Abstract
Plant growth-promoting bacteria (PGPBs) offer a sustainable alternative for enhancing crop productivity in low-fertility tropical soils. In this study, 30 bacterial isolates were screened in vitro for multiple PGP traits, including phosphate solubilization (from aluminum phosphate—AlPO4 and thermophosphate), potassium release from phonolite rock, siderophore production, indole-3-acetic acid (IAA) synthesis, ACC deaminase activity, and antagonism against Fusarium spp. Statistical analysis revealed significant differences (p < 0.05) among the isolates. The most efficient isolates demonstrated a solubilization capacity ranging from 24.0 to 45.2 mg L−1 for thermophosphate and 21.7 to 23.5 mg L−1 for potassium from phonolite. Among them, Pseudomonas azotoformans K22 showed the highest AlPO4 solubilization (16.6 mg L−1). IAA production among the isolates varied widely, from 1.34 to 9.65 µg mL−1. Furthermore, 17 isolates produced carboxylate-type siderophores, and only Pseudomonas aeruginosa SS183 exhibited ACC deaminase activity, coupled with strong antifungal activity (91% inhibition). A composite performance index identified P. azotoformans K22, E. hormaechei SS150, S. sciuri SS120, and B. cereus SS18 and SS17 as the most promising isolates. This study provides a valuable foundation for characterizing plant growth-promoting traits and identifies key candidates for future validation and the development of microbial consortia.
1. Introduction
The continued growth of the global population has driven agricultural production to historically high levels, primarily sustained by the intensive use of agrochemicals. Among these inputs, synthetic fertilizers and pesticides stand out as indispensable pillars of modern agriculture, without which global agricultural production can be reduced by approximately 40–50% [1]. Nitrogen, phosphate, and potassium fertilizers are among the most widely used agricultural inputs, with nitrogen fertilizers accounting for between 55% and 60% of global consumption, followed by phosphate fertilizers at approximately 22% to 25%, and potassium fertilizers, responsible for 18% to 20% [2]. Furthermore, pesticides, including herbicides, insecticides, and fungicides, are essential in reducing losses caused by pests, pathogens, and invasive plants, which can compromise up to 40% of global agricultural production [3].
The intensive use of synthetic fertilizers and pesticides has had significant environmental and economic impacts. The dependence of phosphate and potassium fertilizers on non-renewable natural resources threatens long-term food security. In addition to being depleted, phosphate rock reserves are highly concentrated in a few countries, posing a geopolitical risk. Similarly, potassium deposits are abundant in a few countries, reinforcing the vulnerability of global supply chains [4,5]. Furthermore, nutrient leaching contaminates aquifers and water bodies, leading to eutrophication and, consequently, creating dead zones in aquatic ecosystems [6,7]. Synthetic pesticides, when poorly managed, affect non-target species, including indispensable pollinators such as bees, and compromise soil health by reducing microbial biodiversity [8,9,10].
Therefore, there is an urgent need for sustainable alternatives that allow diversification of nutrient sources and an increase in their efficiency. Most studies on the microbial solubilization of insoluble phosphates have evaluated calcium phosphates, such as hydroxyapatite and tricalcium phosphate, which are predominant in temperate soils; however, the results obtained from this approach have limited applicability in tropical soils [11]. In these environments, low phosphorus availability is strongly related to fixation in iron and aluminum oxides, with aluminum phosphate (AlPO4) being one of the least soluble and bioavailable forms, which remain little explored in research [12]. Furthermore, alternative sources of phosphate fertilizers, such as thermophosphate, offer gradual phosphorus release and reduce losses through fixation and leaching [13,14]. Similarly, a potassic rock abundant in Brazil, phonolite, has emerged as a strategic alternative for reducing external dependence on potassium and enhancing local resources [15,16].
Assessing the ability of native microorganisms to solubilize these mineral sources is an essential step toward developing bioinoculants adapted to tropical soil and climatic conditions [17]. In this context, plant growth-promoting bacteria (PGPBs) have emerged as promising alternatives for promoting agricultural sustainability [18]. These beneficial microorganisms contribute significantly to nutrient cycling, including the mineralization of organic matter, phosphate solubilization, potassium availability, biological nitrogen fixation, and the availability of micronutrients, such as iron, through siderophore production [19,20,21]. Furthermore, they can modulate plant development through the production of phytohormones, especially auxins [19,22], and contribute to tolerance to abiotic stresses through the production of ACC-deaminase, an enzyme that degrades the ethylene precursor (ACC), thereby preventing excessive accumulation of this hormone associated with stress responses [23].
In addition to promoting plant growth, PGPBs play a crucial role in the biological control of pathogens by acting as natural suppressors through multiple mechanisms. These microorganisms can inhibit the development of phytopathogenic fungi, bacteria, and nematodes through the production of antimicrobial compounds (such as antibiotics and lytic enzymes), competition for niches and nutrients, and induction of systemic resistance (ISR) in plants [24]. This multifaceted antagonistic activity is particularly valuable against harmful soil-borne pathogens such as Fusarium species, which cause devastating wilts, rots, and blights in numerous economically important crops. For example, Fusarium oxysporum f. sp. lycopersici causes Fusarium wilt, a disease that can lead to substantial economic losses in tomato production globally [25]. Furthermore, Fusarium species contaminate grains and food products with hazardous mycotoxins, such as fumonisins and deoxynivalenol (DON). These toxins pose a serious risk to food safety and can cause various health issues in humans and livestock, including immunosuppression and esophageal cancer, highlighting the critical need for effective biocontrol strategies [25].
Considering the challenges posed by tropical soils, which are often characterized by high acidity, high levels of toxic aluminum, and low availability of phosphorus and potassium, bioprospecting native PGPBs has become a priority. These microorganisms, which are naturally adapted to such conditions, represent a solid basis for the development of multifunctional bioinoculants capable of optimizing the use of available mineral sources in the soil, modulating plant growth through phytohormone synthesis, increasing tolerance to abiotic stresses, and contributing to the biological control of pathogens [17]. These characteristics position PGPBs as strategic tools for promoting more sustainable agricultural systems, aligning with the recommendations of the Intergovernmental Panel on Climate Change (IPCC) for climate change mitigation and adaptation [26]. Therefore, investing in exploring tropical microbiota transcends the scope of scientific opportunity; it is consolidated as a pressing need to advance toward truly resilient and innovative tropical agriculture. However, translating this potential into practical applications requires a systematic screening of native microorganisms for key agronomic traits. Therefore, this study aimed to evaluate the potential of native bacteria in relation to attributes, such as nutrient solubilization, siderophore production, phytohormone synthesis, ACC deaminase synthesis, and antagonistic activity against phytopathogens, thereby generating useful information to support future research to develop bioinoculants for tropical systems.
