Red Foxes (Vulpes vulpes) and European Badgers (Meles meles) as Overlooked Wildlife Hosts of Canine Parvovirus in Slovakia: First Evidence by Molecular Characterization and Virus Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. DNA Extraction
2.3. Sample Origin Confirmation
2.4. Real-Time PCR Screening and DNA Copy Number Determination
2.5. Conventional PCR Amplification of the VP2 Gene
2.6. Sequencing and Analysis of Amino Acid Residues of the VP2 Protein
2.7. Phylogenetic Analysis
2.8. Cell Culture and Virus Isolation
2.9. Statistical Analysis
3. Results
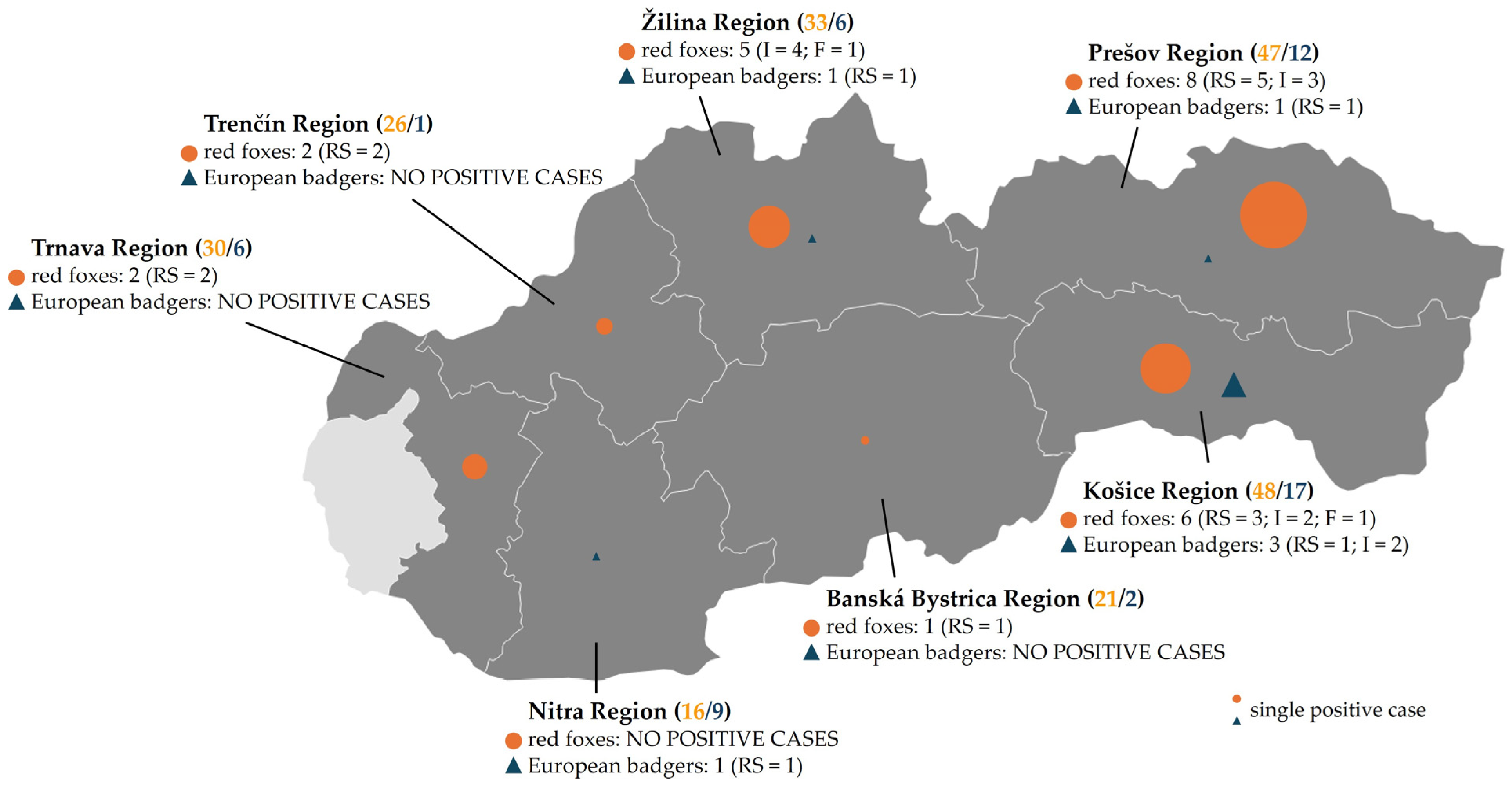
3.1. Study Area and Distribution of Samples
3.2. Fecal Samples Originate from Red Foxes
3.3. Screening for CPV
3.4. VP2 Sequence and Phylogenetic Analysis
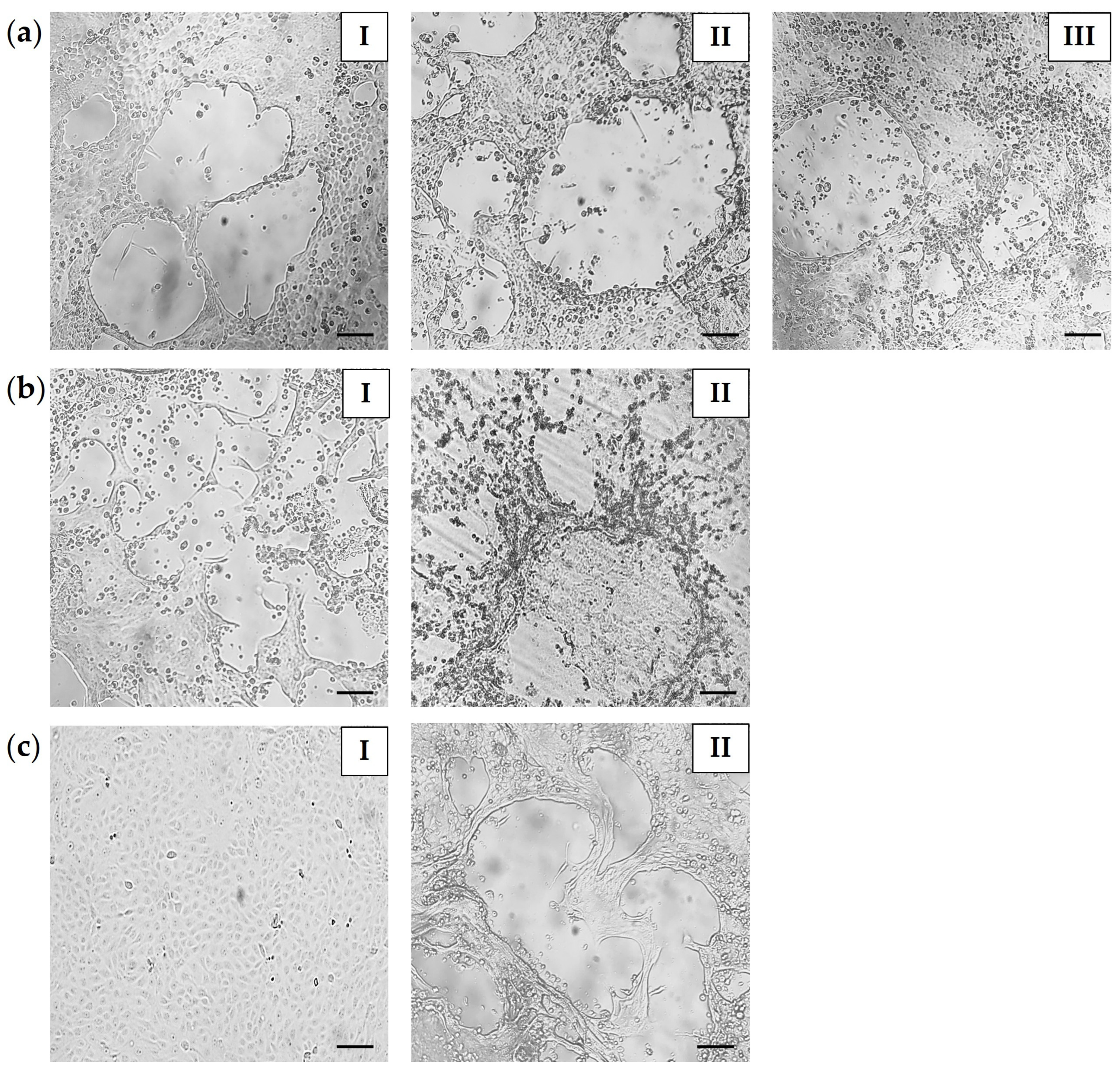
3.5. Virus Isolation on MDCK Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPE | cytopathic effect |
| CPV | canine parvovirus |
| EB | European badger |
| F | fecal sample |
| I | small intestine |
| MDCK | Madin-Darby Canine Kidney |
| MEM | Minimal Essential Medium |
| N/A | not available |
| RF | red fox |
| RS | rectal swab |
References
- Tuteja, D.; Banu, K.; Mondal, B. Canine Parvovirology—A Brief Updated Review on Structural Biology, Occurrence, Pathogenesis, Clinical Diagnosis, Treatment and Prevention. Comp. Immunol. Microbiol. Infect. Dis. 2022, 82, 101765. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.J.; Scott, F.W.; Carmichael, L.E. Isolation and Immunisation Studies of a Canine Parco-like Virus from Dogs with Haemorrhagic Enteritis. Vet. Rec. 1979, 105, 156–159. [Google Scholar] [CrossRef]
- Zhou, H.; Cui, K.; Su, X.; Zhang, H.; Xiao, B.; Li, S.; Yang, B. Overview of Recent Advances in Canine Parvovirus Research: Current Status and Future Perspectives. Microorganisms 2025, 13, 47. [Google Scholar] [CrossRef]
- Decaro, N.; Desario, C.; Parisi, A.; Martella, V.; Lorusso, A.; Miccolupo, A.; Mari, V.; Colaianni, M.L.; Cavalli, A.; Di Trani, L.; et al. Genetic Analysis of Canine Parvovirus Type 2c. Virology 2009, 385, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.P.; Jones, E.V.; Miller, T.J. Nucleotide Sequence and Genome Organization of Canine Parvovirus. J. Virol. 1988, 62, 266–276. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santana, W.; Silveira, V.P.; Wolf, J.M.; Kipper, D.; Echeverrigaray, S.; Canal, C.W.; Truyen, U.; Lunge, V.R.; Streck, A.F. Molecular Phylogenetic Assessment of the Canine Parvovirus 2 Worldwide and Analysis of the Genetic Diversity and Temporal Spreading in Brazil. Infect. Genet. Evol. 2022, 98, 105225. [Google Scholar] [CrossRef]
- Stamenković, G.G.; Ćirković, V.S.; Šiljić, M.M.; Blagojević, J.V.; Knežević, A.M.; Joksić, I.D.; Stanojević, M.P. Substitution Rate and Natural Selection in Parvovirus B19. Sci. Rep. 2016, 6, 35759. [Google Scholar] [CrossRef]
- Cadar, D.; Cságola, A.; Kiss, T.; Tuboly, T. Capsid Protein Evolution and Comparative Phylogeny of Novel Porcine Parvoviruses. Mol. Phylogenet. Evol. 2013, 66, 243–253. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Pan, Y.; Li, H.; He, T.; Dong, Q.; Song, W.; Zhang, W.; Zhang, L.; Kareem, K.; et al. Evolutionary Dynamics and Pathogenicity Analysis of Feline Panleukopenia Virus in Xinjiang, China. Microorganisms 2024, 12, 2205. [Google Scholar] [CrossRef]
- Decaro, N.; Desario, C.; Addie, D.D.; Martella, V.; Vieira, M.J.; Elia, G.; Zicola, A.; Davis, C.; Thompson, G.; Thiry, E.; et al. Molecular Epidemiology of Canine Parvovirus, Europe. Emerg. Infect. Dis. 2007, 13, 1222–1224. [Google Scholar] [CrossRef]
- Shackelton, L.A.; Parrish, C.R.; Truyen, U.; Holmes, E.C. High Rate of Viral Evolution Associated with the Emergence of Carnivore Parvovirus. Proc. Natl. Acad. Sci. USA 2005, 102, 379–384. [Google Scholar] [CrossRef]
