Probiotic Supplementation and Inflammatory Status in Coronary Artery Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Ethics Statement
2.2. Selection Criteria
2.3. Main Outcome Measures and Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
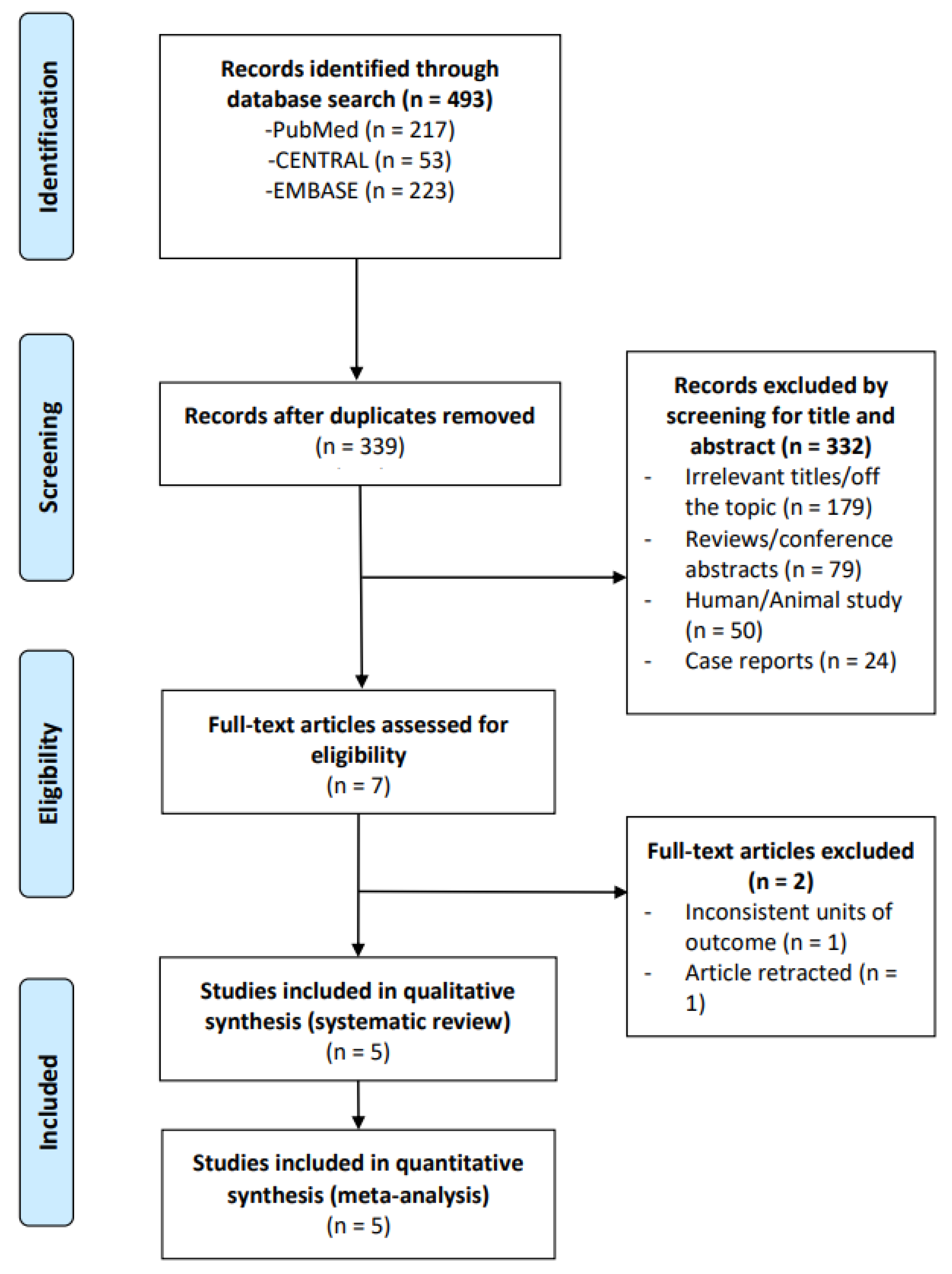
3.1. Study Selection
3.2. Characteristics of the Included Studies
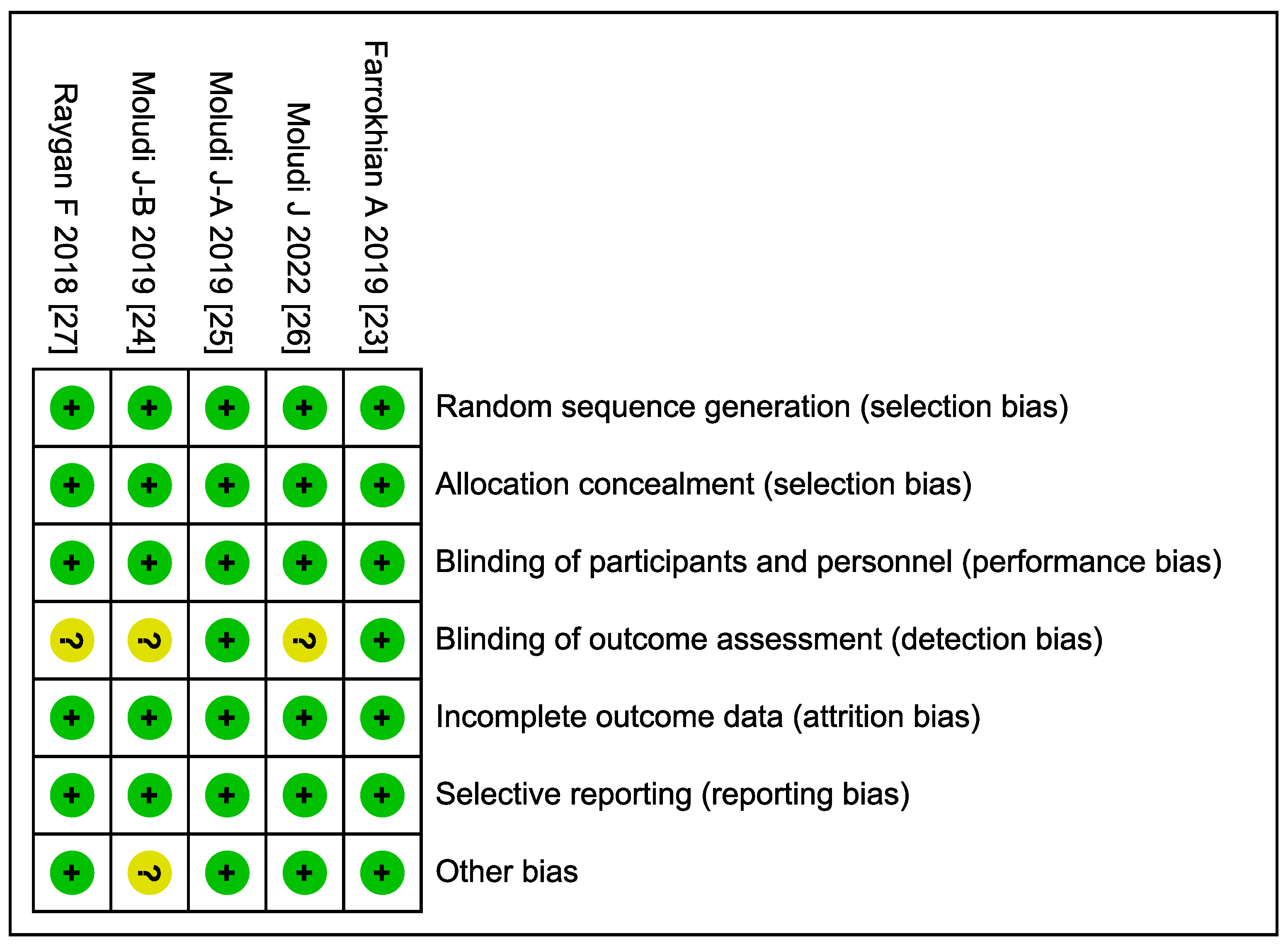
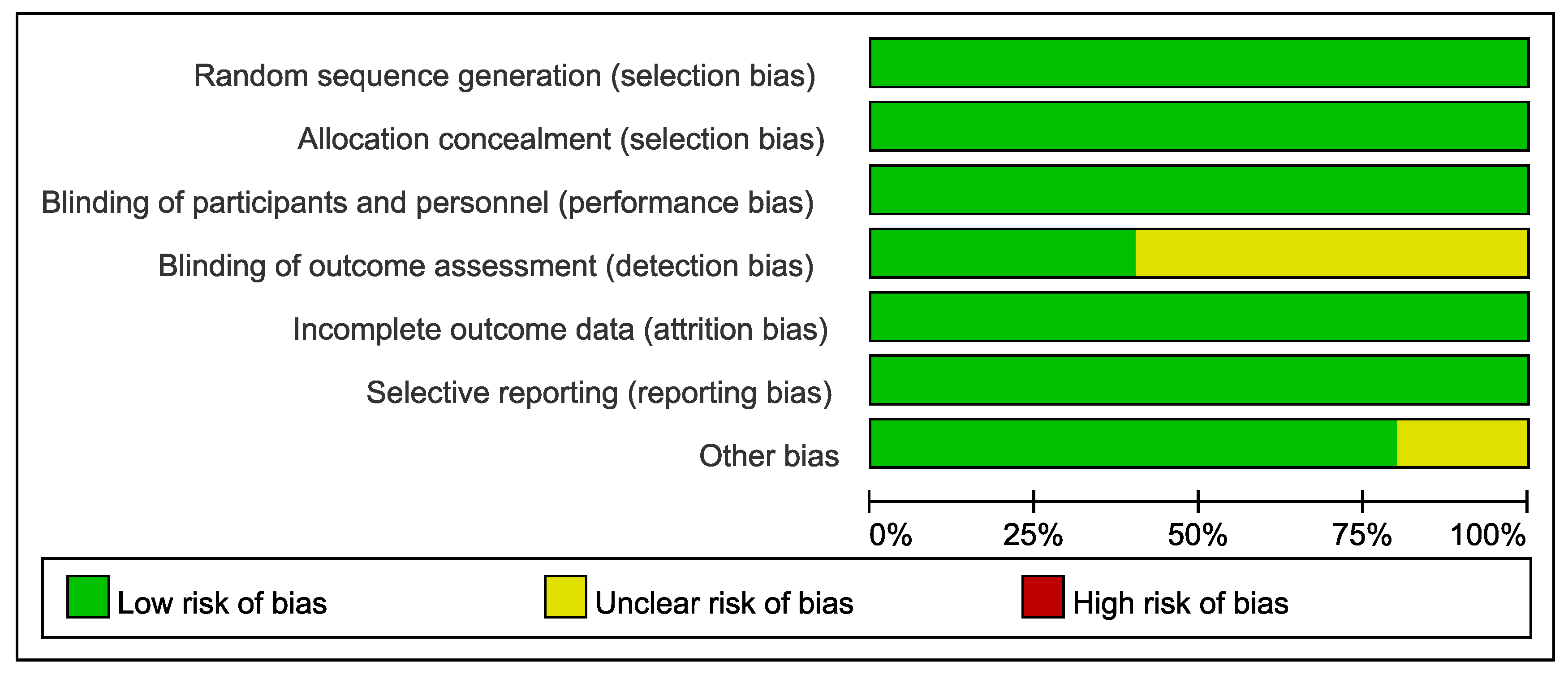
3.3. Quality Assessment (Risk of Bias)
3.4. Meta-Analysis
4. Discussion
4.1. Strengths and Limitations
4.2. Directions of Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.S.; Ghosh, K. Current status of probiotic and related health benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Varela-Trinidad, G.U.; Domínguez-Díaz, C.; Solórzano-Castanedo, K.; Íñiguez-Gutiérrez, L.; Hernández-Flores, T.J.; Fafutis-Morris, M. Probiotics: Protecting Our Health from the Gut. Microorganisms 2022, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hangyu, H.; Naveed, M.; Shabbir, M.A.; Sarwar, A.; Nasbeeb, J.; Zhennai, Y.; Alharbi, M. Genotypic Profiling, Functional Analysis, Cholesterol-Lowering Ability, and Angiotensin I-Converting Enzyme (ACE) Inhibitory Activity of Probiotic Lactiplantibacillus plantarum K25 via Different Approaches. Probiotics Antimicrob. Proteins 2024, 1–15. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views Off. J. Gulf Heart Assoc. 2017, 18, 109–114. [Google Scholar] [CrossRef]
- Tardif, J.C. Coronary artery disease in 2010. Eur. Heart J. Suppl. 2010, 12, C2–C10. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Silvis, M.J.M.; Demkes, E.J.; Fiolet, A.T.L.; Dekker, M.; Bosch, L.; van Hout, G.P.J.; Timmers, L.; de Kleijn, D.P.V. Immunomodulation of the NLRP3 Inflammasome in Atherosclerosis, Coronary Artery Disease, and Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2021, 14, 23–34. [Google Scholar] [CrossRef]