2. Materials and Methods
2.1. Origin of Bacterial Isolates
This study used 30 bacterial strains deposited in the Laboratory of Bacteriology and Molecular Biology of the Federal Rural University of Rio de Janeiro. Of these, 26 were isolated from the rhizospheres of plants naturally established in saline soils in the state of Rio de Janeiro, Brazil [27]. These isolates were selected based on their ability to adapt to adverse environmental conditions, a characteristic considered relevant for developing microbial inoculants. Additionally, four bacterial strains of different origins were included to expand the taxonomic and functional diversity of the study set. One Bacillus pumilus isolate was obtained from the Guandu River in the metropolitan region of Rio de Janeiro. An isolate of Pseudomonas azotroformans was obtained from the Biofertilizer PotaBARVAR-2, produced by Green Biotech Incorporation (Balehonnur, India), and formulated with a focus on potassium solubilization. The Bacillus thiuringiensis isolate was obtained from the commercial product Vectobac (Kirchhoffs, Cape Town, South Africa). The isolate Bacillus altitudinis complex CCT 3116 was acquired from the Culture Collection of the André Tosello Tropical Research and Technology Foundation, as it is a well-studied strain recognized for its biological properties.
2.2. Bacterial Identification by MALDI-TOF Mass Spectrometry
After reactivation on Tryptone Soy Broth (TSB) agar at 30 °C for 24 h, bacterial cultures were transferred to a 96-well microplate (MSP, Bruker, Billerica, MA, USA). A lysis solution comprising 70% formic acid (Sigma-Aldrich®, St. Louis, MO, USA) was added to the bacterial pellet in a volume sufficient to fully cover it. Subsequently, 1 μL of matrix solution comprising alpha-cyano-4-hydroxycinnamic acid diluted in 50% acetonitrile and 2.5% trifluoroacetic acid (Sigma-Aldrich®) was applied to the bacterial extract. Mass spectra were acquired using a MALDI-TOF LT Microflex mass spectrometer (Bruker®, Billerica, MA, USA), operating in linear mode with a 337 nm nitrogen laser, and controlled by the FlexControl software version 3.3 (Bruker®). Spectra were collected in the mass range of 2000–20,000 m/s and subsequently analyzed using the MALDI Biotyper 2.0 program (Bruker®), with standardized settings for bacterial identification. The program compares unknown sample spectra with reference samples in a database.
Values greater than 2.0 indicate high identification reliability. In the range of 1.7 to 1.99, the identification reliability was low. Values below 1.7 indicate no identification reliability.
2.3. Inoculum Preparation
Bacterial isolates were grown on nutrient agar plates (HiMedia, Mumbai, India) and incubated at 30 °C for 24 h. A single colony of each strain was inoculated into test tubes containing 9 mL of TSB (Kasvi, Curitiba, Brazil). The cultures were incubated at 30 °C for 24 h on an orbital shaker at 150 rpm. Then, the absorbance of each culture was measured using a spectrophotometer at 600 nm and adjusted to values between 0.9 and 1.0 by dilution in sterile TSB.
2.4. Solubilization of Phosphate Sources
The isolates were prepared as described in Section 2.3. After this step, 300 µL of each bacterial culture was inoculated into 50 mL Falcon tubes containing 35 mL of NBRIP medium (National Botanic Research Institute’s phosphate) (10 g·L−1 glucose; 0.15 g·L−1 (NH4)2SO4; 0.2 g·L−1 KCl; 5 g·L−1 MgCl2·6H2O; 0.25 g·L−1 MgSO4·7H2O; phosphate source) supplemented with 5 g·L−1 of aluminum phosphate (AlPO4) or 13 g·L−1 of thermophosphate (Yoorin Fertilizers, São Paulo, Brazil), with an initial pH of 7.0, and maintained under stirring at 150 rpm for 7 d [28]. The tests were performed in triplicate.
A 10 mL aliquot of each sample unit was transferred to 15 mL Falcon tubes and centrifuged at 6000 × g for 10 min. The supernatant was filtered using a 0.22-µm membrane syringe filter to remove bacterial cells from the medium. The filtrate was used to determine the pH and diluted as needed to determine the soluble phosphorus. The soluble phosphorus concentration was assessed using the method described by Teixeira et al. [29]. Finally, the OD was measured by spectrophotometry at 660 nm. Control flasks containing uninoculated culture medium were used. Values for inoculated treatments were corrected by subtracting the values of their respective uninoculated controls. The standard curve was generated as described by Teixeira et al. [29]. The Gluconacetobacter diazotrophicus (BR-11281) strain was used as a positive control [30]. This strain was kindly provided by EMBRAPA Agrobiologia (Seropedica, Brazil).
2.5. Potassium Solubilization
The isolates were prepared as described in Section 2.3. After this step, 300 µL of each culture was transferred to 15 mL Falcon tubes containing 10 mL of modified Aleksandrov medium (5 g·L−1 glucose; 0.5 g·L−1 MgSO4.7H2O; 0.005 g·L−1 FeCl3; 0.1 g·L−1 CaCO3; 2.72 g·L−1 Na2 HPO4), supplemented with 2 g·L−1 potassium aluminum silicate (phonolite), with an initial pH of 7.0 [31]. The tubes were incubated at 30 °C and 150 rpm for 7 d. The tests were performed in triplicate.
Subsequently, the samples were centrifuged at 6000× g for 10 min. The supernatant was used to determine the pH and the amount of soluble potassium released into the solution. Flame photometry was used to determine the potassium content of the samples. Control flasks containing uninoculated culture medium were used. Values obtained from the uninoculated controls were subtracted from those of the respective inoculated treatments. The standard curve was prepared with the 1000 mg·L−1 K + standard solution with distilled water, as Silva [32] described.
The phonolite, whose main chemical composition is characterized by the major oxides 52.7% SiO2, 20.0% Al2O3, 8.1% K2O, 7.9% Na2O, 4.0% Fe2O3, 1.3% CaO, and 0.34% MgO, was subjected to comminution in a jaw crusher. This process aimed to reduce the particle size and increase the surface area of the material according to the methodology described by Dias [33].
2.6. Siderophore Production
Siderophore production was evaluated as described by Schwyn and Neilands [34]. The inocula were prepared as described in Section 2.3. After this step, 7 µL of each culture was inoculated into Petri dishes containing solid King B medium (3.0 g·L−1 glycerol; 4.0 g·L−1 bacteriological peptone; 0.23 g·L−1 K2HPO4; 0.30 g·L−1 MgSO4·7H2O; 15.0 g·L−1 Noble agar), adjusted to pH 6.8. The plates were incubated at 30 °C for 24 h. The chromium reagent Azurol S (CAS) was prepared as described below using a combination of three solutions:
Solution I: 60.5 mg of Chrome Azurol S (CAS) dissolved in 50 mL of distilled water.
Solution II: 27 mg of FeCl3·6H2O dissolved in 10 mL of 10 mM HCl.
Solution III: 72.9 mg of hexadecyltrimethylammonium bromide (CTAB) was dissolved in 40 mL of distilled water.
Initially, Solution I was mixed with 9 mL of Solution II. This mixture was then combined with Solution III, resulting in a blue solution. The CAS solution was then autoclaved at 110 °C for 10 min. The sterile CAS solution was added to King B culture medium at approximately 55 °C to prepare the assay medium in a 1:10 (v/v) ratio.
