Inefficiency of Kocher and Caird’s Criteria in Septic Arthritis of the Hip Due to Kingella kingae: A Multicenter Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
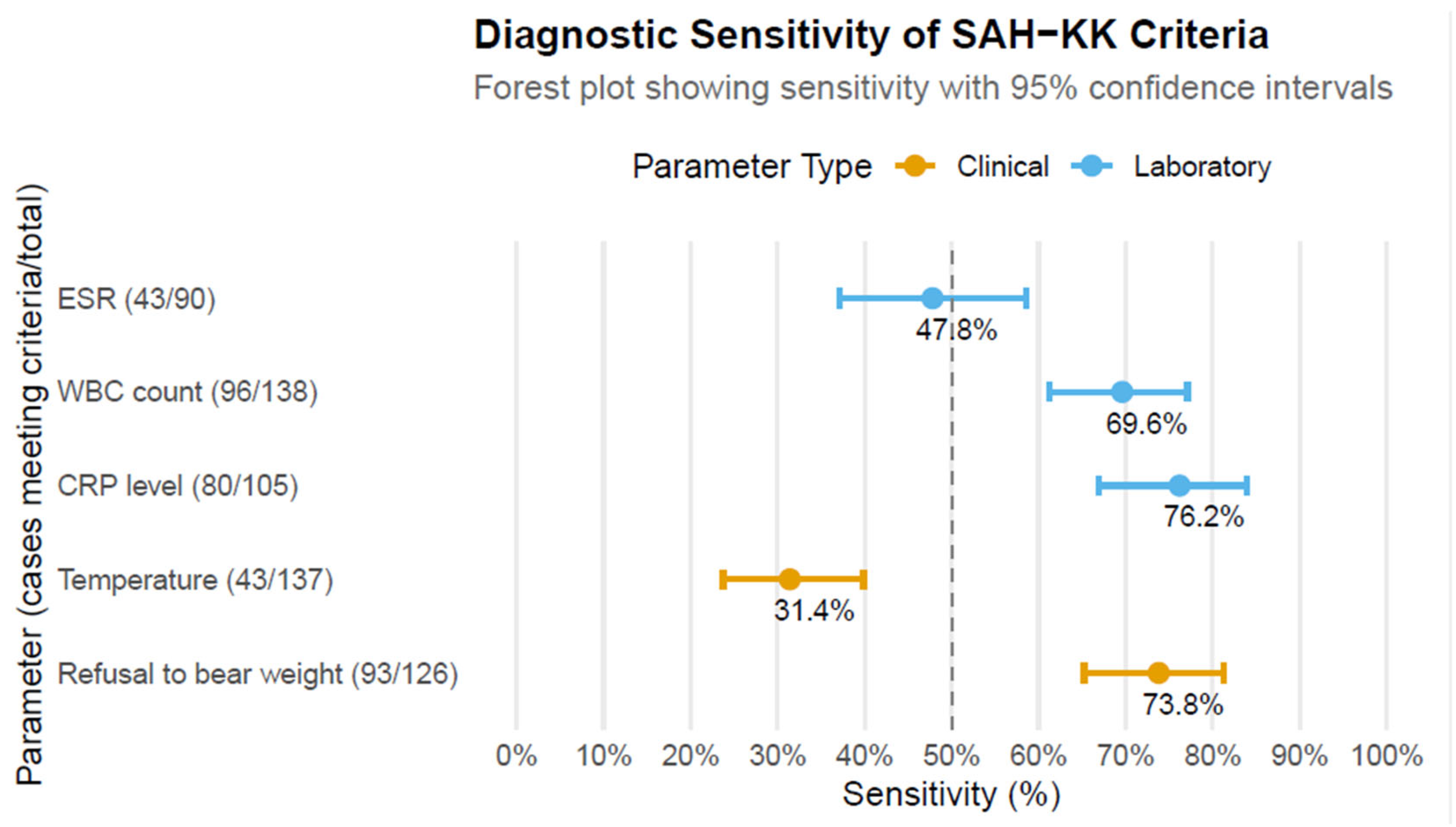
3. Results
3.1. Patient Characteristics and Demographics (Table 1)
| Variables | Values (n = 140) |
|---|---|
| Gender, n (%) | |
| Female | 39 (36.8%) |
| Male | 67 (63.2%) |
| Not reported | 34 |
| Age, months, mean (SD) [median, range] | 16.8 (6.4) [14; 4–56] |
| Age repartition, n (%) | |
| 0–12 months | 50 (35.7%) |
| 13–24 months | 70 (50.0%) |
| 25–36 months | 11 (7.9%) |
| 37–48 months | 7 (5.0%) |
| >48 months | 2 (1.4%) |
3.2. Kocher Criteria and Caird Criteria (Table 2)
| Variables | Kocher Algorithm (n = 4 Criteria *) | Caird Algorithm (n = 5; KC + CRP Level **) |
|---|---|---|
| Patients with complete data | 77/140 (56.7%) | 50/140 (30.0%) |
| Mean (SD) score | 2 (1.1) | 2.8 (1.3) |
| Median score | 2 | 3 |
| Range | 0–4 | 0–5 |
| Number of present criteria | ||
| 0 | 4 (5.2%) | 2 (4%) |
| 1 | 18 (23.4%) | 9 (18%) |
| 2 | 27 (35%) | 9 (18%) |
| 3 | 23 (29.9%) | 10 (20%) |
| 4 | 5 (6.5%) | 17 (34%) |
| 5 | - | 3 (6%) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| K. kingae | Kingella kingae |
| SA | Septic arthritis |
| SAH | Septic arthritis of the hip |
| SAH-KK | Septic arthritis of the hip caused by K. kingae |
| TSH | Transient synovitis of the hip |
| OAI | Osteoarticular infection |
| WBC | White blood cell |
| ESR | Erythrocyte sedimentation rate |
| CRP | C-reactive protein |
References
- Lázaro Carreño, M.I.; Fraile Currius, R.; García Clemente, A. Non-Traumatic Limping in Paediatric Emergencies: Epidemiology, Evaluation and Results. Rev. Esp. Cir. Ortop. Traumatol. 2018, 62, 127–133. [Google Scholar] [CrossRef]
- Fischer, S.U.; Beattie, T.F. The Limping Child: Epidemiology, Assessment and Outcome. J. Bone Jt. Surg. Ser. B 1999, 81, 1029–1034. [Google Scholar] [CrossRef]
- Price, J.; Heinz, P. A Practical Approach to Joint Pain in Children. Paediatr. Child Health 2022, 32, 43–49. [Google Scholar] [CrossRef]
- Dubois-Ferrière, V.; Belaieff, W.; Lascombes, P.; De Coulon, G.; Ceroni, D. Transient Synovitis of the Hip: Which Investigations Are Truly Useful? Swiss Med. Wkly. 2015, 145, w14176-w14176. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Shin, Y.W.; Chung, C.Y.; Cho, T.-J.; Yoo, W.J.; Lee, D.Y. Surgical Treatment of the Severe Sequelae of Infantile Septic Arthritis of the Hip. Clin. Orthop. Relat. Res. 2005, 434, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rutz, E.; Brunner, R. Septic Arthritis of the Hip-Current Concepts. Hip Int. 2009, 19 (Suppl. 6), 9–12. [Google Scholar] [CrossRef]