- Dimitroglou, Y.; Aggeli, C.; Theofilis, P.; Tsioufis, P.; Oikonomou, E.; Chasikidis, C.; Tsioufis, K.; Tousoulis, D. Novel Anti-Inflammatory Therapies in Coronary Artery Disease and Acute Coronary Syndromes. Life 2023, 13, 1669. [Google Scholar] [CrossRef]
- Lorenzatti, A.; Servato, M.L. Role of Anti-inflammatory Interventions in Coronary Artery Disease: Understanding the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Cardiol. 2018, 13, 38–41. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Li, X.; Guo, D.; Hu, Y.; Chen, Y. Oxidative Stress and Inflammation Are Associated with Coexistent Severe Multivessel Coronary Artery Stenosis and Right Carotid Artery Severe Stenosis in Elderly Patients. Oxidative Med. Cell. Longev. 2021, 2021, 2976447. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P.; Parveen, A.; Kim, S.Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. (Updated March, 2011). The Cochrane Collaboration. Available online: http://www.mrc-bsu.cam.ac.uk/cochrane/handbook/ (accessed on 19 July 2025).
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Farrokhian, A.; Raygan, F.; Soltani, A.; Tajabadi-Ebrahimi, M.; Sharifi Esfahani, M.; Karami, A.A.; Asemi, Z. The Effects of Synbiotic Supplementation on Carotid Intima-Media Thickness, Biomarkers of Inflammation, and Oxidative Stress in People with Overweight, Diabetes, and Coronary Heart Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 133–142. [Google Scholar] [CrossRef]
