Dual Benefits of Endophytic Bacillus velezensis Amzn015: Growth Promotion and Root Rot Control in Atractylodes macrocephala
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Microbial Isolation
2.2. Preliminary Screening of Antagonistic Bacteria Against F. oxysporum
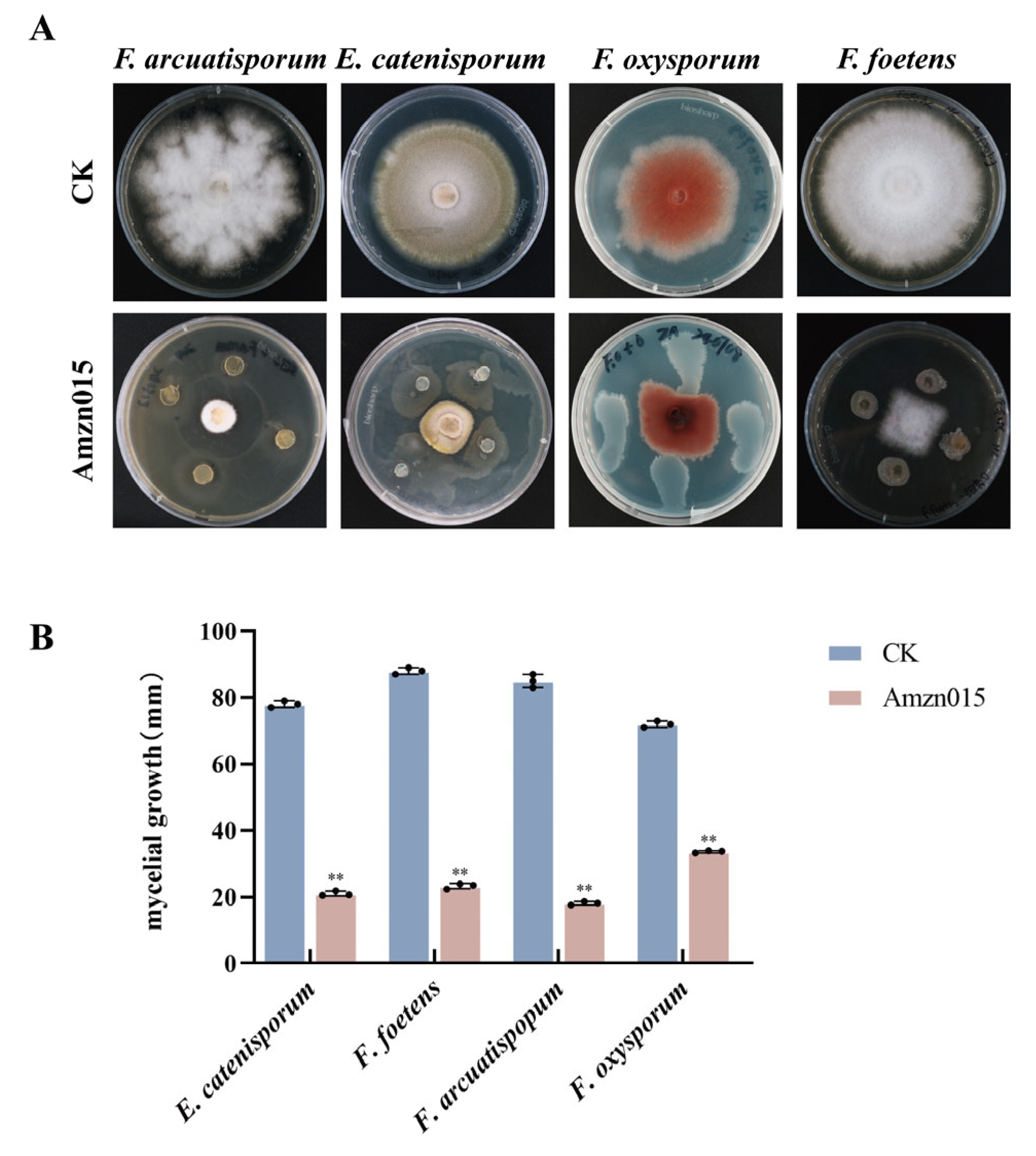
2.3. Verification of Broad-Apectrum Antagonistic Activity
2.4. Morphological and Molecular Identification of Amzn015
2.5. Assessment of Amzn015’s Plant Growth Capabilities
2.6. Effects of Amzn015 on Spore Germination, Viability, and Hyphal Morphology of F. oxysporum
2.6.1. Effect on Spore Germination
2.6.2. Assessment of Spore Viability by Evans Blue Staining
2.6.3. Scanning Electron Microscopy (SEM) Observation of Hyphae
2.7. Assessment of the Effects of Amzn015 on the Germination and Seedling Performance of A. macrocephala
2.8. Assessment of Disease Index in A. macrocephala Following F. oxysporum Infection with or Without Amzn015 Induction
2.9. Determination of Defense Enzyme Activities in A. macrocephala Induced by Amzn015
2.10. RNA Extraction, cDNA Synthesis, and qPCR Analysis
2.11. Statistical Analysis
3. Results
3.1. Isolation of Endophytic Bacteria from A. macrocephala and Screening for Antagonistic Activity
3.2. Amzn015 was Identified as Bacillus velezensis
3.3. Microscopic Evidence Reveals the Multitarget Antagonistic Mechanism of Amzn015
3.4. Amzn015 Enhances Growth and Disease Resistance in A. macrocephala
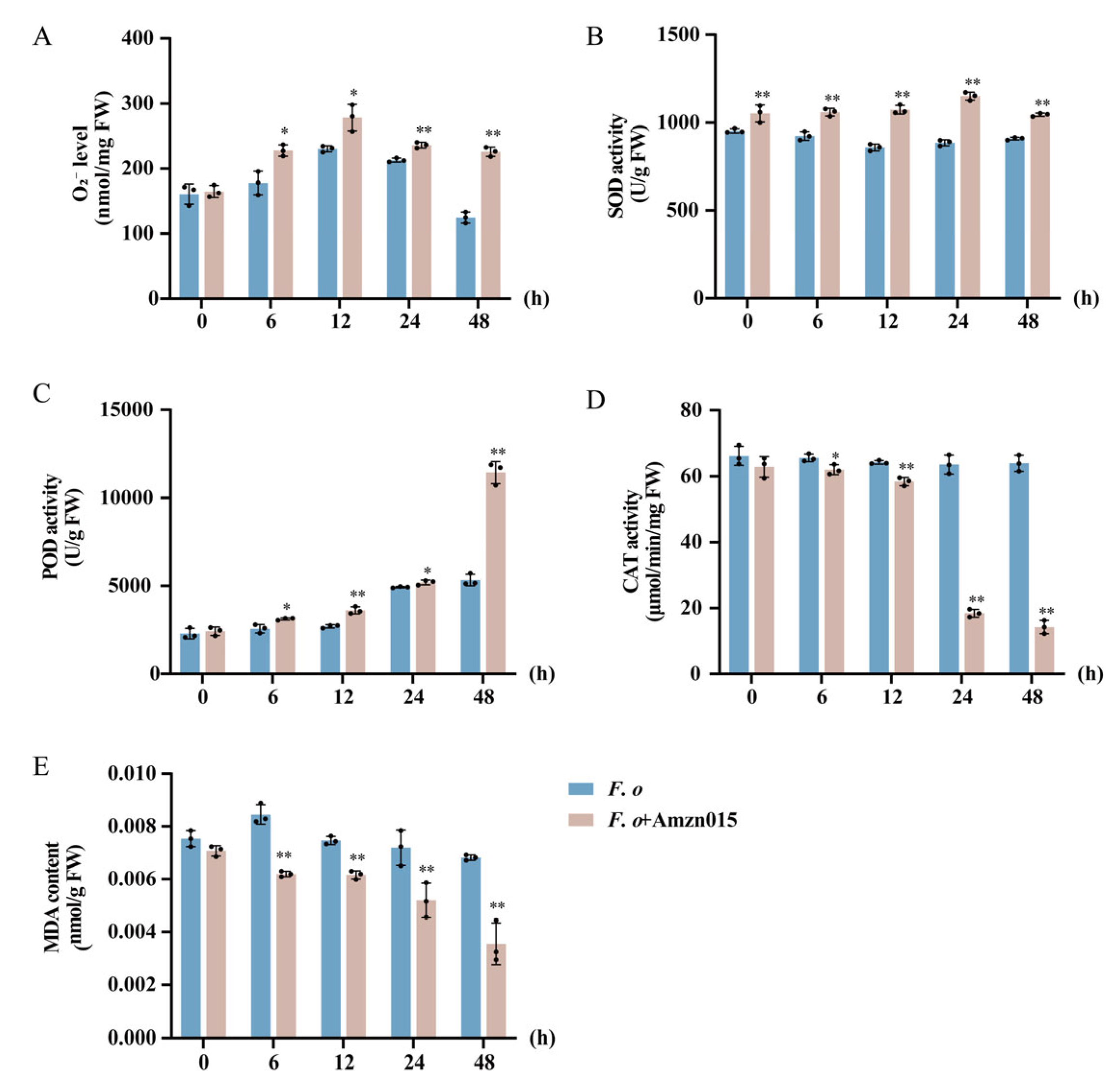
3.5. Amzn015 Enhances Disease Resistance in A. macrocephala by Dynamically Regulating ROS Metabolism
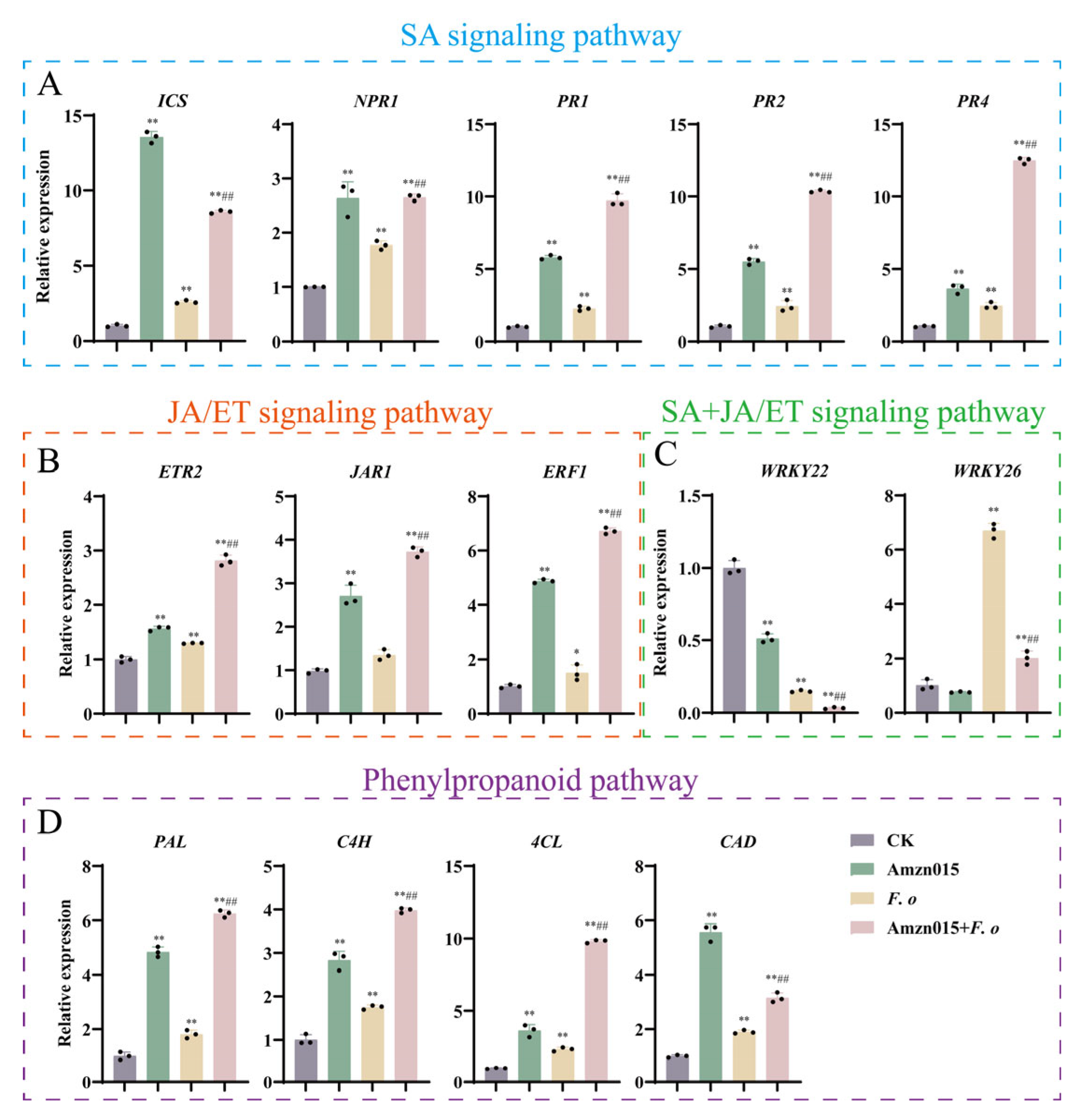
3.6. Amzn015 Activates the Disease Resistance Signaling Network in A. macrocephala