- Pääkkönen, M.; Peltola, H. Treatment of Acute Septic Arthritis. Pediatr. Infect. Dis. J. 2013, 32, 684–685. [Google Scholar] [CrossRef]
- Bennett, O.; Namnyak, S. Acute Septic Arthritis of the Hip Joint in Infancy and Childhood. Clin. Orthop. Relat. Res. 1992, 281, 123–132. [Google Scholar] [CrossRef]
- Kaplan, S. Challenges in the Evaluation and Management of Bone and Joint Infections and the Role of New Antibiotics for Gram Positive Infections. Adv. Exp. Med. Biol. 2009, 634, 111–120. [Google Scholar]
- Grill, F.; Rustler, T. Late Sequelae in Septic Arthritis of the Hip in Infants. Orthopade 1997, 26, 848–857. [Google Scholar] [CrossRef]
- Dobbs, M.; Sheridan, J.; Gordon, J.; Corley, C.; SZYMANSKI, D.; Schoenecker, P. Septic Arthritis of the Hip in Infancy: Long-Term Follow-Up. J. Pediatr. Orthop. 2003, 23, 162–168. [Google Scholar] [CrossRef]
- Peters, W.; Irving, J.; Letts, M. Long-Term Effects of Neonatal Bone and Joint Infection on Adjacent Growth Plates. J. Pediatr. Orthop. 1992, 12, 806–810. [Google Scholar] [CrossRef]
- Pääkkönen, M. Septic Arthritis in Children: Diagnosis and Treatment. Pediatr. Health Med. Ther. 2017, 8, 65–68. [Google Scholar] [CrossRef]
- Choi, I.; Pizzutillo, P.; Bowen, J.; Dragann, R.; Malhis, T. Sequelae and Reconstruction after Septic Arthritis of the Hip in Infants. J. Bone Jt. Surg. Am. 1990, 72, 1150–1165. [Google Scholar] [CrossRef]
- Baghdadi, T.; Saberi, S.; Sobhani Eraghi, A.; Arabzadeh, A.; Mardookhpour, S. Late Sequelae of Hip Septic Arthritis in Children. Acta Med. Iran. 2012, 50, 463–467. [Google Scholar] [PubMed]
- Kocher, M.S.; Zurakowski, D.; Kasser, J.R. Differentiating between Septic Arthritis and Transient Synovitis of the Hip in Children: An Evidence-Based Clinical Prediction Algorithm. J. Bone Jt. Surg. Am. 1999, 81, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Mandiga, R.; Murphy, J.; Goldmann, D.; Harper, M.; Sundel, R.; Ecklung, K.; Kasser, J. A Clinical Practice Guideline for Treatment of Septic Arthritis in Children: Efficacy in Improving Process of Care and Effect on Outcome of Septic Arthritis of the Hip. J. Bone Jt. Surg. Am. 2003, 85, 994–999. [Google Scholar] [CrossRef]