- Moludi, J.; Alizadeh, M.; Behrooz, M.; Maleki, V.; Seyed Mohammadzad, M.H.; Golmohammadi, A. Interactive Effect of Probiotics Supplementation and Weight Loss Diet on Metabolic Syndrome Features in Patients With Coronary Artery Diseases: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Am. J. Lifestyle Med. 2021, 15, 653–663. [Google Scholar] [CrossRef]
- Moludi, J.; Alizadeh, M.; Mohammadzad, M.H.S.; Davari, M. The Effect of Probiotic Supplementation on Depressive Symptoms and Quality of Life in Patients After Myocardial Infarction: Results of a Preliminary Double-Blind Clinical Trial. Psychosom. Med. 2019, 81, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Palathinkara, M.; Aljadah, M.; Thorgerson, A.; Dawson, A.Z.; Widlansky, M.E. Association of probiotic supplementation and cardiovascular risk profiles of patients with coronary artery disease—A cross-sectional analysis of the NHANES database between 1999–2019. Front. Nutr. 2025, 12, 1495633. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Li, Z.; Luo, Y. Association of the gut microbiota with coronary artery disease and myocardial infarction: A Mendelian randomization study. Front. Genet. 2023, 14, 1158293. [Google Scholar] [CrossRef]
- Hofeld, B.C.; Puppala, V.K.; Tyagi, S.; Ahn, K.W.; Anger, A.; Jia, S.; Salzman, N.H.; Hessner, M.J.; Widlansky, M.E. Lactobacillus plantarum 299v probiotic supplementation in men with stable coronary artery disease suppresses systemic inflammation. Sci. Rep. 2021, 11, 3972. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Liu, M.; Tandorost, A.; Moludi, J.; Dey, P. Prebiotics Plus Probiotics May Favorably Impact on Gut Permeability, Endocannabinoid Receptors, and Inflammatory Biomarkers in Patients with Coronary Artery Diseases: A Clinical Trial. Food Sci. Nutr. 2024, 12, 1207–1217. [Google Scholar] [CrossRef]