- Chung, H.-C.; Kim, S.-J.; Nguyen, V.G.; Shin, S.; Kim, J.Y.; Lim, S.-K.; Park, Y.H.; Park, B. New Genotype Classification and Molecular Characterization of Canine and Feline Parvoviruses. J. Vet. Sci. 2020, 21, e43. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Thompson, G. Canine Parvovirus: The Worldwide Occurrence of Antigenic Variants. J. Gen. Virol. 2016, 97, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zeng, W.; Zhang, X.; Li, S. The Genetic Evolution of Canine Parvovirus—A New Perspective. PLoS ONE 2017, 12, e0175035. [Google Scholar] [CrossRef]
- Li, G.; Ji, S.; Zhai, X.; Zhang, Y.; Liu, J.; Zhu, M.; Zhou, J.; Su, S. Evolutionary and Genetic Analysis of the VP2 Gene of Canine Parvovirus. BMC Genom. 2017, 18, 534. [Google Scholar] [CrossRef]
- Mira, F.; Purpari, G.; Lorusso, E.; Di Bella, S.; Gucciardi, F.; Desario, C.; Macaluso, G.; Decaro, N.; Guercio, A. Introduction of Asian Canine Parvovirus in Europe through Dog Importation. Transbound. Emerg. Dis. 2018, 65, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hueffer, K.; Parker, J.S.L.; Weichert, W.S.; Geisel, R.E.; Sgro, J.-Y.; Parrish, C.R. The Natural Host Range Shift and Subsequent Evolution of Canine Parvovirus Resulted from Virus-Specific Binding to the Canine Transferrin Receptor. J. Virol. 2003, 77, 1718–1726. [Google Scholar] [CrossRef]
- Sarabandi, S.; Pourtaghi, H. Whole Genome Sequence Analysis of CPV-2 Isolates from 1998 to 2020. Virol. J. 2023, 20, 138. [Google Scholar] [CrossRef]
- Miranda, C.; Santos, N.; Parrish, C.; Thompson, G. Genetic characterization of canine parvovirus in sympatric free-ranging wild carnivores in Portugal. J. Wildl. Dis. 2017, 53, 824–831. [Google Scholar] [CrossRef]
- Alfano, F.; Dowgier, G.; Valentino, M.P.; Galiero, G.; Tinelli, A.; Nicola, D.; Fusco, G. Identification of Pantropic Canine Coronavirus in a Wolf (Canis lupus italicus) in Italy. J. Wildl. Dis. 2019, 55, 504–508. [Google Scholar] [CrossRef]
- Ndiana, L.A.; Lanave, G.; Desario, C.; Berjaoui, S.; Alfano, F.; Puglia, I.; Fusco, G.; Colaianni, M.L.; Vincifori, G.; Camarda, A.; et al. Circulation of Diverse Protoparvoviruses in Wild Carnivores, Italy. Transbound. Emerg. Dis. 2021, 68, 2489–2502. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, O.; Esperón, F.; Velarde, R.; Oleaga, Á.; Llaneza, L.; Ribas, A.; Negre, N.; de la Torre, A.; Rodríguez, A.; Millán, J. Genetic Characterization of Carnivore Parvoviruses in Spanish Wildlife Reveals Domestic Dog and Cat-Related Sequences. Transbound. Emerg. Dis. 2020, 67, 626–634. [Google Scholar] [CrossRef]
- Leopardi, S.; Milani, A.; Cocchi, M.; Bregoli, M.; Schivo, A.; Leardini, S.; Festa, F.; Pastori, A.; de Zan, G.; Gobbo, F.; et al. Carnivore Protoparvovirus 1 (CPV-2 and FPV) Circulating in Wild Carnivores and in Puppies Illegally Imported into North-Eastern Italy. Viruses 2022, 14, 2612. [Google Scholar] [CrossRef]
- Magliocca, M.; Taddei, R.; Urbani, L.; Bertasio, C.; Facile, V.; Gallina, L.; Sampieri, M.; Rugna, G.; Rubini, S.; Maioli, G.; et al. Molecular Detection of Viral and Bacterial Pathogens in Red Foxes (Vulpes Vulpes) from Italy. Animals 2024, 14, 1969. [Google Scholar] [CrossRef]
- Kurucay, H.N.; Tamer, C.; Muftuoglu, B.; Elhag, A.E.; Gozel, S.; Cicek-Yildiz, Y.; Demirtas, S.; Ozan, E.; Albayrak, H.; Okur-Gumusova, S.; et al. First Isolation and Molecular Characterization of Canine Parvovirus-Type 2b (CPV-2b) from Red Foxes (Vulpes Vulpes) Living in the Wild Habitat of Turkey. Virol. J. 2023, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Cedillo-Rosales, S.; Cedillo-Rosales, D.; Busqueta-Medina, A.; Avalos-Nolazco, D.; Zamora-Ávila, D.; Zaráte-Ramos, J.; Avalos-Ramirez, R. Molecular detection of canine parvovirus-2c (CPV-2C) in diseased coyote pups (Canis latrans) at northeastern Mexico. Int. J. Infect. Dis. 2023, 130, S142–S143. [Google Scholar] [CrossRef]
