Degradation of Dioxins and DBF in Urban Soil Microcosms from Lausanne (Switzerland): Functional Performance of Indigenous Bacterial Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Soil Samples
2.3. Soil Starting Inoculum Preparation
2.4. Selection of Dioxin-Degrading Microbial Strains
2.5. Chemical Analysis of DBF and 2,7-DD
2.6. Quantification, Isolation and Identification of Degrading Microbial Strains
2.7. Degradation Assays with Individual Strains and Defined Co-Cultures
2.8. Antibiotic Susceptibility Testing
2.9. Scale-Up in 2 L Cultures: Contamination Assessment
2.10. Phenotype Stability Assay Following Scale-Up
2.11. Treatment of Real Soil Samples for Bioremediation Experiments
2.12. Statistical Analysis
3. Results and Discussion
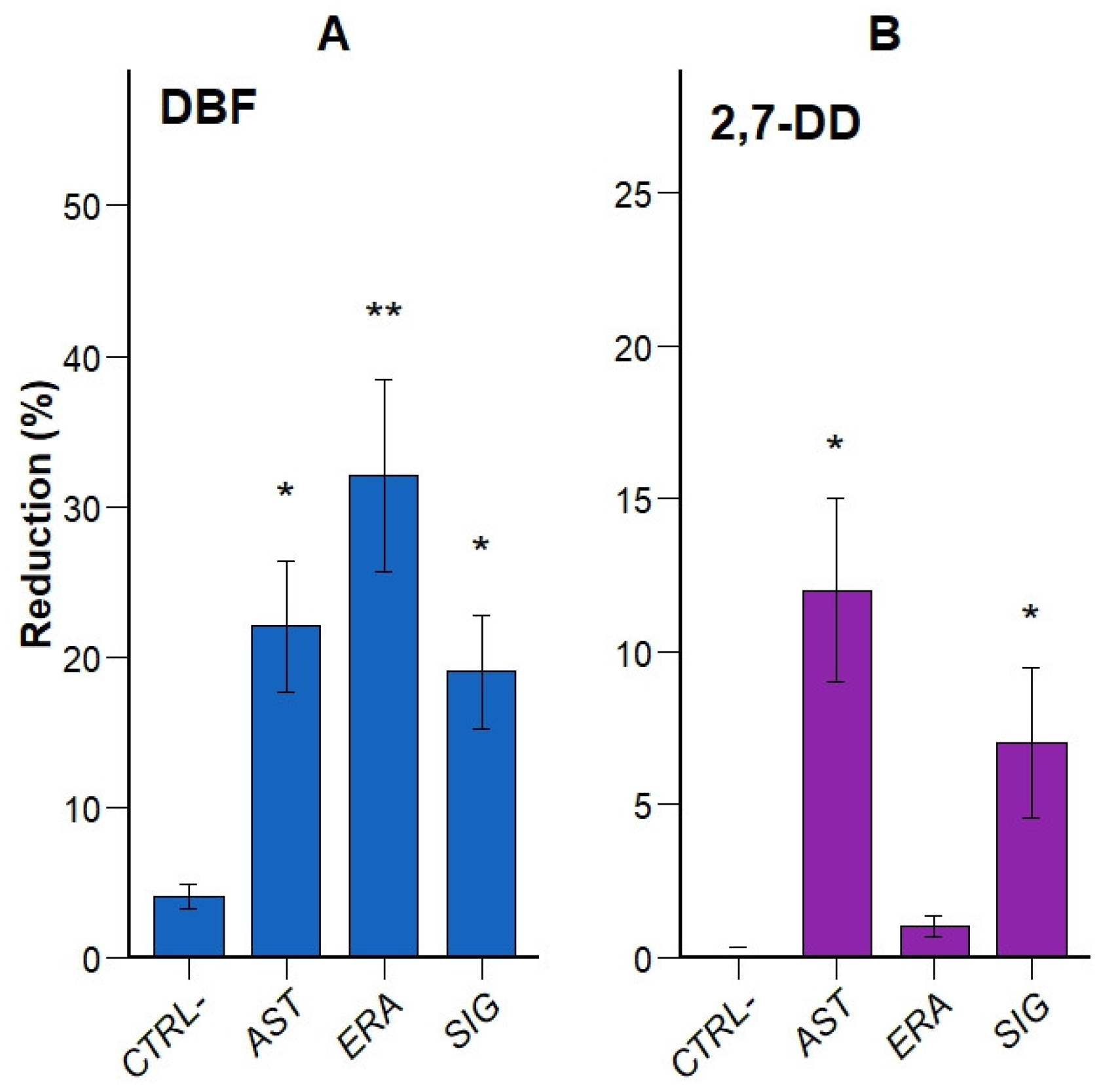
3.1. Enriched Consortia Can Degrade Both DBF and 2,7-DD
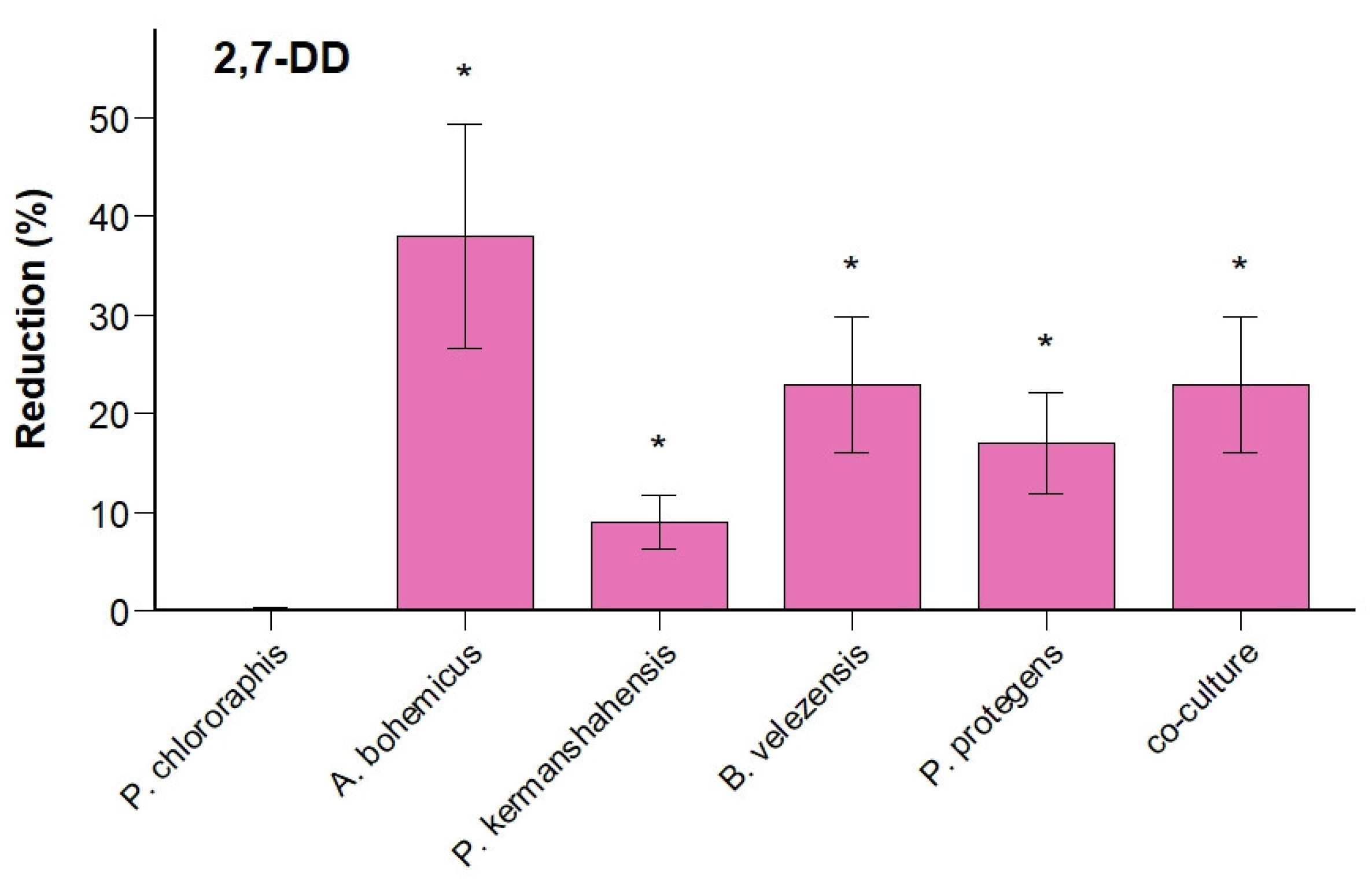
3.2. Five Bacterial Strains Identified for Putatative Degradation Activity
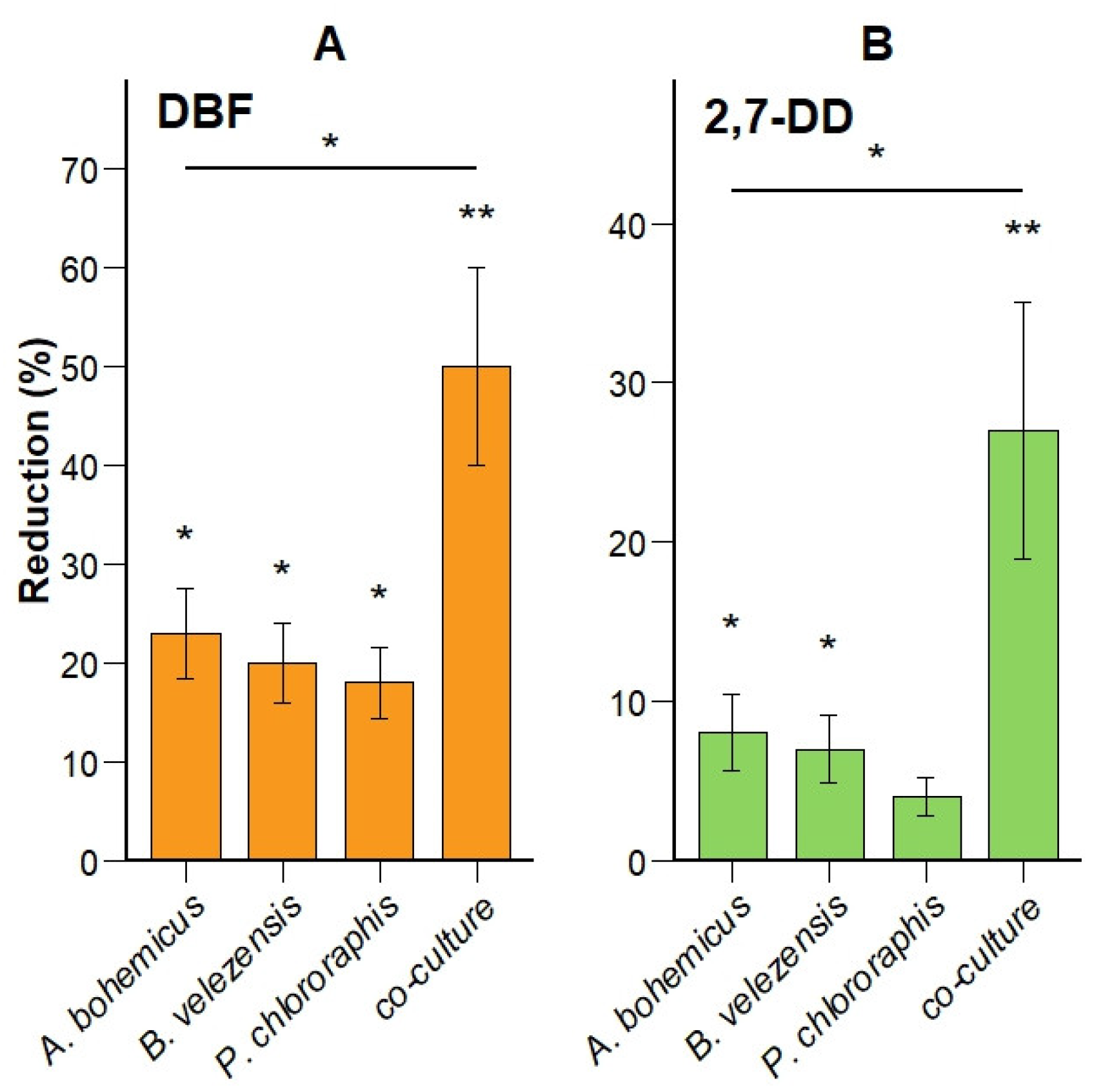
3.3. DBF and 2,7-DD Degradation Efficiency Is Higher in Five-Species Co-Culture Compared to Monocultures
3.4. Antibiotic Susceptibility Results Do Not Compromise Future Use of the Strains
3.5. The Five Selected Strains Are Suitable for Industrial Scale-Up
3.6. Phenotype Stability Is Preserved After Large Biomass Production
3.7. Biodegradation Performance of Selected Bacterial Treatments in Real Soil Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,7-DD | 2,7-Dichlorodibenzo-p-dioxin |
| DBF | Dibenzofuran |
| TEQ | Toxic Equivalent |
| WHO-05 | World Health Organization 2005 Toxic Equivalency Factors |
| PCDD | Polychlorinated dibenzo-p-dioxin |
| PCDF | Polychlorinated dibenzofuran |
| OCDD | Octachlorodibenzo-p-dioxin |
| OCDF | Octachlorodibenzofuran |
| LQ | Lower-bound Quantification Limit |
| CFU | Colony Forming Units |
| TSB | Tryptic Soy Broth |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| ANOVA | Analysis of Variance |
| SIG | Signal site (sampling location) |
| ERA | Eracom site (sampling location) |
| AST | Ancien Stand site (sampling location) |
References
- Kirkok, S.K.; Kibet, J.K.; Kinyanjui, T.K.; Okanga, F.I. A review of persistent organic pollutants: Dioxins, furans, and their associated nitrogenated analogues. SN Appl. Sci. 2020, 2, 1729. [Google Scholar] [CrossRef]
- Mathew, N.; Somanathan, A.; Tirpude, A.; Pillai, A.M.; Mondal, P.; Arfin, T. Dioxins and their impact: A review of toxicity, persistence, and novel remediation strategies. Anal. Methods 2025, 17, 1698–1748. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, R.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gándara, J. A critical review about human exposure to polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) through foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 1590–1617. [Google Scholar] [CrossRef] [PubMed]
- Or, A.B.; Palazzolo, R.; Kaplan, A.; Attia, S.; Haikin, N.; Katoshevski, D. Troubleshooting dioxins stack emissions in an industrial waste gas incinerator. Chemosphere 2023, 342, 139857. [Google Scholar] [CrossRef]
- Aldeli, N.; Murphy, D.; Hanano, A. Impact of dioxins on reproductive health in female mammals. Front. Toxicol. 2024, 6, 1392257. [Google Scholar] [CrossRef]
- Kumbale, C.M.; Zhang, Q.; Voit, E.O. Analysis of systemic effects of dioxin on human health through template-and-anchor modeling. PLoS Comput. Biol. 2025, 21, e1012840. [Google Scholar] [CrossRef]