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Commission, N.P. Pharmacopoeia of the People’s Republic of China Part 1; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Zhang, M.; Fan, H.; Wang, P. Effects of plant immune inducer “Baokangling No.1” on quality and root rot resistance of Atractylodes macrocephala. Plant Med. 2024, 3, 34–42. [Google Scholar]
- Fan, H.; an, J.; Li, X.; Zhou, J.; Zhao, L.; Ying, Y.; Kai, G. Atractylodes macrocephala Root Rot Affects Microbial Communities in Various Root-Associated Niches. Agronomy 2024, 14, 2662. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, J.; Ji, Q.; Wu, W.; Dong, S.; Yu, J.; Zhang, Q.; Qin, L. Diversity of rhizosphere and endophytic fungi in Atractylodes macrocephala during continuous cropping. PeerJ 2020, 8, e8905. [Google Scholar] [CrossRef]
- Huang, X.G.; Li, M.Y.; Yan, X.N.; Yang, J.S.; Rao, M.C.; Yuan, X.F. The potential of Trichoderma brevicompactum for controlling root rot on Atractylodes macrocephala. Can. J. Plant Pathol. 2021, 43, 794–802. [Google Scholar] [CrossRef]
- Al Raish, S.M.; Sourani, O.M.; Abu-Elsaoud, A.M. Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects. Appl. Microbiol. 2025, 5, 44. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, L.; Okyere, S.K.; Wen, J.; Ran, Y.; Nong, X.; Hu, Y. Antibacterial Activity of Two Metabolites Isolated From Endophytic Bacteria Bacillus velezensis Ea73 in Ageratina adenophora. Front. Microbiol. 2022, 13, 860009. [Google Scholar] [CrossRef] [PubMed]
- Nanjani, S.; Soni, R.; Paul, D.; Keharia, H. Genome analysis uncovers the prolific antagonistic and plant growth-promoting potential of endophyte Bacillus velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wang, X.; Song, X.; Zhou, Q.; Li, C.; Chen, Z.; Gao, Q.; Li, H.; Li, J.; Zhang, P.; et al. Effect of Bacillus velezensis JC-K3 on Endophytic Bacterial and Fungal Diversity in Wheat Under Salt Stress. Front. Microbiol. 2021, 12, 802054. [Google Scholar] [CrossRef]