- Sarchese, V.; Di Profio, F.; Robetto, S.; Orusa, R.; Vuillermoz, B.; Pellegrini, F.; Marsilio, F.; Martella, V.; Di Martino, B. Molecular Survey for Major Canine Enteric Viral Pathogens in Wild Carnivores, Northwestern Italy. Vet. Sci 2025, 12, 814. [Google Scholar] [CrossRef]
- Mendenhall, I.H.; Low, D.; Neves, E.S.; Anwar, A.; Oh, S.; Su, Y.C.F.; Smith, G.J.D. Evidence of Canine Parvovirus Transmission to a Civet Cat (Paradoxurus Musangus) in Singapore. One Health 2016, 2, 122–125. [Google Scholar] [CrossRef]
- Xinyu, T.; Min, C.S.; Yifan, W.; Lien, S.M.; Chan, A.; Hui, T.X.; Lee, B.; Yelin, W.; Chia-Da, H.; Oh, S.; et al. Canine Parvovirus-2c (CPV-2c) Infection in Wild Asian Palm Civets (Paradoxurus Hermaphroditus) in Singapore. J. Wildl. Dis. 2019, 55, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Bassi, F.; De Arcangeli, S.; Zobba, R.; Dedola, C.; Alberti, A.; Battilani, M. Molecular Analysis of Carnivore Protoparvovirus Detected in White Blood Cells of Naturally Infected Cats. BMC Vet. Res. 2018, 14, 41. [Google Scholar] [CrossRef]
- Staff, T.P.P. Correction: Host-Specific Parvovirus Evolution in Nature Is Recapitulated by In Vitro Adaptation to Different Carnivore Species. PLOS Pathog. 2014, 10, e1004586. [Google Scholar] [CrossRef]
- Son, K.; Lee, S.-M.; Kim, Y.; Kim, Y.-K.; Lee, S.-Y.; Jheong, W.-H.; Oem, J.-K. Genetic Characterization of Canine Parvovirus Type 2 Detected in Wild Raccoon Dogs (Nyctereutes Procyonoides) in the Republic of Korea. J. Wildl. Dis. 2019, 55, 512–515. [Google Scholar] [CrossRef]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus Infections in Wild Carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef]
- Gordon, J.C.; Angrick, E.J. Canine Parvovirus: Environmental Effects on Infectivity. Am. J. Vet. Res. 1986, 47, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Pluemer, M.; Dubay, S.; Drake, D.; Crimmins, S.; Veverka, T.; Hovanec, H.; Torkelson, M.; Mueller, M. Red Foxes (Vulpes vulpes) and Coyotes (Canis latrans) in an Urban Landscape: Prevalence and Risk Factors for Disease. J. Urban Ecol. 2019, 5, juz022. [Google Scholar] [CrossRef]
- Kimpston, C.N.; Hatke, A.L.; Castelli, B.; Otto, N.; Tiffin, H.S.; Machtinger, E.T.; Brown, J.D.; Van Why, K.R.; Marconi, R.T. High Prevalence of Antibodies against Canine Parvovirus and Canine Distemper Virus among Coyotes and Foxes from Pennsylvania: Implications for the Intersection of Companion Animals and Wildlife. Microbiol. Spectr. 2022, 10, e0253221. [Google Scholar] [CrossRef]
- Van Arkel, A.; Kelman, M.; West, P.; Ward, M.P. The Relationship between Reported Domestic Canine Parvovirus Cases and Wild Canid Distribution. Heliyon 2019, 5, e02511. [Google Scholar] [CrossRef] [PubMed]
- Yon, L.; Duff, J.P.; Ågren, E.O.; Erdélyi, K.; Ferroglio, E.; Godfroid, J.; Hars, J.; Hestvik, G.; Horton, D.; Kuiken, T.; et al. Recent changes in infectious disease in European wildlife. J. Wildl. Dis. 2019, 55, 3–43. [Google Scholar] [CrossRef]
- Brandell, E.E.; Cross, P.C.; Craft, M.E.; Smith, D.W.; Dubovi, E.J.; Gilbertson, M.L.J.; Wheeldon, T.; Stephenson, J.A.; Barber-Meyer, S.; Borg, B.L.; et al. Patterns and Processes of Pathogen Exposure in Gray Wolves across North America. Sci. Rep. 2021, 11, 3722. [Google Scholar] [CrossRef]
- Riley, S.P.D.; Foley, J.; Chomel, B. Exposure to Feline and Canine Pathogens in Bobcats and Gray Foxes in Urban and Rural Zones of a National Park in California. J. Wildl. Dis. 2004, 40, 11–22. [Google Scholar] [CrossRef]
- Garcês, A.; Pires, I. Secrets of the Astute Red Fox (Vulpes vulpes, Linnaeus, 1758): An Inside-Ecosystem Secret Agent Serving One Health. Environments 2021, 8, 103. [Google Scholar] [CrossRef]
- Gürler, A.T.; Gori, F.; Bölükbas¸, C.S.; Umur, Ş.; Açıcı, M.; Deplazes, P. Investigation of Echinococcus multilocularis in Environmental Definitive Host Feces in the Asian and the European Parts of Turkey. Front. Vet. Sci. 2018, 5, 48. [Google Scholar] [CrossRef]