- Tang, X.F.; Zhou, C.Y.; Xia, W.; Liang, Y.T.; Zeng, Y.X.; Zhao, X.Y.; Xiong, W.P.; Cheng, M.; Wang, Z.W. Recent advances in metal-organic framework-based materials for removal of fluoride in water: Performance, mechanism, and potential practical application. Chem. Eng. J. 2022, 446, 137299. [Google Scholar] [CrossRef]
- Kumawat, M.; Pal, N.; Sharma, P.; Verma, V.; Tiwari, R.R.; Singh, S.; Shubham, S.; Sarma, D.K.; Kumar, M. Investigating the presence of dioxins in drinking water: Implications for public health. Int. J. Environ. Health Res. 2024, 34, 3735–3748. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M.; Brambilla, P.; Signorini, S.; Ames, J.; Mocarelli, P. The Seveso accident: A look at 40 years of health research and beyond. Environ. Int. 2018, 121, 71–84. [Google Scholar] [CrossRef]
- Mai, T.A.; Doan, T.V.; Tarradellas, J.; de Alencastro, L.F.; Grandjean, D. Dioxin contamination in soils of Southern Vietnam. Chemosphere 2007, 67, 1802–1807. [Google Scholar] [CrossRef]
- Dopico, M.; Gómez, A. Review of the current state and main sources of dioxins around the world. J. Air Waste Manag. Assoc. 2015, 65, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, H. National PCDD/PCDF release inventories under the Stockholm convention on persistent organic pollutants. Chemosphere 2007, 67, S96–S108. [Google Scholar] [CrossRef] [PubMed]
- Vernez, D.; Oltramare, C.; Sauvaget, B.; Demougeot-Renard, H.; Aicher, L.; Roth, N.; Rossi, I.; Radaelli, A.; Lerch, S.; Marolf, V.; et al. Polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) soil contamination in Lausanne, Switzerland: Combining pollution mapping and human exposure assessment for targeted risk management. Environ. Pollut. 2023, 316, 120441. [Google Scholar] [CrossRef] [PubMed]
- Oltramare, C.; Zennegg, M.; Graille, M.; Lerch, S.; Berthet, A.; Vernez, D. Polychlorinated dibenzo-p-dioxin and dibenzofuran contamination of free-range eggs: Estimation of the laying hen’s soil ingestion based on a toxicokinetic model, and human consumption recommendations. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2024, 41, 1302–1314. [Google Scholar] [CrossRef]
- LaKind, J.S.; Berlin, C.M.; Mattison, D.R. The heart of the matter on breastmilk and environmental chemicals: Essential points for healthcare providers and new parents. Breastfeed. Med. 2008, 3, 251–256. [Google Scholar] [CrossRef]
- Joung, H.T.; Cho, S.J.; Seo, Y.C.; Kim, W.H. Concentration of dioxin congeners in different phase products from a thermal process of end-of-life automobile shredder dusts. Environ. Eng. Sci. 2009, 26, 721–729. [Google Scholar] [CrossRef]
- Kirman, C.; Budinsky, R.A.; Yost, L.; Baker, B.F.; Zabik, J.M.; Rowlands, J.C.; Long, T.F.; Simon, T. Derivation of soil clean-up levels for 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD) toxicity equivalence (TEQ_{D/F}) in soil through deterministic and probabilistic risk assessment of exposure and toxicity. Hum. Ecol. Risk Assess. 2011, 17, 125–158. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wu, J.H.; Lin, Y.Y.; Huang, H.J.; Chang, J.E. Bioremediation potential of soil contaminated with highly substituted polychlorinated dibenzo-p-dioxins and dibenzofurans: Microcosm study and microbial community analysis. J. Hazard. Mater. 2013, 261, 351–361. [Google Scholar] [CrossRef]