After incubation, siderophore production was determined by observing the characteristic color halos around the colonies which were classified as follows: catechol, transition from blue to pink; hydroxamate, transition from blue to orange; and carboxylate, transition from blue to light yellow [35].
2.7. Production of Indole-3-Acetic Acid (IAA)
Salkowski colorimetric assay was used to quantitatively analyze IAA [36]. Bacterial isolates were prepared as described in Section 2.3. Subsequently, a 50 μL aliquot of the culture was inoculated in TSB medium supplemented with 1 g·L−1 of L-tryptophan (Sigma-Aldrich, St. Louis, MO, USA), and the tubes were incubated for 72 h at 30 °C at 150 rpm. The test was performed in triplicate. Samples (2 mL) were collected, and the cells were centrifuged (13,000 × g, 5 min). One milliliter of the resulting supernatants was mixed with 2 mL of Salkowski reagent (1 mL of 0.5 M FeCl3 in 50 mL of 35% (v/v) HClO4) and incubated at 28 °C in the dark for 30 min. The absorbance was measured using a spectrophotometer at 540 nm. A standard curve was constructed as described by Lana et al. [37]. Azospirillum brasilense strain (BR-11001) kindly provided by EMBRAPA Agrobiologia, was used as the positive control [38].
2.8. Production of ACC-Deaminase
Bacterial isolates were prepared as described in Section 2.3. The isolates were then centrifuged at 3000× g for 5 min. The pellet was washed twice with a 0.1 M solution. The pellet was resuspended in 1 mL of 0.1 M Tris-HCl and 2 μL was inoculated in DF medium (2.0 g·L−1 glucose; 2.0 g·L−1 citric acid; 4.0 g·L−1 KH2·PO4; 6.0 g·L−1 Na2·HPO4; 0.2 g·L−1 MgSO4·7H2O; 20 g·L−1 agar) pH 7.2 [39].
DF medium with a nitrogen source (supplemented with 2 g·L−1 (NH4)2SO4) was used as a negative control, and DF medium without a nitrogen source was used. To evaluate the ACC-deaminase production capacity, DF medium without a nitrogen source was used, applying 120 μL of a 0.5-M ACC-deaminase solution to the surface of the plate. The plates were incubated at 30 °C for 7 d. Isolates that grew well in medium without nitrogen and with ACC deaminase solution were considered positive. All experiments were performed in triplicate. The Herbaspirillum seropedicae strain (BR-11790) provided by EMBRAPA Agrobiologia was used as the positive control [40].
2.9. Antagonist Activity
The bacterial isolates, prepared as described in Section 2.3, were evaluated for antagonistic activity against the phytopathogenic fungus Fusarium oxysporum f. sp. lycopersici (L3304), obtained from the Seed Epidemiology Laboratory of the Federal Rural University of Rio de Janeiro. The Fusarium sp. isolate was cultured on Potato Dextrose Agar (PDA) medium (HiMedia, Mumbai, India) for 7 d at 28 °C. PDA was selected for its optimal support of fungal mycelial growth, ensuring robust fungal biomass for antagonism assays.
Antagonism was determined using the dual-culture containment technique following the methodology of Fernandes et al. [41]. The bacterial strains were plated on PDA, forming four parallel strips spaced 40 mm apart. A 7 mm diameter disk of the culture medium containing the fungal mycelium was then placed in the center of the plate, perpendicular to and equidistant from the bacterial strips.
The plates were incubated at room temperature for 15 d. The experimental design included control plates consisting of fungal disks incubated in the absence of bacteria. Antagonistic activity was interpreted by analyzing colony area and percentage inhibition. The fungal colony area was obtained by measuring radial growth along the orthogonal axes. The mean of these two measurements was calculated and assumed as the radius (r) for area calculation using the formula πr2. The results are expressed in mm2. The percentage inhibition was determined as the ratio of the pathogen growth area in the presence of each bacterium to the area occupied by the pathogen in the control treatment. Using these values, the percentage inhibition (% In) was calculated using the following formula adapted from Fernandes et al. [41]:
Based on the percentage inhibition values, bacterial isolates were classified into three levels of antagonism: low (<40%), moderate (40–80%), and high (>80%).
2.10. Statistical Analysis
The experiment was conducted using a completely randomized design. All statistical analyses were performed using R Core Team (2024) software. The normality of residuals was verified using the Shapiro–Wilk test, and the homoscedasticity of variances was verified using the Bartlett test. Data transformation was performed through automatic selection using the BestNormalize function in R software (version 4.4.2; R Core Team, 2024) when necessary. The transformed data were subjected to assumption verification. Subsequently, analysis of variance was applied, and when significant by the F test (p < 0.05), multiple comparisons of means were performed using the Scott-Knott cluster test at 5% probability.
The mean value of each variable was calculated per isolate and standardized using the Z-score method (mean = 0, standard deviation = 1). A composite index was constructed from this standardization by averaging the Z-scores and used to rank the isolates. For multidimensional comparative analysis, the mean data for each variable were normalized to a 0–1 (min–max) scale and used to construct the radar plots. Five isolates with the highest composite indices were selected, and the radar plots represented their profiles.
3. Results
Of the 30 bacterial isolates, 25 (83.3%) were identified with high reliability by the MALDI-TOF technique, presenting scores higher than 2.00 (Table 1). Three isolates (Bp2, K22, and SS120) were identified with intermediate reliability scores between 1.70 and 1.99. One isolate (SS11) did not reach a reliable level of identification, with a score lower than 1.70. The isolates from reference culture collections CCT 3116 (Bacillus altitudini), BR11281 (Gluconacetobacter diazotrophicus), BR11001 (Azospirillum brasilense), and BR11790 (Herbaspirillum seropedicae) were not subjected to re-identification by MALDI-TOF, as their taxonomic identities had been previously validated and maintained under strict quality control by the institutions of origin.

Table 1.
Bacterial species identified by MALDI-TOF MS and their respective identification scores.
Regarding the solubilization of phosphorus from aluminum phosphate (AlPO4), the bacterial isolates showed statistically significant differences. The Pseudomonas azotoformans isolate (K22) demonstrated remarkable performance, reaching 16.5 mg·L−1. Despite this high value, its performance was statistically inferior to that of the positive control, Gluconacetobacter diazotrophicus (BR11281), which solubilized 50.6 mg·L−1. In the thermophosphate evaluation, significant differences in solubilization capacity were observed between the isolates. Bacillus isolates cereus (SS18, SS17, SS80, SS68, SS36, SS101, SS29, SS89), Bacillus altitudinis (CCT 3116), and Pseudomonas azotoformans (K22) stood out with the highest solubilization values, ranging from 24.0 to 45.2 mg·L−1. All isolates performed better than the positive control, which solubilized 19.9 mg·L−1. Regarding potassium availability from phonolites, the highest solubilization was observed in the Enterobacter sp. SS11 (23.5 mg·L−1) and SS186 (23.5 mg·L−1), followed by Pseudomonas aeruginosa sp. SS183, Pantoea sp. SS150, Enterobacter hormaechei SS15, and Staphylococcus sciuri SS120, ranging from 22.8 mg·L−1 to 21.7 mg·L−1 (Figure 1).