- Wilson, J.J.; Rougerie, R.; Schonfeld, J.; Janzen, D.H.; Hallwachs, W.; Hajibabaei, M.; Kitching, I.J.; Haxaire, J.; Hebert, P.D. When Species Matches Are Unavailable Are DNA Barcodes Correctly Assigned to Higher Taxa? An Assessment Using Sphingid Moths. BMC Ecol. 2011, 11, 18. [Google Scholar] [CrossRef]
- Lan, T.M.; Lin, Y.; Njaramba-Ngatia, J.; Guo, X.S.; Li, R.G.; Li, H.M.; Kumar-Sahu, S.; Wang, X.; Yang, X.J.; Guo, H.B.; et al. Improving Species Identification of Ancient Mammals Based on Next-Generation Sequencing Data. Genes 2019, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Trani, L.D.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A Real-Time PCR Assay for Rapid Detection and Quantitation of Canine Parvovirus Type 2 in the Feces of Dogs. Vet. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef]
- Hu, W.; Xu, X.; Liu, Q.; Ji, J.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Molecular Characterisation and Genetic Diversity of Canine Parvovirus Type 2 Prevalent in Central China. J. Vet. Res. 2020, 64, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Pelegrinová, A.; Petroušková, P.; Korytár, Ľ.; Ondrejková, A.; Drážovská, M.; Vojtek, B.; Mojžišová, J.; Prokeš, M.; Kostičák, M.; Zákutná, Ľ.; et al. The First Evidence of Asian-like CPV-2b in Slovakia in a Vaccinated Dog with an Acute Fatal Course of Parvovirus Infection: A Case Report. Vet. Res. Commun. 2024, 48, 3253–3262. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Hao, Y.; Zhang, G.; Lyu, Y.; Wang, J.; Liu, W.; Qin, T. Characterization of the VP2 and NS1 Genes from Canine Parvovirus Type 2 (CPV-2) and Feline Panleukopenia Virus (FPV) in Northern China. Front. Vet. Sci. 2022, 9, 934849. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Parrish, C.R.; Aquadro, C.F.; Strassheim, M.L.; Evermann, J.F.; Sgro, J.Y.; Mohammed, H.O. Rapid Antigenic-Type Replacement and DNA Sequence Evolution of Canine Parvovirus. J. Virol. 1991, 65, 6544–6552. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, C.; Martella, V.; Pratelli, A.; Tempesta, M.; Cavalli, A.; Buonavoglia, D.; Bozzo, G.; Elia, G.; Decaro, N.; Carmichael, L. Evidence for Evolution of Canine Parvovirus Type 2 in Italy. J. Gen. Virol. 2001, 82, 3021–3025. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Behdenna, A.; Lembo, T.; Calatayud, O.; Cleaveland, S.; Halliday, J.E.B.; Packer, C.; Lankester, F.; Hampson, K.; Craft, M.E.; Czupryna, A.; et al. Transmission Ecology of Canine Parvovirus in a Multi-Host, Multi-Pathogen System. Proc. Biol. Sci. 2019, 286, 20182772. [Google Scholar] [CrossRef]
- Vieira, F.V.; Hoffmann, D.J.; Fabri, C.U.F.; Bresciani, K.D.S.; Gameiro, R.; Flores, E.F.; Cardoso, T.C. Circulation of Canine Parvovirus among Dogs Living in Human-Wildlife Interface in the Atlantic Forest Biome, Brazil. Heliyon 2017, 3, e00491. [Google Scholar] [CrossRef]
- Kelman, M.; Harriott, L.; Carrai, M.; Kwan, E.; Ward, M.P.; Barrs, V.R. Phylogenetic and Geospatial Evidence of Canine Parvovirus Transmission between Wild Dogs and Domestic Dogs at the Urban Fringe in Australia. Viruses 2020, 12, 663. [Google Scholar] [CrossRef]
- Monterroso, P.; Castro, D.; Silva, T.L.; Ferreras, P.; Godinho, R.; Alves, P.C. Factors Affecting the (in)Accuracy of Mammalian Mesocarnivore Scat Identification in South-Western Europe. J. Zool. 2013, 289, 243–250. [Google Scholar] [CrossRef]
- Davison, A.; Birks, J.D.S.; Brookes, R.C.; Braithwaite, T.C.; Messenger, J.E. On the Origin of Faeces: Morphological versus Molecular Methods for Surveying Rare Carnivores from Their Scats. J. Zool. 2002, 257, 141–143. [Google Scholar] [CrossRef]