- Hanano, A.; Ammouneh, H.; Almousally, I.; Alorr, A.; Shaban, M.; Abu Alnaser, A.; Ghanem, I. Traceability of polychlorinated dibenzo-dioxins/furans pollutants in soil and their ecotoxicological effects on genetics, functions and composition of bacterial community. Chemosphere 2014, 108, 326–333. [Google Scholar] [CrossRef]
- Hiraishi, A.; Miyakoda, H.; Lim, B.R.; Hu, H.Y.; Fujie, K.; Suzuki, J. Toward the bioremediation of dioxin-polluted soil: Structural and functional analyses of in situ microbial populations by quinone profiling and culture-dependent methods. Appl. Microbiol. Biotechnol. 2001, 57, 248–256. [Google Scholar] [CrossRef]
- Nhung, N.T.H.; Nguyen, X.T.; Long, V.D.; Wei, Y.; Fujita, T. A review of soil contaminated with dioxins and biodegradation technologies: Current status and future prospects. Toxics 2022, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Saibu, S.; Adebusoye, S.A.; Oyetibo, G.O. Aerobic bacterial transformation and biodegradation of dioxins: A review. Bioresour. Bioproc. 2020, 7, 7. [Google Scholar] [CrossRef]
- Tran, H.T.; Hoang, H.G.; Chacha, W.E.; Mukherjee, S.; Duong, T.V.H.; Nguyen, N.S.H.; Nguyen, K.N.; Naidu, R. A review of advanced bioremediation technologies for dioxin-contaminated soil treatment: Current and future outlook. Chemosphere 2024, 366, 143400. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.R.; Hartmann, E.M.; Halden, R.U. Proteomic profiling of the dioxin-degrading bacterium Sphingomonas wittichii RW1. J. Biomed. Biotechnol. 2012, 2012, 408690. [Google Scholar] [CrossRef]
- Lu, Q.; Liang, Q.; Wang, S. Burning question: Rethinking organohalide degradation strategy for bioremediation applications. Microb. Biotechnol. 2024, 17, e14539. [Google Scholar] [CrossRef]
- Dutta, N.; Usman, M.; Ashraf, M.A.; Luo, G.; Zhang, S.C. Efficacy of emerging technologies in addressing reductive dechlorination for environmental bioremediation: A review. J. Hazard. Mater. Lett. 2022, 3, 100065. [Google Scholar] [CrossRef]
- Terzaghi, E.; Vergani, L.; Mapelli, F.; Borin, S.; Raspa, G.; Zanardini, E.; Morosini, C.; Anelli, S.; Nastasio, P.; Sale, V.M.; et al. New data set of polychlorinated dibenzo-dioxin and dibenzofuran half-lives: Natural attenuation and rhizoremediation using several common plant species in a weathered contaminated soil. Environ. Sci. Technol. 2020, 54, 10000–10011. [Google Scholar] [CrossRef]
- Chang, Y.S. Recent developments in microbial biotransformation and biodegradation of dioxins. J. Mol. Microbiol. Biotechnol. 2008, 15, 152–171. [Google Scholar] [CrossRef]
- Bôto, M.L.; Magalhaes, C.; Perdigao, R.; Alexandrino, D.A.M.; Fernandes, J.P.; Bernabeu, A.M.; Ramos, S.; Carvalho, M.F.; Semedo, M.; LaRoche, J.; et al. Harnessing the potential of native microbial communities for bioremediation of oil spills in the Iberian Peninsula NW coast. Front. Microbiol. 2021, 12, 633659. [Google Scholar] [CrossRef]
- Sarkar, J.; Kazy, S.K.; Gupta, A.; Dutta, A.; Mohapatra, B.; Roy, A.; Bera, P.; Mitra, A.; Sar, P. Biostimulation of indigenous microbial community for bioremediation of petroleum refinery sludge. Front. Microbiol. 2025, 16, 1551928. [Google Scholar] [CrossRef]
- Armengaud, J.; Timmis, K.N. Biodegradation of dibenzo-p-dioxin and dibenzofuran by bacteria. J. Microbiol. 1997, 35, 241–252. [Google Scholar]