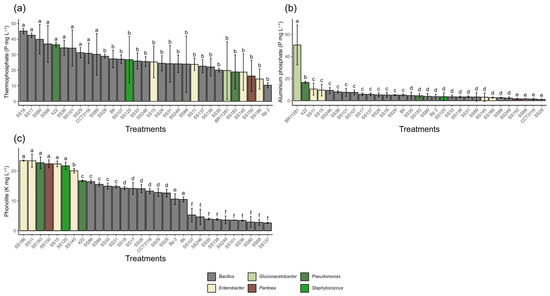
Figure 1.
Solubilization of different mineral sources by different bacterial isolates after 7 days of incubation. (a) Phosphorus solubilization from thermophosphate. (b) Phosphorus solubilization from aluminum phosphate (AlPO4). (c) Potassium solubilization from phonolite. All values are expressed in mg L−1. Means followed by the same letter do not differ statistically from each other according to the Scott-Knott test at 5% probability.
All isolates promoted marked acidification of the medium with AlPO4 after seven days of incubation, with a final pH ranging from 3.5 to 5.2. Culture media inoculated with nine isolates had a final pH between 3.5 and 3.9, including SS11 and SS89 (both at pH 3.5). Culture media inoculated with the remaining 19 isolates had a final pH of 4.0 and 5.0. Despite this acidification, the correlation between pH and solubilization was very weak and not significant (R = −0.173; p = 0.097), suggesting that other mechanisms may be more relevant in the AlPO4 solubilization process. In the medium supplemented with thermophosphate, the final pH ranged from 5.0 to 8.6. Most isolates (13/30) acidified the medium, presenting a pH between 5.0 and 6.0, while 12 maintained the pH range between 6.0 and 7.0. Only three isolates remained close to the initial pH of the culture medium (7.0), and two exhibited results higher than the initial pH, including Enterobacter sp. (SS11, pH 8.6) and Bacillus cereus (SS89, pH 8.3). The correlation between pH and phosphorus solubilization from thermophosphate was moderately negative and statistically significant (R = −0.541; p < 0.000001), indicating that acidification favored the release of phosphorus from this source. In the media containing phonolite, the final pH ranged from 4.2 to 6.4, with the media inoculated with most isolates (19/30) maintaining a pH close to 5.0, eight resulting in a pH drop to near 4.0, and three maintaining a pH close to the initial value, such as Bacillus cereus (SS101, pH 6.4) and Enterobacter hormaechei (SS145, pH 6.3). The correlation between acidification and solubilization was moderately positive and highly significant (R = 0.391; p = 0.0003), confirming that pH reduction favors K release from phonolites (Figure 2).
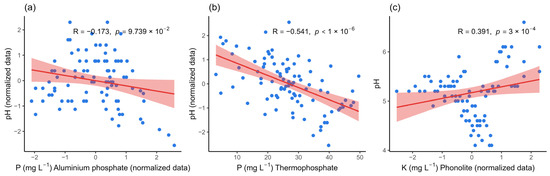
Figure 2.
Correlation between pH values and solubilization of mineral sources by bacterial isolates after 7 days of incubation. (a) Correlation between culture medium pH and the solubilization of aluminum phosphate, (b) thermophosphate, and (c) potassium. The blue points represent the observations. The red line indicates the linear trend, and the pink band represents the 95% confidence interval for that trend. R is the Pearson correlation coefficient, and p is the p-value for the two-tailed test. p-value less than 0.05 indicate statistically significant correlations.
Siderophore production: 17 isolates produced carboxylate-type siderophores, whereas the others did not produce this molecule or any other type of siderophore (Table 2). Regarding ACC deaminase activity, only Pseudomonas aeruginosa (SS183) tested positive, whereas all other isolates tested negative (Table S1—Supplementary Material).

Table 2.
Siderophore production by bacterial isolates.
Pseudomonas azotoformans K22 and Staphylococcus sciuri SS120 stood out in IAA production (Figure 3), with 8.94 µg·mL−1 and 9.65 µg·mL−1, respectively. Still, these values were statistically lower than those of the positive control Azospirillum brasilense (BR11001), which produced 29.4 µg·mL−1, indicating that the reference strain has greater metabolic efficiency in synthesizing the phytohormone. In contrast, Bacillus cereus SS88, SS26, and SS31 showed very low production (1.56 µg·mL−1 to 1.43 µg·mL−1), suggesting limited capabilities in this plant growth promotion mechanism.
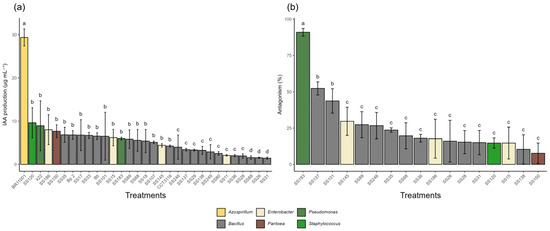
Figure 3.
Indoleacetic acid (IAA) production and antagonistic activity by bacterial isolates. (a) IAA production (µg·mL−1) by bacterial isolates grown in tryptophan-supplemented medium. (b) Antagonistic activity (% inhibition) against Fusarium spp. Isolates that did not show antagonistic activity (% inhibition = 0%) are not represented in the graph. Different letters above the bars indicate statistical differences by the Scott-Knott test at 5% probability.
Regarding antagonistic activity against the phytopathogenic fungus Fusarium spp., the isolate Pseudomonas aeruginosa (SS183) showed the greatest inhibitory effect, with 91% inhibition of mycelial growth (Figure 3). Two isolates of Bacillus cereus (SS101 and SS137) exhibited moderate activity with 44% and 52%, respectively. The remaining isolates showed low inhibition percentages, less than 20%, with nine isolates showing no inhibitory effect.
Analysis of the 30 bacterial isolates based on the composite performance index (average standardized Z-scores) revealed differences in the functional potential of the evaluated strains (Figure 4). This index integrated key variables related to plant growth promotion and biocontrol, including antagonism, indole acetic acid (IAA) production, aluminum phosphate solubilization (P_AL), thermophosphate solubilization (P_T), and potassium solubilization (K). The ranking obtained allowed us to identify isolates with superior performance, highlighting the isolates of P. azotoformans K22, E. hormaechei SS150, and S. sciuri SS120, and two isolates of B. cereus SS18 and SS17, which presented the highest positive index values, suggesting that they are promising candidates for future biotechnological applications. Notably, owing to their qualitative nature, siderophore production and ACC deaminase attributes were not included in previous quantitative statistical analyses. However, we observed that, among the five isolates with superior performance, P. azotoformans K22 and two B. cereus isolates, SS18 and SS17, showed siderophore (carboxylate) production.