- Duarte, M.D.; Henriques, A.M.; Barros, S.C.; Fagulha, T.; Mendonça, P.; Carvalho, P.; Monteiro, M.; Fevereiro, M.; Basto, M.P.; Rosalino, L.M.; et al. Snapshot of Viral Infections in Wild Carnivores Reveals Ubiquity of Parvovirus and Susceptibility of Egyptian Mongoose to Feline Panleukopenia Virus. PLoS ONE 2013, 8, e59399. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine Parvovirus—A Review of Epidemiological and Diagnostic Aspects, with Emphasis on Type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Segev, G.; Yaaran, T.; Maurice, S.; Baneth, G. Effect of Sampling Site on the Diagnosis of Canine Parvovirus Infection in Dogs Using Polymerase Chain Reaction. J. Vet. Intern. Med. 2022, 36, 591–598. [Google Scholar] [CrossRef]
- Mech, L.D.; Almberg, E.S.; Smith, D.; Goyal, S.; Singer, R.S. Use of Real-Time PCR to Detect Canine Parvovirus in Feces of Free-Ranging Wolves. J. Wildl. Dis. 2012, 48, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors—Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Martella, V.; Elia, G.; Desario, C.; Campolo, M.; Lorusso, E.; Colaianni, M.L.; Lorusso, A.; Buonavoglia, C. Tissue Distribution of the Antigenic Variants of Canine Parvovirus Type 2 in Dogs. Vet. Microbiol. 2007, 121, 39–44. [Google Scholar] [CrossRef]
- Decaro, N.; Desario, C.; Billi, M.; Lorusso, E.; Colaianni, M.L.; Colao, V.; Elia, G.; Ventrella, G.; Kusi, I.; Bo, S.; et al. Evaluation of an In-Clinic Assay for the Diagnosis of Canine Parvovirus. Vet. J. 2013, 198, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Proksch, A.L.; Unterer, S.; Speck, S.; Truyen, U.; Hartmann, K. Influence of Clinical and Laboratory Variables on Faecal Antigen ELISA Results in Dogs with Canine Parvovirus Infection. Vet. J. 2015, 204, 304–308. [Google Scholar] [CrossRef]
- Pereira, C.A.D.; Leal, E.S.; Durigon, E.L. Selective Regimen Shift and Demographic Growth Increase Associated with the Emergence of High-Fitness Variants of Canine Parvovirus. Infect. Genet. Evol. 2007, 7, 399–409. [Google Scholar] [CrossRef]
- Ogbu, K.I.; Mira, F.; Purpari, G.; Nwosuh, C.; Loria, G.R.; Schirò, G.; Chiaramonte, G.; Tion, M.T.; Di Bella, S.; Ventriglia, G.; et al. Nearly Full-length Genome Characterization of Canine Parvovirus Strains Circulating in Nigeria. Transbound. Emerg. Dis. 2020, 67, 635–647. [Google Scholar] [CrossRef]
- Allison, A.B.; Organtini, L.J.; Zhang, S.; Hafenstein, S.L.; Holmes, E.C.; Parrish, C.R. Single Mutations in the VP2 300 Loop Region of the Three-Fold Spike of the Carnivore Parvovirus Capsid Can Determine Host Range. J. Virol. 2016, 90, 753–767. [Google Scholar] [CrossRef]
- Voorhees, I.E.H.; Lee, H.; Allison, A.B.; Lopez-Astacio, R.; Goodman, L.B.; Oyesola, O.O.; Omobowale, O.; Fagbohun, O.; Dubovi, E.J.; Hafenstein, S.L.; et al. Limited Intrahost Diversity and Background Evolution Accompany 40 Years of Canine Parvovirus Host Adaptation and Spread. J. Virol. 2019, 94, e01162-19. [Google Scholar] [CrossRef]
- Balboni, A.; Niculae, M.; Di Vito, S.; Urbani, L.; Terrusi, A.; Muresan, C.; Battilani, M. The Detection of Canine Parvovirus Type 2c of Asian Origin in Dogs in Romania Evidenced Its Progressive Worldwide Diffusion. BMC Vet. Res. 2021, 17, 206. [Google Scholar] [CrossRef]
- Mira, F.; Dowgier, G.; Purpari, G.; Vicari, D.; Di Bella, S.; Macaluso, G.; Gucciardi, F.; Randazzo, V.; Decaro, N.; Guercio, A. Molecular Typing of a Novel Canine Parvovirus Type 2a Mutant Circulating in Italy. Infect. Genet. Evol. 2018, 61, 67–73. [Google Scholar] [CrossRef]
- Umar, S.; Gao, D.; Kim, S.; Cheng, Y.; Fang, Z.; Zhongqi, Q.; Yu, W.; Anderson, B.D. Molecular Characterization of Canine Parvovirus Type 2 (CPV2) Reveals a High Prevalence of the CPV2c Genotype among Dogs Suffering from Diarrhea. Anim. Dis. 2024, 4, 1. [Google Scholar] [CrossRef]
- Silva, L.M.N.; Santos, M.R.; Carvalho, J.A.; Carvalho, O.V.; Favarato, E.S.; Fietto, J.L.R.; Bressan, G.C.; Silva-Júnior, A. Molecular Analysis of the Full-Length VP2 Gene of Brazilian Strains of Canine Parvovirus 2 Shows Genetic and Structural Variability between Wild and Vaccine Strains. Virus Res. 2022, 313, 198746. [Google Scholar] [CrossRef]