- Kasuga, K.; Nojiri, H.; Yamane, H.; Omori, T. Genes of enzymes involved in the biodegradation of carbazole, dibenzofuran, fluorene, and dibenzo-p-dioxin by bacteria. Water Sci. Technol. 1997, 36, 9–16. [Google Scholar] [CrossRef]
- Miyauchi, K.; Sukda, P.; Nishida, T.; Ito, E.; Matsumoto, Y.; Masai, E.; Fukuda, M. Isolation of dibenzofuran-degrading bacterium, Nocardioides sp. DF412, and characterization of its dibenzofuran degradation genes. J. Biosci. Bioeng. 2008, 105, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Nagata, S.; Taniguchi, H. Isolation and characterization of dibenzofuran-degrading bacteria. FEMS Microbiol. Lett. 2002, 208, 179–185. [Google Scholar] [CrossRef]
- Habe, H.; Chung, J.S.; Lee, J.H.; Kasuga, K.; Yoshida, T.; Nojiri, H.; Omori, T. Degradation of chlorinated dibenzofurans and dibenzo-dioxins by two types of bacteria having angular dioxygenases with different features. Appl. Environ. Microbiol. 2001, 67, 3610–3617. [Google Scholar] [CrossRef]
- Hong, H.B.; Chang, Y.S.; Nam, I.H.; Fortnagel, P.; Schmidt, S. Biotransformation of 2,7-dichloro- and 1,2,3,4-tetrachlorodibenzo-dioxin by RW1. Appl. Environ. Microbiol. 2002, 68, 2584–2588. [Google Scholar] [CrossRef]
- Hu, K.K.; Bunce, N.J. Metabolism of polychlorinated dibenzo-dioxins and related dioxin-like compounds. J. Toxicol. Environ. Health B Crit. Rev. 1999, 2, 183–210. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Bottoni, P.; Grenni, P. Microcosm studies to evaluate microbial potential to degrade pollutants in soil and water ecosystems. Microchem. J. 2013, 107, 126–130. [Google Scholar] [CrossRef]
- Charlestra, L.; Amirbahman, A.; Courtemanch, D.L.; Alvarez, D.A.; Patterson, H. Estimating pesticide sampling rates by the polar organic chemical integrative sampler (POCIS) in the presence of natural organic matter and varying hydrodynamic conditions. Environ. Pollut. 2012, 169, 98–104. [Google Scholar] [CrossRef]
- Grenni, P.; Falconi, F.; Caracciolo, A.B. Microcosm experiments for evaluating natural bioremediation of contaminated ecosystems. Chem. Eng. Trans. 2012, 28, 7–12. [Google Scholar] [CrossRef]
- Omrani, R.; Spini, G.; Puglisi, E.; Saidane, D. Modulation of microbial consortia enriched from different polluted environments during petroleum biodegradation. Biodegradation 2018, 29, 187–209. [Google Scholar] [CrossRef]
- Lelieveld, H.L.M.; Boon, B.; Bennett, A.; Brunius, G.; Cantley, M.; Chmiel, A.; Collins, C.H.; Crooy, P.; Dolblhoff-Dier, O.; Economidis, I.; et al. Safe biotechnology. 7. Classification of microorganisms on the basis of hazard. Appl. Microbiol. Biotechnol. 1996, 45, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, A.; Franzetti, A.; Formicola, F.; Ambaye, T.G.; Gomez, F.H.; Murena, B.; De Marco, E.; Beltrani, T.; Sbaffoni, S.; Vaccari, M. Insights into rhamnolipid-based soil remediation technologies by safe microorganisms: A critical review. J. Clean. Prod. 2022, 367, 133088. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef]
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef]
- Nzila, A.; Razzak, S.A.; Zhu, J. Bioaugmentation: An emerging strategy of industrial wastewater treatment for reuse and discharge. Int. J. Environ. Res. Public Health 2016, 13, 846. [Google Scholar] [CrossRef]