- Han, V.C.; Yu, N.H.; Yoon, H.; Ahn, N.H.; Son, Y.K.; Lee, B.H.; Kim, J.C. Identification, Characterization, and Efficacy Evaluation of Bacillus velezensis for Shot-Hole Disease Biocontrol in Flowering Cherry. Plant Pathol. J. 2022, 38, 115–130. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.M.; Yang, J.E.; Jeong, S.-G.; Kim, Y.Y.; Hwang, I.M.; Yu, N.H.; Kim, J.-C.; Park, H.W. Biological control of the shot-hole disease in flowering cherry tree using antimicrobial compounds produced by Bacillus velezensis 8–2. Chem. Biol. Technol. Agric. 2024, 11, 87. [Google Scholar] [CrossRef]
- Wang, J.; Qu, F.; Liang, J.; Yang, M.; Hu, X. Bacillus velezensis SX13 promoted cucumber growth and production by accelerating the absorption of nutrients and increasing plant photosynthetic metabolism. Sci. Hortic. 2022, 301, 111151. [Google Scholar] [CrossRef]
- Ta, Y.; Fu, S.; Liu, H.; Zhang, C.; He, M.; Yu, H.; Ren, Y.; Han, Y.; Hu, W.; Yan, Z.; et al. Evaluation of Bacillus velezensis F9 for Cucumber Growth Promotion and Suppression of Fusarium wilt Disease. Microorganisms 2024, 12, 1882. [Google Scholar] [CrossRef]
- Zhang, J.; Islam, M.S.; Wang, J.; Zhao, Y.; Dong, W. Isolation of Potato Endophytes and Screening of Chaetomium globosum Antimicrobial Genes. Int. J. Mol. Sci. 2022, 23, 4611. [Google Scholar] [CrossRef]
- Skidmore, A.M.; Dickinson, C.H. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 1976, 66, 57–64. [Google Scholar] [CrossRef]
- Jin, M.J.; Li, S.S.; Tian, W.B.; Yang, C.D.; Wang, Y.Q. Screening, identification and detection of growth-promoting antagonistic endophytic bacteria from Carex moorcroftii in alpine grassland. J. Plant Prot. 2019, 46, 779–786. [Google Scholar]
- Kumar, S.; Yadav, A.K.; Chambel, P.; Kaur, R. Molecular and functional characterization of myxobacteria isolated from soil in India. 3 Biotech. 2017, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Sarangi, B.K.; Thul, S.T. Identification of arsenic resistant endophytic bacteria from Pteris vittata roots and characterization for arsenic remediation application. J. Env. Manag. 2016, 180, 359–365. [Google Scholar] [CrossRef]