- Schirò, G.; Mira, F.; Canuti, M.; Vullo, S.; Purpari, G.; Chiaramonte, G.; Di Bella, S.; Cannella, V.; Randazzo, V.; Castronovo, C.; et al. Identification and Molecular Characterization of a Divergent Asian-like Canine Parvovirus Type 2b (CPV-2b) Strain in Southern Italy. Int. J. Mol. Sci. 2022, 23, 11240. [Google Scholar] [CrossRef]
- Battilani, M.; Modugno, F.; Mira, F.; Purpari, G.; Di Bella, S.; Guercio, A.; Balboni, A. Molecular Epidemiology of Canine Parvovirus Type 2 in Italy from 1994 to 2017: Recurrence of the CPV-2b Variant. BMC Vet. Res. 2019, 15, 393. [Google Scholar] [CrossRef]
- Boros, Á.; Albert, M.; Urbán, P.; Herczeg, R.; Gáspár, G.; Balázs, B.; Cságola, A.; Pankovics, P.; Gyenesei, A.; Reuter, G. Unusual “Asian-Origin” 2c to 2b Point Mutant Canine Parvovirus (Parvoviridae) and Canine Astrovirus (Astroviridae) Co-Infection Detected in Vaccinated Dogs with an Outbreak of Severe Haemorrhagic Gastroenteritis with High Mortality Rate in Hungary. Vet. Res. Commun. 2022, 46, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, O.; Esperón, F.; Cleaveland, S.; Biek, R.; Keyyu, J.; Eblate, E.; Neves, E.; Lembo, T.; Lankester, F. Carnivore Parvovirus Ecology in the Serengeti Ecosystem: Vaccine Strains Circulating and New Host Species Identified. J. Virol. 2019, 93, e02220-18. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, A.M.; Moreno, K.; Chaves, A.; Ibarra-Cerdeña, C.N.; Rubio, A.; Foley, J.; List, R.; Suzán, G.; Sarmiento, R.E. Carnivore Protoparvovirus 1 at the Wild–Domestic Carnivore Interface in Northwestern Mexico. EcoHealth 2019, 16, 502–511. [Google Scholar] [CrossRef]

| Primer/Probe | Sequence (5′–3′) | Position | Amplicon Size | Reference |
|---|---|---|---|---|
| Sample-origin confirmation | ||||
| VV-COI-F | CACTGTAGGAATAGATGTGG | 6228–6247 a | 503 bp | this study |
| VV-COI-R | AGATGAATGAGCCTATAGATG | 6710–6730 a | ||
| CPV screening | ||||
| qPCR-CPV-F | AAACAGGAATTAACTATACTAATATATTTA | 4101–4130 b | 93 bp | [45] |
| qPCR-CPV-R | AAATTTGACCATTTGGATAAACT | 4171–4193 b | ||
| qPCR-CPV-P | FAM–TGGTCCTTTAACTGCATTAAATAATGTACC-BHQ1 | 4138–4167 b | ||
| VP2 sequencing | ||||
| PCR I | ||||
| VP2-F c | AGAGACAATCTTGCACCAAT | 2768–2787 b | 554 bp | [46] |
| CPV-VP2-INT-R1 | CTATCTAATGCAACCATCAATG | 3300–3321 b | [47] | |
| PCR II | ||||
| CPV-VP2-INT-F1 | GTTGCATTTAGTTAGTTTTGAACA | 3190–3213 b | 541 bp | [47] |
| CPV-VP2-INT-R2 | ACCACGTCTTTTATCTTGTTG | 3710–3730 b | ||
| PCR III | ||||
| CPV-VP2-INT-F2 | GATTGTAAACCATGTAGACTAACA | 3590–3613 b | 563 bp | [47] |
| CPV-VP2-INT-R3 | GCAGTTAAAGGACCATAAGTA | 4132–4152 b | ||
| PCR IV | ||||
| CPV-VP2-INF-F3 | GAAGATATCCAGAAGGAGATTGG | 4005–4027 b | 539 bp | [47] |
| VP2-R d | ATGTTAATATAATTTTCTAGGTGCT | 4519–4543 b | [46] | |
| Confirmation of virus isolation | ||||
| degCPV-VP2-F | TGATGGAGSAGTWCAACCAGA | 2791–2811 b | 573 bp | [47] |
| CPV-VP2-R | TCAGATCTCATAGCTGCTGGA | 3343–3363 b | [48] | |
| Host Species | No. of Samples | Sample Type | Total Positives | Prevalence (95% CI) | ||
|---|---|---|---|---|---|---|
| Rectal Swabs Pos./Total (%) | Intestines Pos./Total (%) | Feces Pos./Total (%) | ||||
| red fox | 221 | 13/84 (15.5%) | 9/72 (12.5%) | 2/65 (3.1%) | 24/221 | 10.9% (7.4–15.6) |
| European badger | 53 | 4/39 (10.3%) | 2/14 (14.3%) | – | 6/53 | 11.3% (5.3–22.6) |
| Total | 274 | 17/123 (13.8%) | 11/86 (12.8%) | 2/65 (3.1%) | 30/274 | 10.9% (7.8–15.2) |
| Region | Host Species | No. of Samples | Sample Type | Total CPV Positives | Positivity Rate | ||
|---|---|---|---|---|---|---|---|
| Rectal Swabs Pos./Total (%) | Intestines Pos./Total (%) | Feces Pos./Total (%) | |||||
| Banská Bystrica | Red fox | 21 | 1/9 | 0/5 | 0/7 | 1/21 | 4.8% |
| European badger | 2 | 0/2 | N/A | – | 0/2 | – | |
| Total | 23 | 1/11 | 0/5 | 0/7 | 1/23 | 4.3% | |
