired urinary tract infection. BMC Infect Dis. 2021, 21, 1232. [Google Scholar] [CrossRef]
- Golli, A.L.; Nițu, F.M.; Balasoiu, M.; Rascu, S.; Lungu, M.A.; Dinescu, S.N.; Ciobanu Mitrache, L.; Glodeanu, A.; Vacaru, M.; Olteanu, M. Microbiological profile and antibiotic resistance pattern of bacterial uropathogens among hospitalized patients. Farmacia 2019, 67, 167–173. [Google Scholar] [CrossRef]
- Yalçın, T.Y.; Salgür, F.; Sarı, N.; Azap, Ö.K.; Doruk, H. Evaluation of the Antimicrobial Resistance Rates in Urine Samples of the Elderly. Eur. J. Geriatr. Gerontol. 2022, 4, 129–134. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; RodríguezBaño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20, 1–55. [Google Scholar] [CrossRef] [PubMed]




| Patients | Year | ||
|---|---|---|---|
| 2022 | 2023 | 2024 | |
| Male | 142 (43.56%) | 294 (47.57%) | 382 (41.52%) |
| Female | 184 (54.44%) | 324 (52.43%) | 538 (58.48%) |
| Total | 326 | 618 | 920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golli, A.-L.; Zlatian, O.M.; Popa, S.-G.; Turcu, F.L.; Balasoiu, A.T. Trends in the Antimicrobial Resistance Pattern of Bacterial Gram-Negative Pathogens in Elderly Patients Admitted to the Intensive Care Unit. Microorganisms 2025, 13, 2330. https://doi.org/10.3390/microorganisms13102330
Golli A-L, Zlatian OM, Popa S-G, Turcu FL, Balasoiu AT. Trends in the Antimicrobial Resistance Pattern of Bacterial Gram-Negative Pathogens in Elderly Patients Admitted to the Intensive Care Unit. Microorganisms. 2025; 13(10):2330. https://doi.org/10.3390/microorganisms13102330
Chicago/Turabian StyleGolli, Andreea-Loredana, Ovidiu Mircea Zlatian, Simona-Georgiana Popa, Flavia Liliana Turcu, and Andrei Theodor Balasoiu. 2025. "Trends in the Antimicrobial Resistance Pattern of Bacterial Gram-Negative Pathogens in Elderly Patients Admitted to the Intensive Care Unit" Microorganisms 13, no. 10: 2330. https://doi.org/10.3390/microorganisms13102330
APA StyleGolli, A.-L., Zlatian, O. M., Popa, S.-G., Turcu, F. L., & Balasoiu, A. T. (2025). Trends in the Antimicrobial Resistance Pattern of Bacterial Gram-Negative Pathogens in Elderly Patients Admitted to the Intensive Care Unit. Microorganisms, 13(10), 2330. https://doi.org/10.3390/microorganisms13102330