- Harding, M.W.; Hill, T.B.; Yang, Y.; Daniels, G.C.; Hwang, S.F.; Strelkov, S.E.; Howard, R.J.; Feng, J. An Improved Evans Blue Staining Method for Consistent, Accurate Assessment of Plasmodiophora brassicae Resting Spore Viability. Plant Dis. 2019, 103, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S.; Sharma, S. Decoding the Plant Growth Promotion and Antagonistic Potential of Bacterial Endophytes From Ocimum sanctum Linn. Against Root Rot Pathogen Fusarium oxysporum in Pisum sativum. Front. Plant Sci. 2022, 13, 813686. [Google Scholar] [CrossRef]
- Rewers, M.; Sliwinska, E. Endoreduplication intensity as a marker of seed developmental stage in the Fabaceae. Cytom. A 2012, 81, 1067–1075. [Google Scholar] [CrossRef]
- Tang, Y.; Li, R.; Jiang, Z.; Cheng, Z.; Li, W.; Shao, Y. Combined effect of Debaryomyces hansenii and Bacillus atrophaeus on the physicochemical attributes, defense-related enzyme activity, and transcriptomic profile of stored litchi fruit. Biol. Control 2022, 172, 104975. [Google Scholar] [CrossRef]
- Sultana, S.; Adhikary, S.K.; Islam, M.M.; Rahman, S.M.M. Evaluation of Pathogenic Variability Based on Leaf Blotch Disease Development Components of Bipolaris sorokiniana in Triticum aestivum and Agroclimatic Origin. Plant Pathol. J. 2018, 34, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tang, Y.; Zhu, N.; Meng, Q.; Zhou, Y.; Zhao, Y.; Xu, J.; Gu, C.; Dai, S.; Zhu, B.; et al. Analyzing the defense response mechanism of Atractylodes macrocephala to Fusarium oxysporum through small RNA and degradome sequencing. Front. Plant Sci. 2024, 15, 1415209. [Google Scholar] [CrossRef]