- Moludi, J.; Kafil, H.S.; Qaisar, S.A.; Gholizadeh, P.; Alizadeh, M.; Vayghyan, H.J. Effect of probiotic supplementation along with calorie restriction on metabolic endotoxemia, and inflammation markers in coronary artery disease patients: A double blind placebo controlled randomized clinical trial. Nutr. J. 2021, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Cantero, M.A.; Guedes, M.R.A.; Fernandes, R.; Lollo, P.C.B. Trimethylamine N-oxide reduction is related to probiotic strain specificity: A systematic review. Nutr. Res. 2022, 104, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Popa-Fotea, N.M.; Ferdoschi, C.E.; Micheu, M.M. Molecular and cellular mechanisms of inflammation in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1200341. [Google Scholar] [CrossRef]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Zakhary, N.I.; Aleya, L.; Bungǎu, S.G.; Bohara, R.A.; Siddiqi, N.J. Aging, Metabolic, and Degenerative Disorders: Biomedical Value of Antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 2098123. [Google Scholar] [CrossRef]
- Zaha, D.C.; Bungau, S.; Aleya, S.; Tit, D.M.; Vesa, C.M.; Popa, A.R.; Pantis, C.; Maghiar, O.A.; Bratu, O.G.; Furau, C.; et al. What antibiotics for what pathogens? The sensitivity spectrum of isolated strains in an intensive care unit. Sci. Total Environ. 2019, 687, 118–127. [Google Scholar] [CrossRef]

| Study | Number of Patients | Mean Age (Years) | Sex (%) | Diagnosis | Type of Probiotic | Dosage (CFU) | Duration of Intervention (Weeks) |
|---|---|---|---|---|---|---|---|
| Moludi J (2022) [26] | Probiotic: 24 Placebo: 24 | 51.5 | Female: 35.4 Male: 64.6 | CAD | Lactobacillus rhamnosus | 1.9 × 109 | 8 |