- Caird, M.S.; Flynn, J.M.; Leung, Y.L.; Millman, J.E.; D’Italia, J.G.; Dormans, J.P. Factors Distinguishing Septic Arthritis from Transient Synovitis of the Hip in Children. A Prospective Study. J. Bone Jt. Surg. Am. 2006, 88, 1251–1257. [Google Scholar] [CrossRef]
- Kocher, M.S.; Mandiga, R.; Zurakowski, D.; Barnewolt, C.; Kasser, J.R. Validation of a Clinical Prediction Rule for the Differentiation between Septic Arthritis and Transient Synovitis of the Hip in Children. J. Bone Jt. Surg. Am. 2004, 86, 1629–1635. [Google Scholar] [CrossRef]
- Yagupsky, P. Kingella Kingae: From Medical Rarity to an Emerging Paediatric Pathogen. Lancet Infect. Dis. 2004, 4, 358–367. [Google Scholar] [CrossRef]
- Juchler, C.; Spyropoulou, V.; Wagner, N.; Merlini, L.; Dhouib, A.; Manzano, S.; Tabard-Fougère, A.; Samara, E.; Ceroni, D. The Contemporary Bacteriologic Epidemiology of Osteoarticular Infections in Children in Switzerland. J. Pediatr. 2018, 194, 190–196.e1. [Google Scholar] [CrossRef]
- Samara, E.; Spyropoulou, V.; Tabard-Fougère, A.; Merlini, L.; Valaikaite, R.; Dhouib, A.; Manzano, S.; Juchler, C.; Dayer, R.; Ceroni, D. Kingella Kingae and Osteoarticular Infections. Pediatrics 2019, 144, e20191509. [Google Scholar] [CrossRef] [PubMed]
- Coulin, B.; Demarco, G.; Spyropoulou, V.; Juchler, C.; Vendeuvre, T.; Habre, C.; Tabard-Fougère, A.; Dayer, R.; Steiger, C.; Ceroni, D. Osteoarticular Infection in Children. Bone Jt. J. 2021, 103, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Ilharreborde, B.; Bidet, P.; Lorrot, M.; Even, J.; Mariani-Kurkdjian, P.; Liguori, S.; Vitoux, C.; Lefevre, Y.; Doit, C.; Fitoussi, F.; et al. New Real-Time PCR-Based Method for Kingella Kingae DNA Detection: Application to Samples Collected from 89 Children with Acute Arthritis. J. Clin. Microbiol. 2009, 47, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, D.; Dubois-Ferrière, V.; Cherkaoui, A.; Lamah, L.; Renzi, G.; Lascombes, P.; Wilson, B.; Schrenzel, J. 30 Years of Study of Kingella Kingae: Post Tenebras, Lux. Future Microbiol. 2013, 8, 233–245. [Google Scholar] [CrossRef]