| Košice | Red fox | 48 | 3/15 | 2/19 | 1/14 | 6/48 | 12.5% |
| European badger | 17 | 1/12 | 2/5 | – | 3/17 | 17.6% | |
| Total | 65 | 4/27 | 4/24 | 1/14 | 9/65 | 13.8% | |
| Nitra | Red fox | 16 | 0/7 | 0/4 | 0/5 | 0/16 | – |
| European badger | 9 | 1/7 | 0/2 | – | 1/9 | 11.1% | |
| Total | 25 | 1/14 | 0/6 | 0/5 | 1/25 | 4% | |
| Prešov | Red fox | 47 | 5/21 | 3/17 | 0/9 | 8/47 | 17% |
| European badger | 12 | 1/9 | 0/3 | – | 1/12 | 8.3% | |
| Total | 59 | 6/30 | 3/20 | 0/9 | 9/59 | 15.2% | |
| Trenčín | Red fox | 26 | 2/11 | 0/7 | 0/8 | 2/26 | 7.7% |
| European badger | 1 | N/A | 0/1 | – | 0/1 | 0 | |
| Total | 27 | 2/11 | 0/8 | 0/8 | 2/27 | 7.4% | |
| Trnava | Red fox | 30 | 2/10 | 0/8 | 0/12 | 2/30 | 6.7% |
| European badger | 6 | 0/3 | 0/3 | – | 0/6 | – | |
| Total | 36 | 2/13 | 0/11 | 0/12 | 2/36 | 5.5% | |
| Žilina | Red fox | 33 | 0/11 | 4/12 | 1/10 | 5/33 | 15.1% |
| European badger | 6 | 1/6 | N/A | – | 1/6 | 16.6% | |
| Total | 39 | 1/17 | 4/12 | 1/10 | 6/39 | 15.4% | |
| Region | Host Species | Tested Sample | Sample ID | Mean Ct | DNA Copy Number/ µL Template | DNA Copy Number/ Input Material * | CPV-2 Variant |
|---|---|---|---|---|---|---|---|
| Banská Bystrica Region | red fox | rectal swab | RF-RS-7 | 27.92 | 1.47 × 103 | 3.68 × 102 | CPV-2b |
| Košice Region | red fox | rectal swab | RF-RS-5 | 28.01 | 1.38 × 103 | 3.45 × 102 | CPV-2b |
| red fox | rectal swab | RF-RS-32 | 27.62 | 1.81 × 103 | 4.54 × 102 | CPV-2b | |
| red fox | rectal swab | RF-RS-64 | 28.27 | 1.15 × 103 | 2.88 × 102 | CPV-2b | |
| red fox | intestine | RF-I-12 | 27.2 | 2.44 × 103 | 9.75 × 103 | CPV-2b | |
| red fox | intestine | RF-I-27 | 27.64 | 1.79 × 103 | 7.15 × 103 | CPV-2b | |
| red fox | feces | RF-F-26 | 29.61 | 4.47 × 102 | 2.23 × 102 | CPV-2b | |
| European badger | rectal swab | EB-RS-8 | 28.39 | 1.05 × 103 | 2.64 × 102 | CPV-2b | |
| European badger | intestine | EB-I-2 | 21.42 | 1.43 × 105 | 5.73 × 105 | CPV-2b | |
| European badger | intestine | EB-I-11 | 23.22 | 4.02 × 104 | 1.61 × 104 | CPV-2a | |
| Nitra Region | European badger | rectal swab | EB-RS-29 | 25.22 | 9.86 × 103 | 2.47 × 103 | CPV-2a |
| Prešov Region | red fox | rectal swab | RF-RS-14 | 27.48 | 2 × 103 | 5 × 102 | CPV-2b |
| red fox | rectal swab | RF-RS-23 | 28.26 | 1.16 × 103 | 2.9 × 102 | CPV-2c | |
| red fox | rectal swab | RF-RS-39 | 28.7 | 8.51 × 102 | 2.13 × 102 | CPV-2b | |
| red fox | rectal swab | RF-RS-47 | 27.35 | 2.19 × 103 | 5.48 × 102 | CPV-2b | |
| red fox | rectal swab | RF-RS-73 | 28.28 | 1.14 × 103 | 2.85 × 102 | CPV-2b | |
| red fox | intestine | RF-I-17 | 29.4 | 5.2 × 102 | 2.08 × 103 | CPV-2b | |
| red fox | intestine | RF-I-32 | 28.94 | 7.19 × 102 | 2.88 × 103 | CPV-2a | |
| red fox | intestine | RF-I-64 | 24.61 | 1.52 × 104 | 6.06 × 104 | CPV-2b | |
| European badger | rectal swab | EB-RS-13 | 29.97 | 3.48 × 102 | 8.7 × 101 | CPV-2b | |
| Trenčín Region | red fox | rectal swab | RF-RS-2 | 25.5 | 8.10 × 103 | 2.02 × 103 | CPV-2b |
| red fox | rectal swab | RF-RS-51 | 24.46 | 1.68 × 104 | 4.21 × 103 | CPV-2a | |
| Trnava Region | red fox | rectal swab | RF-RS-28 | 25.3 | 9.29 × 103 | 2.32 × 103 | CPV-2a |
| red fox | rectal swab | RF-RS-82 | 23.8 | 2.68 × 104 | 6.7 × 103 | CPV-2a | |
| Žilina Region | red fox | intestine | RF-I-6 | 27.39 | 2.13 × 103 | 8.53 × 103 | CPV-2b |
| red fox | intestine | RF-I-15 | 26.81 | 3.21 × 103 | 1.28 × 104 | CPV-2b | |
| red fox | intestine | RF-I-40 | 27.36 | 2.19 × 103 | 8.74 × 103 | CPV-2a | |
| red fox | intestine | RF-I-66 | 25.18 | 1.01 × 104 | 4.06 × 104 | CPV-2b | |
| red fox | feces | RF-F-58 | 28.14 | 1.26 × 103 | 6.31 × 102 | CPV-2a | |
| European badger | rectal swab | EB-RS-31 | 27.04 | 2.74 × 103 | 6.85 × 102 | CPV-2b |
| CPV-2 Variant | Host | Country | Year | Accession No. | Amino Acid Residues | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 267 | 297 | 300 | 324 | 370 | 426 | 440 | 447 | 552 | |||||