- Kubota, M.; Kawahara, K.; Sekiya, K.; Uchida, T.; Hattori, Y.; Futamata, H.; Hiraishi, A. Nocardioides aromaticivorans sp. nov., a dibenzofuran-degrading bacterium isolated from dioxin-polluted environments. Syst. Appl. Microbiol. 2005, 28, 165–174. [Google Scholar] [CrossRef]
- Spinnel, E.; Danielsson, C.; Haglund, P. Rapid and cost-effective analysis of polychlorinated dibenzo-dioxins and polychlorinated dibenzofurans in soil, fly ash and sediment certified reference materials using pressurized liquid extraction with an integrated carbon trap. Anal. Bioanal. Chem. 2008, 390, 411–417. [Google Scholar] [CrossRef]
- Arif, S.; Reitner, J.; Hoppert, M. Composition, diversity and functional analysis of the modern microbiome of the Middle Triassic Cava Superiore Beds (Monte San Giorgio, Switzerland). Sci. Rep. 2019, 9, 20394. [Google Scholar] [CrossRef]
- Araujo, W.J.; Oliveira, J.S.; Araujo, S.C.S.; Minnicelli, C.F.; Silva-Portela, R.C.B.; da Fonseca, M.M.B.; Freitas, J.F.; Silva-Barbalho, K.K.; Napp, A.P.; Pereira, J.E.S.; et al. Microbial culture in minimal medium with oil favors enrichment of biosurfactant producing genes. Front. Bioeng. Biotechnol. 2020, 8, 962. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Roden, E.E.; Churchill, P.F. Changes in bacterial species composition in enrichment cultures with various dilutions of inoculum as monitored by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 1998, 64, 5046–5048. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Chen, W.M.; Wang, E.T.; Wang, J.M.; Liu, Z.S.; Li, Y.N.; Wei, G.H. Microbial succession in response to pollutants in batch-enrichment culture. Sci. Rep. 2016, 6, 21791. [Google Scholar] [CrossRef] [PubMed]
- Naloka, K.; Kuntaveesuk, A.; Muangchinda, C.; Chavanich, S.; Viyakarn, V.; Chen, B.; Pinyakong, O. Pseudomonas and Pseudarthrobacter are the key players in synergistic phenanthrene biodegradation at low temperatures. Sci. Rep. 2024, 14, 11976. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Krause, S.; Le Roux, X.; Niklaus, P.A.; Van Bodegom, P.M.; Lennon, J.T.; Bertilsson, S.; Grossart, H.P.; Philippot, L.; Bodelier, P.L.E. Trait-based approaches for understanding microbial biodiversity and ecosystem functioning. Front. Microbiol. 2014, 5, 251. [Google Scholar] [CrossRef]
- Tzamali, E.; Poirazi, P.; Tollis, I.G.; Reczko, M. A computational exploration of bacterial metabolic diversity identifying metabolic interactions and growth-efficient strain communities. BMC Syst. Biol. 2011, 5, 167. [Google Scholar] [CrossRef]
- Ito, T.; Sekizuka, T.; Kishi, N.; Yamashita, A.; Kuroda, M. Conventional culture methods with commercially available media unveil the presence of novel culturable bacteria. Gut Microbes 2019, 10, 77–91. [Google Scholar] [CrossRef]
- Yoosathaporn, S.; Tiangburanatham, P.; Bovonsombut, S.; Chaipanich, A.; Pathom-aree, W. A cost-effective cultivation medium for biocalcification of Bacillus pasteurii sp. KCTC 3558 and its effect on cement cubes properties. Microbiol. Res. 2016, 186, 132–138. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Durán, A.; Nogueira, M.; Menduíña, A.; Antunes, J.; Freitas, A.C.; Gomes, A.M. Production of marine probiotic bacteria in a cost-effective marine media based on peptones obtained from discarded fish by-products. Microorganisms 2020, 8, 1121. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Vangnai, A. Modeling degradation kinetics of profenofos using Acinetobacter sp. 33F. Environ. Technol. Innov. 2021, 21, 101367. [Google Scholar] [CrossRef]