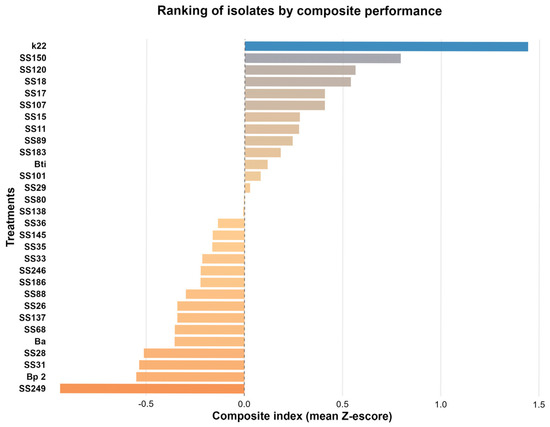
Figure 4.
Ranking of bacterial isolates based on the composite performance index. The composite index was calculated by standardizing (Z-score method) the mean values of all analyzed variables (P solubilization from aluminum phosphate, P solubilization from thermophosphate, potassium solubilization from phonolite, indoleacetic acid production, and antagonist activity). The final index corresponds to the mean Z-score of each isolate, allowing the ordering of treatments according to their multifunctional performance. Isolates are organized from highest (top) to lowest (bottom) composite performance. The colors represent the relative values of the composite performance index. A divergent color scale was applied (orange–beige–blue), where warmer colors (orange) indicate isolates with lower index values, beige indicates values close to the median, and blue indicates isolates with higher index values.
The five isolates that presented the best results in the evaluated growth-promoting attributes were compared using a radar chart highlighting their specific functional characteristics (Figure 5). The P. azotoformans K22 isolate presented the highest composite index, emphasizing IAA production and phosphorus solubilization in both sources analyzed. The E. hormaechei SS150 and S. sciuri SS120 isolates showed balanced performance across all metrics, with consistent values across all variables analyzed. The isolate B. cereus SS18 excelled in K availability, whereas B. cereus SS17 had an intermediate profile. The shape of the polygons in the radar plot indicated that these isolates had significant peaks, but with a notable contribution to antagonism. The polygon configuration in the graph highlights that these isolates have complementary functional potential, reinforcing their viability for use in bacterial consortium formulations capable of integrating multiple mechanisms for plant growth promotion and biocontrol.
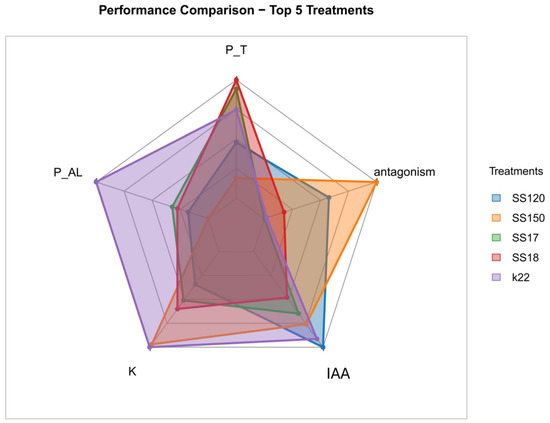
Figure 5.
Multivariate comparative analysis of the five bacterial isolates with the highest composite performance indices. The variables represented are P solubilization from aluminum phosphate (P_AL), P solubilization from thermophosphate (P_T), K solubilization from phonolite (K), indole-3-acetic acid (IAA) production, and antagonist activity.
4. Discussion
Bacterial identification using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry has become a fast, reliable, and low-cost method for routine microbiology laboratories with revolutionary application in microbial taxonomy [42]. The high scores obtained in this study, especially for isolates of the Bacillus cereus complex, corroborate the robustness of this technique for discriminating between morphologically and genetically complex groups [43].
The functional attribute analyses of the studied bacteria revealed remarkable diversity in the metabolic capacity of the isolates, reinforcing that efficiency is a strain-dependent characteristic. In the solubilization of P from AlPO4, the P. azotoformans isolate K22 showed significant solubilization of P from AlPO4. This finding is strategic in tropical soils, characterized by high Al3+ contents and low P availability, where the solubilization of compounds such as AlPO4 is essential for agricultural sustainability. The absence of a linear correlation between acidification and solubilization suggests the participation of other complementary mechanisms in phosphorus solubilization in addition to the production of organic acids, such as the production of exopolysaccharides (EPS), which act synergistically to facilitate the release of adsorbed phosphate [11,19].
In the solubilization of P from thermophosphate, the results demonstrate that several bacteria, particularly strains of B. cereus (SS17, SS18, SS68), P. azotoformans (K22), and Enterobacter sp. (SS11), exhibited high solubilization capacity, releasing significant amounts of phosphorus (P) while modulating the pH of the medium. This process enhances the agronomic advantages of thermophosphate as a slow-release fertilizer, complementing its gradual dissolution and reducing losses because of fixation in highly weathered tropical soils [44]. Thus, the interaction between alternative fertilizers and solubilizing microorganisms can increase phosphorus use efficiency, reduce applied doses, and contribute to more sustainable agricultural practices.
Regarding potassium release from phonolites, the isolates of E. hormaechei (SS186) and P. aeruginosa (SS183) showed high solubilization capacities, which were positively correlated with environmental acidification. Phonolite has been studied as a strategic option to reduce dependence on conventional soluble fertilizers, such as potassium chloride (KCl). Research has shown that phonolites can gradually release their nutrients when subjected to fine grinding or microbial activation processes [45,46,47].
The production of IAA by some investigated bacterial strains, particularly S. sciuri (SS120) and P. azotoformans (K22), was comparable to that reported for commercial strains of Azospirillum brasilense, as documented by Fukami et al. [48], demonstrating remarkable potential for agricultural applications. IAA plays crucial roles in plant development by regulating cell elongation and lateral and adventitious root formation and mediating responses to environmental stresses. However, excessive IAA concentrations can have phytotoxic effects, highlighting the need to evaluate physiologically relevant doses in plant experiments [49]. Recent studies have explored potential applications of IAA-producing bacteria in agriculture [50]. Alori and Babalola (2017) reviewed the use of microbial inoculants to improve crop quality and health in Africa and highlighted the importance of microbial auxins [51]. Fukami et al. [48,49] demonstrate that the benefits of Azospirillum go far beyond biological nitrogen fixation, including significant contributions through the production of phytohormones.
The detection of the ACC deaminase enzyme only in Pseudomonas aeruginosa (SS183) deserves attention because this mechanism is recognized as central to mitigating biotic and abiotic stresses in plants. This enzyme degrades 1-aminocyclopropane-1-carboxylic acid (ACC), the immediate precursor of ethylene, and negatively regulates its synthesis under stress conditions. Bacterial ACC deaminase activity reduces ACC levels, decreases ethylene production, and allows plants to grow even under adverse conditions [19,52].
Producing carboxylate-type siderophores by 17 isolates reflects a fundamental adaptive strategy in tropical soils, where low ferric iron (Fe3+) availability is a limited factor. Rhizospheric bacteria use these high-affinity chelators to solubilize and sequester iron, making it bioavailable. Notably, the genus Pseudomonas is a ubiquitous and efficient producer of pyoverdine-type siderophores, mixed-structure carboxylates whose biosynthesis is tightly regulated by iron deficiency and confers a crucial competitive advantage in oligotrophic environments, as detailed in Schalk et al.’s review [53]. This mechanism of “war by iron” (iron warfare) is a potent means of biocontrol, where deprivation of this essential nutrient directly suppresses the growth of fungal pathogens [53,54].