- Bubner, B.; Baldwin, I.T. Use of real-time PCR for determining copy number and zygosity in transgenic plants. Plant Cell Rep. 2004, 23, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Z.; Feng, Z.; Feng, H.; Wei, F.; Zhao, L. Overview of cotton Verticillium wilt research over the past decades in China and its Prospect in future. Cotton Sci. 2017, 29, 37–50. [Google Scholar]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Moreno-Ramirez, L.; Valdez-Salas, B.; Seleiman, M.F.; El-Hendawy, S.; Aldhuwaib, K.J.; Alotaibi, M.; Gonzalez-Mendoza, D. New Bacillus subtilis Strains Isolated from Prosopis glandulosa Rhizosphere for Suppressing Fusarium Spp. and Enhancing Growth of Gossypium hirsutum L. Biology 2022, 12, 73. [Google Scholar] [CrossRef]
- Berte, R.; Teixeira, G.M.; de Oliveira, J.P.; Nicoletto, M.L.A.; da Silva, D.V.; de Godoy, G.G.; Sanches, D.S.; de Resende, J.T.V.; Pereira, U.P.; Nunes da Rocha, U.; et al. Genome Mining Reveals High Biosynthetic Potential of Biocontrol Agent Bacillus velezensis B.BV10. Genes 2022, 13, 1984. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, W.; Wu, X.; Ye, J. Bacillus velezensis JK-XZ8 prevents and controls crown gall disease on Prunus subhirtella by colonizing and inducing resistance. J. For. Res. 2021, 33, 1019–1031. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, M.; Kong, Z.; Chen, X.; Wang, X.; Ding, W.; Lai, H.; Guo, Q. Genomic Analysis Reveals Potential Mechanisms Underlying Promotion of Tomato Plant Growth and Antagonism of Soilborne Pathogens by Bacillus amyloliquefaciens Ba13. Microbiol. Spectr. 2021, 9, e0161521. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, G.; Wang, L.; Xing, Y. Bacillus velezensis BY6 Promotes Growth of Poplar and Improves Resistance Contributing to the Biocontrol of Armillaria solidipes. Microorganisms 2022, 10, 2472. [Google Scholar] [CrossRef]