- Thangaraj, K.; Kapley, A.; Purohit, H.J. Characterization of diverse Pseudomonas isolates for utilization of multiple aromatic compounds. Bioresour. Technol. 2008, 99, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, S.; Mansour, G.; Hanano, A. Compositional, genetic and functional characterization of soil culturable microbial communities in polychlorinated dibenzo-dioxins/furans contaminated soil. Front. Environ. Sci. 2022, 10, 1008900. [Google Scholar] [CrossRef]
- Dinh, M.T.N.; Nguyen, V.; Nguyen, L.T.H. The potential application of carbazole-degrading bacteria for dioxin bioremediation. Bioresour. Bioproc. 2023, 10, 56. [Google Scholar] [CrossRef]
- Hu, F.H.; Wang, P.L.; Li, Y.H.; Ling, J.H.; Ruan, Y.Q.; Yu, J.J.; Zhang, L.H. Bioremediation of environmental organic pollutants by microorganisms: Mechanisms, methods and challenges. Environ. Res. 2023, 239, 117211. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.J. Microbial consortia are needed to degrade soil pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef]
- Zhao, K.; Si, T.T.; Liu, S.H.; Liu, G.L.; Li, D.H.; Li, F.X. Co-metabolism of microorganisms: A study revealing the mechanism of antibiotic removal, progress of biodegradation transformation pathways. Sci. Total Environ. 2024, 954, 176561. [Google Scholar] [CrossRef]
- Wong, J.Y.; Ngieng, N.S.; Husaini, A.; Saat, R.; Hussain, H. Influence of pH on the biodegradation efficiency of fats, oils, and grease by biosurfactant-producing bacterial consortia. Biodegradation 2025, 36, 50. [Google Scholar] [CrossRef]
- Pulami, D.; Schwabe, L.; Blom, J.; Schwengers, O.; Wilharm, G.; Kämpfer, P.; Glaeser, S.P. Genomic plasticity and adaptive capacity of the quaternary alkyl-ammonium compound and copper tolerant strain QAC-21b isolated from pig manure. Antonie Van Leeuwenhoek 2023, 116, 327–342. [Google Scholar] [CrossRef]
- Heo, G.; Kong, H.; Kim, N.; Lee, S.; Sul, S.; Jeong, D.W.; Lee, J.H. Antibiotic susceptibility of Bacillus velezensis. FEMS Microbiol. Lett. 2022, 369, fnac017. [Google Scholar] [CrossRef]
- Lewandowska, W.; Mahillon, J.; Drewnowska, J.M.; Swiecicka, I. Insight into the phylogeny and antibiotic resistance of Bacillus spp. originating from soil of the Białowieża National Park in Northeastern Poland. Front. Microbiol. 2025, 16, 1454510. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Elfadadny, A.; Ragab, R.F.; AlHarbi, M.; Badshah, F.; Ibáñez-Arancibia, E.; Farag, A.; Hendawy, A.O.; De los Ríos-Escalante, P.R.; Aboubakr, M.; Zakai, S.A.; et al. Antimicrobial resistance of Pseudomonas aeruginosa: Navigating clinical impacts, current resistance trends, and innovations in breaking therapies. Front. Microbiol. 2024, 15, 1374466. [Google Scholar] [CrossRef] [PubMed]