In antagonism toward Fusarium sp., the high inhibition rates observed for P. aeruginosa SS183 and, to a lesser extent, for Bacillus cereus SS137 confirm the potential of these strains as biocontrol agents. While Pseudomonas is recognized for the production of antifungal metabolites such as phenazines and pyocyanin [55], Bacillus species stand out for their arsenal of lipopeptides and hydrolytic enzymes with proven action against filamentous fungi [56,57]
Fusarium species are responsible for many diseases, such as vascular wilt and root rot, which result in significant global agricultural losses of essential crops. Furthermore, their ability to produce mycotoxins poses a serious risk to human and animal health [25]. Historical reliance on chemical control has proven increasingly unsustainable. The emergence of Fusarium spp. populations resistant to important fungicide classes such as triazoles is a widespread and documented problem that compromises control effectiveness [58]. This resistance emerges through an evolutionary process, driven by intense selective pressure from the repeated application of fungicides. This issue is further exacerbated by practices such as sublethal dosing and a lack of rotation among fungicide classes, ultimately leading to widespread control failures, rising production costs, and increased environmental damage [59].
Additionally, growing environmental and food safety concerns related to pesticide residues drive the global demand for safer and more sustainable alternatives within sustainable agriculture. Biological control is a fundamental pillar of Integrated Disease Management (IDM) [60]. Our results position the isolate Pseudomonas aeruginosa SS183 as a promising biocontrol candidate. The inhibition rate of 91% was remarkable and was in line with the best results reported for antagonistic strains of this genus [61].
In the present study, integrated statistical analysis using a composite index (average Z-score) identified five isolates with superior overall performance, each exhibiting distinct and complementary combinations of plant growth-promoting attributes. Pseudomonas azotoformans K22 demonstrated significant solubilization capacity for multiple phosphate and potassium sources (36.5 mg·L−1 thermophosphate, 16.6 mg·L−1 aluminum phosphate, and 16.7 mg·L−1 phonolite), associated with the production of IAA (8.94 mg·L−1) and carboxylate-type siderophore. However, it did not exhibit antagonist or ACC-deaminase activity. Similarly, Bacillus cereus SS17 stood out in the solubilization of thermophosphate (42.5 mg·L−1) and phonolite (14.2 mg·L−1), in addition to producing IAA (6.79 mg·L−1) and siderophore, but with low antagonist activity (5%) and absence of ACC-deaminase. The isolate B. cereus SS18 was the most efficient in the solubilization of thermophosphate (45.2 mg·L−1), although with moderate performance in the other sources and production of IAA (6.76 mg·L−1), but also lacking other attributes. Staphylococcus sciuri SS120 stood out as the largest producer of IAA (9.65 mg·L−1) and in the solubilization of phonolite (21.7 mg·L−1). In comparison, Enterobacter hormaechei SS150 exhibited the highest efficiency for phonolite (22.4 mg·L−1), although it had low performance on aluminum phosphate (0.8 mg·L−1), and intermediate IAA production (7.68 mg·L−1). Neither strain produced ACC-deaminase or siderophores and had low antagonist activity (15% and 8%, respectively). This analysis demonstrated that although no isolate individually presented excellence in all evaluated attributes, selection based on the composite index identified those with the most balanced and complementary combination of characteristics. These isolates compensated for specific deficiencies with high efficiency in key mechanisms of plant growth promotion. This approach revealed that integrated performance constitutes the most appropriate criterion for selecting microorganisms with potential applications as inoculants, as it reflects the multifactorial nature inherent in plant growth-promotion processes in real environments.
Considering the complementary nature of these attributes among isolates, strategies such as forming bacterial consortia and synergistic combinations between different genera or species have emerged as promising alternatives to overcome individual limitations [62,63,64,65]. Previous studies have demonstrated that consortia involving Azospirillum brasilense and Pseudomonas fluorescens promote synergistic positive effects on maize growth [64], just as microbial production of IAA can enhance other growth-promoting mechanisms [65].
This diverse functional profile represents strategic potential for developing bioinoculants adapted to the edaphoclimatic conditions of tropical soils. This perspective aligns with the targets of Sustainable Development Goal (SDG) 2 (Zero Hunger), which promotes more productive and nutrient-efficient agricultural systems, and SDG 13 (Climate Action), which contributes to reducing dependence on chemical fertilizers with a high environmental footprint [66,67].
Despite advances in the study of plant growth-promoting bacteria, it is important to recognize that in vitro assays often fail to capture the complexity of rhizosphere interactions under natural conditions. Factors such as microbial competition, soil variability, and the influence of the native microbiome can limit the efficacy observed in the laboratory [68]. Therefore, translating these promising findings into practical applications requires additional studies integrating agronomic efficiency, sustainability, and ecosystem resilience to develop robust and environmentally friendly microbial solutions.
5. Conclusions
This study shows that the analyzed bacteria produce plant growth attributes ranging from nutrient solubilization to hormonal modulation and biological control of pathogens. Of particular note are Pseudomonas azotoformans (K22), Enterobacter hormaechei SS150, Staphylococcus sciuri SS120, and two Bacillus cereus isolates (SS18 and SS17), which demonstrated the most balanced and complementary profiles of attributes in relation to the characteristics tested. Additionally, the SS183 strain of Pseudomonas aeruginosa, owing to its production of the ACC deaminase enzyme, an exclusive attribute in this study, and its high antagonistic capacity against the phytopathogen Fusarium sp., exhibit characteristics that, although not present in the isolates with the best overall performance, represent valuable functionalities for the composition of synergistic microbial consortia.
Future work should explore the synergy between these lineages in planta experiments and investigate the genes underlying their functional capabilities, paving the way for the bioengineering of even more efficient microorganisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13102321/s1, Table S1: Code, taxonomic identification (by MALDI-TOF MS), score values, and functional characterization of bacterial isolates. Traits include solubilization of thermophosphate, aluminum phosphate, and phonolite (expressed as released P or K in mg·L−1, with corresponding pH); auxin production (µg·mL−1); ACC deaminase activity (presence indicated by +); siderophore production and type (e.g., carboxylate); and antagonistic activity against phytopathogens (percentage of inhibition).
Author Contributions
Conceptualization, J.F.N., I.S.C. and E.Z.; methodology, J.F.N.; validation, J.F.N., M.S.R.A.d.S. and I.S.C.; formal analysis, M.S.R.A.d.S.; investigation, J.F.N., N.F.R.d.O., C.R.d.S., F.S.A. and B.A.T.d.L.; resources, I.S.C. and E.Z.; data curation, J.F.N., M.S.R.A.d.S. and I.S.C.; writing—original draft preparation, J.F.N.; writing—review and editing, J.F.N. and I.S.C.; visualization, J.F.N.; supervision, I.S.C., E.Z.; project administration, I.S.C., E.Z.; funding acquisition, I.S.C. and E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant numbers 140689/2023-0; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Finance Code 001; and by the MAI DAI Agribio Program (Process: 23083.012439/2021-41).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in Zenodo at https://doi.org/10.5281/zenodo.17020394.