- Cochard, B.; De Marco, G.; Bazin, L.; Vazquez, O.; Di Laura Frattura, G.; Steiger, C.N.; Dayer, R.; Ceroni, D. Biological Predictors of Osteoarticular Infection Due to K. Kingae-A Retrospective Cohort Study of 247 Cases. Microorganisms 2023, 11, 2130. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Scheuerman, O.; Chodick, G.; Finkelstein, Y.; Samra, Z.; Garty, B.Z. Invasive Kingella Kingae Infections in Children: Clinical and Laboratory Characteristics. Pediatrics 2008, 122, 1305–1309. [Google Scholar] [CrossRef]
- Yagupsky, P.; Dubnov-Raz, G.; Gené, A.; Ephros, M. Differentiating Kingella Kingae Septic Arthritis of the Hip from Transient Synovitis in Young Children. J. Pediatr. 2014, 165, 985–989.e1. [Google Scholar] [CrossRef]
- Hagedoorn, N.N.; Olijve, L.; Kang, L.; Walls, T.; Davis, J. Comparison of Clinical Prediction Rules in Pre-School Aged Children With Septic Hip Arthritis Due to Different Pathogens. J. Pediatr. Orthop. 2023, 43, E608–E613. [Google Scholar] [CrossRef]
- Valisena, S.; De Marco, G.; Vazquez, O.; Cochard, B.; Steiger, C.; Dayer, R.; Ceroni, D. The Kocher–Caird Criteria for Pediatric Septic Arthritis of the Hip: Time for a Change in the Kingella Era? Microorganisms 2024, 12, 550. [Google Scholar] [CrossRef]
- Coulin, B.; DeMarco, G.; Vazquez, O.; Spyropoulou, V.; Gavira, N.; Vendeuvre, T.; Tabard-Fougère, A.; Dayer, R.; Steiger, C.; Ceroni, D. Osteoarticular Infections in Children: Accurately Distinguishing between MSSA and Kingella Kingae. Microorganisms 2022, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Demarco, G.; Chargui, M.; Coulin, B.; Borner, B.; Steiger, C.; Dayer, R.; Ceroni, D. Kingella Kingae Osteoarticular Infections Approached through the Prism of the Pediatric Orthopedist. Microorganisms 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Dubnov-Raz, G.; Ephros, M.; Garty, B.Z.; Schlesinger, Y.; Maayan-Metzger, A.; Hasson, J.; Kassis, I.; Schwartz-Harari, O.; Yagupsky, P. Invasive Pediatric Kingella Kingae Infections: A Nationwide Collaborative Study. Pediatr. Infect. Dis. J. 2010, 29, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.J.T.; Unkila-Kallio, L.; Aalto, K.; Peltola, H. Serum C-Reactive Protein, Erythrocyte Sedimentation Rate and White Blood Cell Count in Septic Arthritis of Children. Pediatr. Infect. Dis. J. 1997, 16, 411–413. [Google Scholar] [CrossRef]
- Eich, G.F.; Superti-Furga, A.; Umbricht, F.S.; Willi, U.V. The Painful Hip: Evaluation of Criteria for Clinical Decision-Making. Eur. J. Pediatr. 1999, 158, 923–928. [Google Scholar] [CrossRef]