- Calvayrac, C.; Romdhane, S.; Barthelmebs, L.; Rocaboy, E.; Cooper, J.F.; Bertrand, C. Growth abilities and phenotype stability of a sulcotrione-degrading Pseudomonas sp. isolated from soil. Int. Biodeterior. Biodegrad. 2014, 91, 104–110. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Dutilloy, E.; Benchlih, S.; Lahlali, R.; Ait-Barka, E.; Esmaeel, Q. Bacillus velezensis: A versatile ally in the battle against phytopathogens—Insights and prospects. Appl. Microbiol. Biotechnol. 2024, 108, 439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, H.T.; Pan, R.X.; Long, Y.; Zhao, Y.N.; Yu, M.B.; Peng, J.J.; Ma, Y. Optimisation of cultivation conditions for Streptomyces G7 from mangrove plants and exploration of potential bacteriocins. Front. Pharmacol. 2025, 16, 1530043. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of environmental wastes: The role of microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- van Rij, E.T.; Wesselink, M.; Chin, A.W.T.F.; Bloemberg, G.V.; Lugtenberg, B.J. Influence of environmental conditions on the production of phenazine-1-carboxamide by Pseudomonas chlororaphis PCL1391. Mol. Plant Microbe Interact. 2004, 17, 557–566. [Google Scholar] [CrossRef]
- Ghergab, A.; Selin, C.; Tanner, J.; Brassinga, A.K.; de Kievit, T. Pseudomonas chlororaphis PA23 metabolites protect against protozoan grazing by the predator Acanthamoeba castellanii. PeerJ 2021, 9, e10756. [Google Scholar] [CrossRef]
- Reva, O.N.; Swanevelder, D.Z.H.; Mwita, L.A.; Mwakilili, A.D.; Muzondiwa, D.; Joubert, M.; Chan, W.Y.; Lutz, S.; Ahrens, C.H.; Avdeeva, L.V.; et al. Genetic, epigenetic and phenotypic diversity of four Bacillus velezensis strains used for plant protection or as probiotics. Front. Microbiol. 2019, 10, 2610. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Karnaushenko, D.; Baraban, L.; Ye, D.; Uguz, I.; Mendes, R.G.; Rummeli, M.H.; de Visser, J.A.; Schmidt, O.G.; Cuniberti, G.; Makarov, D. Monitoring microbial metabolites using an inductively coupled resonance circuit. Sci. Rep. 2015, 5, 12878. [Google Scholar] [CrossRef]
- Singh, A.K.; Sherry, A.; Gray, N.D.; Jones, D.M.; Bowler, B.F.; Head, I.M. Kinetic parameters for nutrient enhanced crude oil biodegradation in intertidal marine sediments. Front. Microbiol. 2014, 5, 160. [Google Scholar] [CrossRef]
- Byrne, E.; Schum, S.; Schaerer, L.; Techtmann, S.M. Impacts of nutrients on alkene biodegradation rates and microbial community composition in enriched consortia from natural inocula. Microbiol. Spectr. 2023, 11, e0031622. [Google Scholar] [CrossRef]
- Ruiz-Manzano, A.; Yuste, L.; Rojo, F. Levels and activity of the Pseudomonas putida global regulatory protein Crc vary according to growth conditions. J. Bacteriol. 2005, 187, 3678–3686. [Google Scholar] [CrossRef]
- Maddela, N.R.; Ramakrishnan, B.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Major contaminants of emerging concern in soils: A perspective on potential health risks. RSC Adv. 2022, 12, 12396–12415. [Google Scholar] [CrossRef]
- Weber, R.; Gaus, C.; Tysklind, M.; Johnston, P.; Forter, M.; Hollert, H.; Heinisch, E.; Holoubek, I.; Lloyd-Smith, M.; Masunaga, S.; et al. Dioxin- and POP-contaminated sites—Contemporary and future relevance and challenges. Environ. Sci. Pollut. Res. 2008, 15, 363–393. [Google Scholar] [CrossRef]
- Belles, A.; Pardon, P.; Budzinski, H. Development of an adapted version of polar organic chemical integrative samplers (POCIS-Nylon). Anal. Bioanal. Chem. 2014, 406, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Reiner, E.J. The analysis of dioxins and related compounds. Mass Spectrom. Rev. 2010, 29, 526–559. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.A.; Minnich, M.M. Extreme short-range variability in VOC-contaminated soils. Environ. Sci. Technol. 2000, 34, 3611–3616. [Google Scholar] [CrossRef]