- Jin, W.; Peng, L.; Zhang, X.; Sun, H.; Yuan, Z. Effects of endophytic and ectomycorrhizal basidiomycetes on Quercus virginiana seedling growth and nutrient absorption. J. Sustain. For. 2019, 38, 457–470. [Google Scholar] [CrossRef]
- Hong, P.; Hao, W.; Luo, J.; Chen, S.; Hu, M.; Zhong, G. Combination of hot water, Bacillus amyloliquefaciens HF-01 and sodium bicarbonate treatments to control postharvest decay of mandarin fruit. Postharvest Biol. Technol. 2014, 88, 96–102. [Google Scholar] [CrossRef]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 2018, 93, 592–613, Erratum in Plant J. 2018, 94, 450. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Shen, H.; Yang, L. Effect of Exogenous Plant Growth Regulators and Rejuvenation Measures on the Endogenous Hormone and Enzyme Activity Responses of Acer mono Maxim in Cuttage Rooting. Int. J. Mol. Sci. 2023, 24, 11883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hao, H.; Wang, L.; Liu, Z.; Ma, L. Endophytes Bacillus amyloliquefaciens AW3 (CGMCC1.16683) improves the growth of Populus davidiana × Populus bolleana (PdPap) and induces its resistance to wilt disease by Fusarium oxysporum Fox68 (CFCC86068). Eur. J. Plant Pathol. 2021, 162, 1–17. [Google Scholar] [CrossRef]
- Wang, X.; Liang, L.; Shao, H.; Ye, X.; Yang, X.; Chen, X.; Shi, Y.; Zhang, L.; Xu, L.; Wang, J. Isolation of the Novel Strain Bacillus amyloliquefaciens F9 and Identification of Lipopeptide Extract Components Responsible for Activity against Xanthomonas citri subsp. citri. Plants 2022, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, B.; Narisawa, K.; Nong, Q.; Qin, L.; Xie, L. The Dark Septate Endophyte, Phialocephala fortinii J2PC4, Mitigates Southern Rice Black-Streaked Dwarf Disease and Impacts the Mortality of White Back Planthopper. Biol. Control 2022, 170, 104911. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Gheysen, G.; Hofte, M. Hormone defense networking in rice: Tales from a different world. Trends Plant Sci. 2013, 18, 555–565. [Google Scholar] [CrossRef]