- Rose, S.; Petersen, N.; Gardner, T.; Hamill, R.; Trautner, B. Etiology of Thrombocytosis in a General Medicine Population: Analysis of 801 Cases with Emphasis on Infectious Causes. J. Clin. Med. Res. 2012, 4, 415. [Google Scholar] [CrossRef][Green Version]
- Harrison, C.N.; Bareford, D.; Butt, N.; Campbell, P.; Conneally, E.; Drummond, M.; Erber, W.; Everington, T.; Green, A.R.; Hall, G.W.; et al. Guideline for Investigation and Management of Adults and Children Presenting with a Thrombocytosis. Br. J. Haematol. 2010, 149, 352–375. [Google Scholar] [CrossRef]
- Garoufi, A.; Voutsioti, K.; Tsapra, H.; Karpathios, T.; Zeis, P. Reactive Thrombocytosis in Children with Upper Urinary Tract Infections. Acta Paediatr. 2001, 90, 448–449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, G.; Vazquez, O.; Cochard, B.; Foland, P.; Meinzer, U.; Mallet, C.; Ilharreborde, B.; Haumont, E.; Pejin-Arroyo, Z.; Yagupsky, P.; et al. Inefficiency of Kocher and Caird’s Criteria in Septic Arthritis of the Hip Due to Kingella kingae: A Multicenter Retrospective Cohort Study. Microorganisms 2025, 13, 2323. https://doi.org/10.3390/microorganisms13102323
De Marco G, Vazquez O, Cochard B, Foland P, Meinzer U, Mallet C, Ilharreborde B, Haumont E, Pejin-Arroyo Z, Yagupsky P, et al. Inefficiency of Kocher and Caird’s Criteria in Septic Arthritis of the Hip Due to Kingella kingae: A Multicenter Retrospective Cohort Study. Microorganisms. 2025; 13(10):2323. https://doi.org/10.3390/microorganisms13102323
Chicago/Turabian StyleDe Marco, Giacomo, Oscar Vazquez, Blaise Cochard, Piotr Foland, Ulrich Meinzer, Cindy Mallet, Brice Ilharreborde, Edouard Haumont, Zagorka Pejin-Arroyo, Pablo Yagupsky, and et al. 2025. "Inefficiency of Kocher and Caird’s Criteria in Septic Arthritis of the Hip Due to Kingella kingae: A Multicenter Retrospective Cohort Study" Microorganisms 13, no. 10: 2323. https://doi.org/10.3390/microorganisms13102323
APA StyleDe Marco, G., Vazquez, O., Cochard, B., Foland, P., Meinzer, U., Mallet, C., Ilharreborde, B., Haumont, E., Pejin-Arroyo, Z., Yagupsky, P., Gené, A., Velasco Arnaiz, E., Gouveia, C., Arcangelo, J., Mainard, N., Gravel, J., Walls, T., Hagedoorn, N., Khatami, A., ... Ceroni, D. (2025). Inefficiency of Kocher and Caird’s Criteria in Septic Arthritis of the Hip Due to Kingella kingae: A Multicenter Retrospective Cohort Study. Microorganisms, 13(10), 2323. https://doi.org/10.3390/microorganisms13102323