| CPV-2 | dog | USA | 1979 | EU659116 | A | F | S | A | Y | Q | N | T | I | S |
| CPV-2a | dog | Italy | 2009 | KX434454 | A | F | A | G | Y | Q | N | A | I | S |
| raccoon | Canada | 2016 | MF069443 | A | F | A | D | Y | Q | N | T | I | S | |
| red fox 1 | Slovakia | 2023–2025 | PX146837, PX146840–PX146843 | A | F | A | G | Y | Q | N | T | I | S | |
| red fox | Slovakia | 2023 | PX146838 | A | F | A | G | Y | Q | N | T | M | S | |
| E. badger 1 | Slovakia | 2024 | PX146845 | A | F | A | G | Y | Q | N | T | I | S | |
| CPV-2a “Asian-like markers” | dog | China | 2011 | JX660690 | A | Y | A | G | I | Q | N | A | I | S |
| red fox | Italy | 2022 | PP551646 | - | - | A | G | I | Q | N | A | I | - | |
| red fox | Slovakia | 2024 | PX146839 | A | Y | A | G | I | Q | N | T | I | S | |
| E. badger | Slovakia | 2024 | PX146844 | A | Y | A | G | I | Q | N | T | I | S | |
| CPV-2b | dog | Portugal | 2013 | KR559895 | A | F | A | G | Y | Q | D | T | I | S |
| badger | Italy | 2019 | MT353762 | A | F | A | G | Y | Q | D | T | I | S | |
| red fox 2 | Slovakia | 2023–2025 | PX146846–PX146857 | A | F | A | G | Y | Q | D | T | I | S | |
| E. badger 3 | Slovakia | 2023–2024 | PX146858–PX146860 | A | F | A | G | Y | Q | D | T | I | I | |
| “Asian-like” CPV-2b | dog | Italy | 2022 | ON677437 | G | Y | A | G | I | R | D | T | I | S |
| red fox 4 | Slovakia | 2023–2024 | PX146861–PX146864 | G | Y | A | G | I | R | D | T | I | S | |
| E. badger 4 | Slovakia | 2023 | PX146865 | G | Y | A | G | I | R | D | T | I | S | |
| CPV-2c | dog | Italy | 2000 | FJ005195 | A | F | A | G | Y | Q | E | T | I | S |
| E. badger | Spain | 2012 | KP682530 | A | F | A | G | Y | Q | E | T | I | S | |
| “Asian” CPV-2c | dog | Italy | 2022 | OR463608 | G | Y | A | G | I | R | E | T | I | S |
| pangolin | Taiwan | 2018 | MN832850 | G | Y | A | G | I | R | E | T | I | S | |
| wolf | Italy | 2022 | OP595742 | G | Y | A | G | I | R | E | T | I | S | |
| red fox | Slovakia | 2023 | PX146866 | G | Y | A | G | I | R | E | T | I | S | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroušková, P.; Pelegrinová, A.; Lazár, J.; Lipinský, J.; Drážovská, M.; Prokeš, M.; Korytár, Ľ.; Vojtek, B.; Kostičák, M.; Molnár, L.; et al. Red Foxes (Vulpes vulpes) and European Badgers (Meles meles) as Overlooked Wildlife Hosts of Canine Parvovirus in Slovakia: First Evidence by Molecular Characterization and Virus Isolation. Microorganisms 2025, 13, 2325. https://doi.org/10.3390/microorganisms13102325
Petroušková P, Pelegrinová A, Lazár J, Lipinský J, Drážovská M, Prokeš M, Korytár Ľ, Vojtek B, Kostičák M, Molnár L, et al. Red Foxes (Vulpes vulpes) and European Badgers (Meles meles) as Overlooked Wildlife Hosts of Canine Parvovirus in Slovakia: First Evidence by Molecular Characterization and Virus Isolation. Microorganisms. 2025; 13(10):2325. https://doi.org/10.3390/microorganisms13102325
Chicago/Turabian StylePetroušková, Patrícia, Andrea Pelegrinová, Jozef Lazár, Jakub Lipinský, Monika Drážovská, Marián Prokeš, Ľuboš Korytár, Boris Vojtek, Maroš Kostičák, Ladislav Molnár, and et al. 2025. "Red Foxes (Vulpes vulpes) and European Badgers (Meles meles) as Overlooked Wildlife Hosts of Canine Parvovirus in Slovakia: First Evidence by Molecular Characterization and Virus Isolation" Microorganisms 13, no. 10: 2325. https://doi.org/10.3390/microorganisms13102325
APA StylePetroušková, P., Pelegrinová, A., Lazár, J., Lipinský, J., Drážovská, M., Prokeš, M., Korytár, Ľ., Vojtek, B., Kostičák, M., Molnár, L., Vaščinec, J. M., & Ondrejková, A. (2025). Red Foxes (Vulpes vulpes) and European Badgers (Meles meles) as Overlooked Wildlife Hosts of Canine Parvovirus in Slovakia: First Evidence by Molecular Characterization and Virus Isolation. Microorganisms, 13(10), 2325. https://doi.org/10.3390/microorganisms13102325