- Kalsi, H.S.; Karkhanis, A.A.; Natarajan, B.; Bhide, A.J.; Banerjee, A.K. AUXIN RESPONSE FACTOR 16 (StARF16) regulates defense gene StNPR1 upon infection with necrotrophic pathogen in potato. Plant Mol. Biol. 2022, 109, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, M.; Mei, C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hai, J.; Wang, Z.; Deng, J.; Liang, T.; Su, L.; Liu, D. Lilium regale Wilson WRKY2 Regulates Chitinase Gene Expression During the Response to the Root Rot Pathogen Fusarium oxysporum. Front. Plant Sci. 2021, 12, 741463. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Wu, J.; Fan, S.; Meng, Q.; Dai, S.; Mao, M.; Zhao, W.; Yuan, X. Dual Benefits of Endophytic Bacillus velezensis Amzn015: Growth Promotion and Root Rot Control in Atractylodes macrocephala. Microorganisms 2025, 13, 2300. https://doi.org/10.3390/microorganisms13102300
Zhu N, Wu J, Fan S, Meng Q, Dai S, Mao M, Zhao W, Yuan X. Dual Benefits of Endophytic Bacillus velezensis Amzn015: Growth Promotion and Root Rot Control in Atractylodes macrocephala. Microorganisms. 2025; 13(10):2300. https://doi.org/10.3390/microorganisms13102300
Chicago/Turabian StyleZhu, Na, Jiongyi Wu, Sen Fan, Qingling Meng, Shijie Dai, Mingjiang Mao, Weichun Zhao, and Xiaofeng Yuan. 2025. "Dual Benefits of Endophytic Bacillus velezensis Amzn015: Growth Promotion and Root Rot Control in Atractylodes macrocephala" Microorganisms 13, no. 10: 2300. https://doi.org/10.3390/microorganisms13102300
APA StyleZhu, N., Wu, J., Fan, S., Meng, Q., Dai, S., Mao, M., Zhao, W., & Yuan, X. (2025). Dual Benefits of Endophytic Bacillus velezensis Amzn015: Growth Promotion and Root Rot Control in Atractylodes macrocephala. Microorganisms, 13(10), 2300. https://doi.org/10.3390/microorganisms13102300