- Nording, M.; Denison, M.S.; Baston, D.; Persson, Y.; Spinnel, E.; Haglund, P. Analysis of dioxins in contaminated soils with the CALUX and CAFLUX bioassays, an immunoassay, and gas chromatography/high-resolution mass spectrometry. Environ. Toxicol. Chem. 2007, 26, 1122–1129. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Romantschuk, M.; Lahti-Leikas, K.; Kontro, M.; Galitskaya, P.; Talvenmäki, H.; Simpanen, S.; Allen, J.A.; Sinkkonen, A. Bioremediation of contaminated soil and groundwater by biostimulation. Front. Microbiol. 2023, 14, 1258148. [Google Scholar] [CrossRef]
- Alori, E.T.; Gabasawa, A.I.; Elenwo, C.E.; Agbeyegbe, O.O. Bioremediation techniques as affected by limiting factors in soil environment. Front. Soil Sci. 2022, 2, 937186. [Google Scholar] [CrossRef]

| Site | Identified Species | Risk Group |
|---|---|---|
| ERA | Pseudomonas kermanshahensis | 1 |
| ERA | Stenotrophomonas nematodicola | ND |
| AST | Acinetobacter bohemicus | 1 |
| AST | Bacillus velezensis | 1 |
| AST | Pseudomonas shirazica | ND |
| AST | Achromobacter aegrifaciens | 2 |
| AST | Pseudomonas chlororaphis | 1 |
| AST | Stenotrophomonas genomosp. | 2 |
| SIG | Pseudomonas protegens | 1 |
| Specie | Doubling Time (h) |
|---|---|
| Acinetobacter bohemicus | 1.27 |
| Bacillus velezensis | 1.21 |
| Pseudomonas chlororaphis | 3.32 |
| Pseudomonas kermanshahensis | 2.03 |
| Pseudomonas protegens | 1.50 |
| Specie/Condition | % Degradation per 1 × 107 Cells/mL |
|---|---|
| Pseudomonas chlororaphis | ND |
| Acinetobacter bohemicus | 1.0% |
| Pseudomonas kermanshahensis | 0.5% |
| Bacillus velezensis | 57.9% |
| Pseudomonas protegens | 1.0% |
| Co-culture (all strains) | 1.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Martino, R.; Soudani, M.; Castiglioni, P.; Rime, C.; Gillioz, Y.; Sartori, L.; Proust, T.; Neves Dos Santos, F.; Lucarini, F.; Staedler, D. Degradation of Dioxins and DBF in Urban Soil Microcosms from Lausanne (Switzerland): Functional Performance of Indigenous Bacterial Strains. Microorganisms 2025, 13, 2306. https://doi.org/10.3390/microorganisms13102306
Di Martino R, Soudani M, Castiglioni P, Rime C, Gillioz Y, Sartori L, Proust T, Neves Dos Santos F, Lucarini F, Staedler D. Degradation of Dioxins and DBF in Urban Soil Microcosms from Lausanne (Switzerland): Functional Performance of Indigenous Bacterial Strains. Microorganisms. 2025; 13(10):2306. https://doi.org/10.3390/microorganisms13102306
Chicago/Turabian StyleDi Martino, Rita, Mylène Soudani, Patrik Castiglioni, Camille Rime, Yannick Gillioz, Loïc Sartori, Tatiana Proust, Flavio Neves Dos Santos, Fiorella Lucarini, and Davide Staedler. 2025. "Degradation of Dioxins and DBF in Urban Soil Microcosms from Lausanne (Switzerland): Functional Performance of Indigenous Bacterial Strains" Microorganisms 13, no. 10: 2306. https://doi.org/10.3390/microorganisms13102306
APA StyleDi Martino, R., Soudani, M., Castiglioni, P., Rime, C., Gillioz, Y., Sartori, L., Proust, T., Neves Dos Santos, F., Lucarini, F., & Staedler, D. (2025). Degradation of Dioxins and DBF in Urban Soil Microcosms from Lausanne (Switzerland): Functional Performance of Indigenous Bacterial Strains. Microorganisms, 13(10), 2306. https://doi.org/10.3390/microorganisms13102306