Acknowledgments
The authors would like to thank Agribio for its financial support, scholarship, and logistical assistance during the development of this research. We would also like to thank the Integrated Microbiology Laboratory (LIM) of the Paulo Góes Microbiology Institute at the Federal University of Rio de Janeiro (UFRJ) for performing bacterial identification analyses using MALDI-TOF mass spectrometry.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- International Fertilizer Association. Fertilizer Outlook 2022–2026; IFA: Paris, France, 2022; Available online: https://www.ifastat.org/publications/new?title=Outlook (accessed on 15 August 2025).
- International Fertilizer Association. Fertilizer Statistics—Preliminary Consumption Results 2022/2023; IFA: Paris, France, 2023; Available online: https://www.ifastat.org/market-reports (accessed on 15 August 2025).
- FAO on Behalf of the IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests; FAO on Behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- United States Geological Survey. Mineral Commodity Summaries 2023; U.S. Geological Survey: Reston, VA, USA, 2023. [CrossRef]
- United Nations Environment Programme. A Snapshot of the World’s Water Quality: Towards a Global Assessment; UNEP: Nairobi, Kenya, 2016; Available online: https://wesr.unep.org/media/docs/assessments/unep_wwqa_report_web.pdf (accessed on 15 August 2025).
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing Nitrogen for Sustainable Development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the Peer Review of the Pesticide Risk Assessment for Bees for the Active Substance Clothianidin. EFSA J. 2018, 16, 5170. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural Soils, Pesticides and Microbial Diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium Phosphate is Inappropriate as a Universal Selection Factor for Isolating and Testing Phosphate-Solubilizing Bacteria that Enhance Plant Growth: A Proposal for an Alternative Procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Novais, R.F.; Smyth, T.J.; Nunes, F.N. Fósforo. In Fertilidade do Solo; Novais, R.F., Alvarez, V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 275–374. [Google Scholar]
- Guelfi, D.; Nunes, A.P.P.; Sarkis, L.F.; Oliveira, D.P. Innovative Phosphate Fertilizer Technologies to Improve Phosphorus Use Efficiency in Agriculture. Sustainability 2022, 14, 14266. [Google Scholar] [CrossRef]
- Zapata, F.; Roy, R.N. Use of Phosphate Rocks for Sustainable Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; Available online: http://www.fao.org/3/y5053e/y5053e00.htm (accessed on 26 August 2025).
- Empresa Brasileira de Pesquisa Agropecuária. Fonolito: Rocha para Produção de Fertilizante Potássico; Comunicado Técnico 256; Embrapa: Brasília, Brazil, 2021; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1137873/fonolito-rocha-para-producao-de-fertilizante-potassico (accessed on 16 August 2025).
- Santos, W.O.; Mattiello, E.M.; Vergütz, L.; Costa, R.F. Production and Evaluation of Potassium Fertilizers from Silicate Rock. J. Plant Nutr. Soil Sci. 2015, 179, 547–556. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Babalola, O.O.; Adejumo, T.O. Harnessing of Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungi in Agroecosystem Sustainability. CABI Agric. Biosci. 2023, 4, 26. [Google Scholar] [CrossRef]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects a Review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Soares, E.V. Perspective on the Biotechnological Production of Bacterial Siderophores and Their Use. Appl. Microbiol. Biotechnol. 2022, 106, 3985–4004. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-Acetic Acid in Plant–Microbe Interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Glick, B.R.; Nascimento, F.X. 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase from Pseudomonas and its Role in Beneficial Plant-Microbe Interactions. Microorganisms 2021, 9, 2467. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Simões, D.; Andrade, E.D. Fusarium Species Responsible for Tomato Diseases and Mycotoxin Contamination and Biocontrol Opportunities. In Fusarium—Recent Studies; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Xavier, J.F. Isolamento e Caracterização de Bactérias Associadas À Rizosfera de Plantas Halófitas. Master’s Thesis, Universidade Federal Rural do Rio de Janeiro, Seropédica, Brazil, 2021. [Google Scholar]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Ceballos-Aguirre, N.; Restrepo, G.M.; Patiño, S.; Cuéllar, J.A.; Sánchez, Ó.J. Utilization of Gluconacetobacter diazotrophicus in Tomato Crop: Interaction with Nitrogen and Phosphorus Fertilization. Agriculture 2025, 15, 1191. [Google Scholar] [CrossRef]
- Rajawat, M.V.S.; Singh, S.; Tyagi, S.P.; Saxena, A.K. A Modified Plate Assay for Rapid Screening of Potassium-Solubilizing Bacteria. Pedosphere 2016, 26, 768–773. [Google Scholar] [CrossRef]
- Silva, F.G. Determinação de Potássio em Solos Tropicais via Fotometria de Chama: Construção de Curva Padrão e Aplicações Metodológicas. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2009. [Google Scholar]
- Dias, R.D.C. Potencial e Eficiência da Utilização de Rochas Silicáticas como Fonte de Potássio na Agricultura. Ph.D. Thesis, Universidade Federal Rural do Rio de Janeiro, Seropédica, Brazil, 2022. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária. Bioprospecção de Microrganismos para o Uso em Bioinsumos: Métodos para Triagem Inicial de Bioativos Visando à Nutrição de Plantas e à Tolerância a Estresses Abióticos e Bióticos; Documentos 456; Embrapa: Brasília, Brazil, 2024; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1164913/bioprospeccao-de-microrganismos-para-o-uso-em-bioinsumos-metodos-para-triagem-inicial-de-bioativos-visando-a-nutricao-de-plantas-e-a-tolerancia-a-estresses-abioticos-e-bioticos (accessed on 16 August 2025).
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation of Indoleacetic Acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Lana, U.G.P.; Gomes, E.A.; Ribeiro, V.P.; Santos, F.M.; Marriel, I.E. Seleção em Larga Escala de Bactérias Produtoras do Hormônio Ácido Indolacético (AIA), Auxina Associada à Promoção de Crescimento em Plantas; Comunicado Técnico 126; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2016; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/166896/1/CT-126.pdf (accessed on 26 November 2024).