| Farrokhian A (2019) [23] | Synbiotic: 30 Placebo: 30 | 64.1 | Female: 63.3 Male: 36.7 | CAD with overweight and T2DM | Lactobacillus acidophilus; Lactobacillus casei;
Bifidobacterium bifidum | 2 × 109 | 12 |
| Moludi J (2019)-A [25] | Probiotic: 22 Placebo: 22 | 56.9 | Female: 6.8 Male: 93.2 | CAD with myocardial infarction | Lactobacillus rhamnosus | 1.6 × 109 | 12 |
| Moludi J (2019)-B [24] | Probiotic: 22 Placebo: 22 | 52.6 | Female: 6.8 Male: 93.2 | CAD | Lactobacillus rhamnosus | 1.6 × 109 | 12 |
| Raygan F (2018) [27] | Synbiotic: 30 Placebo: 30 | 69.4 | Female: 50.0 Male: 50.0 | 2- and 3-vessel CAD with T2DM | Lactocare® (ZistTakhmir Company) | 8 × 109 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, Y.-Y.; Chiu, T.-Y.; Chen, M.-J. Probiotic Supplementation and Inflammatory Status in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Microorganisms 2025, 13, 2303. https://doi.org/10.3390/microorganisms13102303
Chiou Y-Y, Chiu T-Y, Chen M-J. Probiotic Supplementation and Inflammatory Status in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Microorganisms. 2025; 13(10):2303. https://doi.org/10.3390/microorganisms13102303
Chicago/Turabian StyleChiou, Yuan-Yow, Tsu-Yun Chiu, and Mei-Ju Chen. 2025. "Probiotic Supplementation and Inflammatory Status in Coronary Artery Disease: A Systematic Review and Meta-Analysis" Microorganisms 13, no. 10: 2303. https://doi.org/10.3390/microorganisms13102303
APA StyleChiou, Y.-Y., Chiu, T.-Y., & Chen, M.-J. (2025). Probiotic Supplementation and Inflammatory Status in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Microorganisms, 13(10), 2303. https://doi.org/10.3390/microorganisms13102303