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Foster, J.W. Experiments with Some Microorganisms Which Utilize Ethane and Hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Pedrosa, F.O.; Monteiro, R.A.; Wassem, R.; Cruz, L.M.; Ayub, R.A.; Colauto, N.B.; Fernandez, M.A.; Fungaro, M.H.P.; Grisard, E.C.; Hungria, M.; et al. Genome of Herbaspirillum seropedicae Strain SmR1, a Specialized Diazotrophic Endophyte of Tropical Grasses. PLoS Genet. 2011, 7, e1002064. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.F.R.; Ribeiro, T.G.; Rouws, J.R.C.; Soares, L.H.B.; Zilli, J.É. Biotechnological Potential of Bacteria from Genera Bacillus, Paraburkholderia, and Pseudomonas to Control Seed Fungal Pathogens. Braz. J. Microbiol. 2021, 52, 705–714. [Google Scholar] [CrossRef]
- Bizzini, A.; Greub, G. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry, a Revolution in Clinical Microbial Identification. Clin. Microbiol. Infect. 2010, 16, 1614–1619. [Google Scholar] [CrossRef]
- Branquinho, R.; Sousa, C.; Lopes, J.; Pintado, M.E.; Peixe, L.V.; Osório, H. Differentiation of Bacillus pumilus and Bacillus safensis Using MALDI-TOF-MS. PLoS ONE 2014, 9, e110127. [Google Scholar] [CrossRef]
- Dutra, M.P.; Baldotto, M.A.; Silva, L.F.; Oliveira, V.C.; Baldotto, L.E.B. Bactérias Solubilizadoras de Fosfato em Associação com Termofosfato e Fertilizante Organomineral. In O Equilíbrio da Natureza: Explorando a Complexidade do Meio Ambiente; Baldotto, M.A., Ed.; Atena Editora: Ponta Grossa, Brazil, 2023; pp. 41–50. Available online: https://atenaeditora.com.br/catalogo/post/bacterias-solubilizadoras-de-fosfato-em-associacao-com-termofosfato-e-fertilizante-organomineral (accessed on 16 August 2025).
- Silva, U.C.; Marriel, I.E.; Paiva, C.A.O.; Gomes, E.A.; Resende, Á.V.D.; Lana, U.G.D.P. Biossolubilização de Potássio in Vitro a Partir da Rocha Fonolito por Microrganismos do Solo; Documentos 177; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2015; Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1024827/1/doc177.pdf (accessed on 16 August 2025).
- Pádua, S.D.; Florentino, L.A. Uso do Fonolito e Bactérias Solubilizadoras de Potássio na Cultura do Feijoeiro. Res. Soc. Dev. 2022, 11, e26248. [Google Scholar] [CrossRef]
- Miranda, C.C.B.; Florentino, L.A.; de Rezende, A.V.; Nogueira, D.A.; Leite, R.F.; Naves, L.P. Desenvolvimento de Urochloa brizantha Adubada com Fonolito e Inoculada com bactérias diazotróficas Solubilizadoras de potássio. Rev. Ciências Agrárias 2018, 41, 625–632. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far Beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and Induction of Plant-Stress Tolerance and Defense Genes by Seed and Foliar Inoculation with Azospirillum brasilense Cells and Metabolites Promote Maize Growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef]
- Pappalettere, L.; Bartolini, S.; Toffanin, A. Auxin-Producing Bacteria Used As Microbial Biostimulants Improve the Growth of Tomato (Solanum lycopersicum L.) Seedlings in Hydroponic Systems. BioTech 2024, 13, 32. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial Inoculants for Improving Crop Quality and Human Health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Rigouin, C.; Godet, J. An Overview of Siderophore Biosynthesis Among Fluorescent Pseudomonas and New Insights into Their Complex Cellular Organization. Environ. Microbiol. 2020, 22, 1447–1466. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Holmström, S.J.M. Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Serafim, B.; Bernardino, A.R.; Freitas, F.; Torres, C.A.V. Recent Developments in the Biological Activities, Bioproduction, and Applications of Pseudomonas spp. Phenazines. Molecules 2023, 28, 1368. [Google Scholar] [CrossRef]
- Hussain, S.; Tai, B.; Ali, M.; Jahan, I.; Sakina, S.; Wang, G.; Zhang, X.; Yin, Y.; Xing, F. Antifungal Potential of Lipopeptides Produced by the Bacillus siamensis Sh420 Strain Against Fusarium graminearum. Microbiol. Spectr. 2024, 12, e04008-23. [Google Scholar] [CrossRef]
- Diniz, G.D.F.D.; Figueiredo, J.E.F.; Canuto, K.M.; Cota, L.V.; Souza, A.S.D.Q.; Simeone, M.L.F.; Tinoco, S.M.D.S.; Ribeiro, P.R.V.; Ferreira, L.V.S.; Marins, M.S.; et al. Chemical and Genetic Characterization of Lipopeptides from Bacillus velezensis and Paenibacillus ottowii with Activity Against Fusarium verticillioides. Front. Microbiol. 2024, 15, 1443327. [Google Scholar] [CrossRef]
- Poloni, N.M.; Carvalho, G.; Vicentini, S.N.C.; Dorigan, A.F.; Maciel, J.L.N.; McDonald, B.A.; Moreira, S.I.; Hawkins, N.; Fraaije, B.A.; Kelly, D.E.; et al. Widespread Distribution of Resistance to Triazole Fungicides in Brazilian Populations of the Wheat Blast Pathogen. Plant Pathol. 2021, 70, 436–448. [Google Scholar] [CrossRef]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance: The Assessment of Risk, 2nd ed.; FRAC Monograph No. 2; Fungicide Resistance Action Committee: Brussels, Belgium, 2007; Available online: https://www.frac.info/media/23rp130k/monograph-2.pdf (accessed on 20 September 2025).
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a Reduced Reliance on Conventional Pesticides in European Agriculture. Plant Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef]
- Thammasittirong, S.N.-R.; Thammasittirong, A.; Saechow, S. Biocontrol and Growth Promotion of Rice by Pseudomonas aeruginosa SNTKU16: Beneficial Properties and Genomic Potential. J. Microbiol. Biotechnol. 2025, 35, e2411067. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhuang, L.; Yu, Y.; Liu, J.; Zhang, L.; Gao, Z.; Wu, Y.; Gao, W.; Ding, G.; et al. A Bacillus subtilis and Trichoderma harzianum Consortium Derived from the Rhizosphere Suppresses Common Scab and Increases Potato Yield. Comput. Struct. Biotechnol. J. 2019, 17, 645–653. [Google Scholar] [CrossRef]
- de Oliveira-Paiva, C.A.; Bini, D.; de Sousa, S.M.; Ribeiro, V.P.; dos Santos, F.C.; de Paula Lana, U.G.; de Souza, F.F.; Gomes, E.A.; Marriel, I.E. Inoculation with Bacillus megaterium CNPMS B119 and Bacillus subtilis CNPMS B2084 Improve P-Acquisition and Maize Yield in Brazil. Front. Microbiol. 2024, 15, 1426166. [Google Scholar] [CrossRef]
- Sandini, I.E.; Pacentchuk, F.; Franco, D.A.D.S.; Sandini, A.H. Bacterial Consortium of Azospirillum brasilense and Pseudomonas fluorescens on the Stimulation of Growth of Corn Culture. Ciênc. Rural 2024, 54, e20230123. [Google Scholar] [CrossRef]
- de Lima, J.D.; Monteiro, P.H.R.; Rivadavea, W.R.; Barbosa, M.; Cordeiro, R.D.; Garboggini, F.F.; Auer, C.G.; da Silva, G.J. Potential of Endophytic Bacteria from Acacia mearnsii: Phosphate Solubilization, Indole Acetic Acid Production, and Application in Wheat. Appl. Soil Ecol. 2024, 196, 105315. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 20 June 2024).
- Food and Agriculture Organization of the United Nations. Brief The State of Food and Agriculture 2021; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).