Cyclodextrin Counteracts Coxsackievirus-Induced Cardiac Damage by Protecting Desmosome Integrity and Suppressing Proinflammatory Cytokine Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Cell Culture and Viral Infection
2.2. Cell Transfection
2.3. Cyclodextrin Treatment
2.4. RNA Extraction and RT-qPCR
2.5. Western Blotting
2.6. Transmission Electron Microscopy (TEM)
2.7. Immunohistochemistry (IHC)
2.8. Immunofluorescence (IF)
2.9. MTS Cell Viability Assay
2.10. Viral Plaque Assay
2.11. Statistical Analysis
3. Results
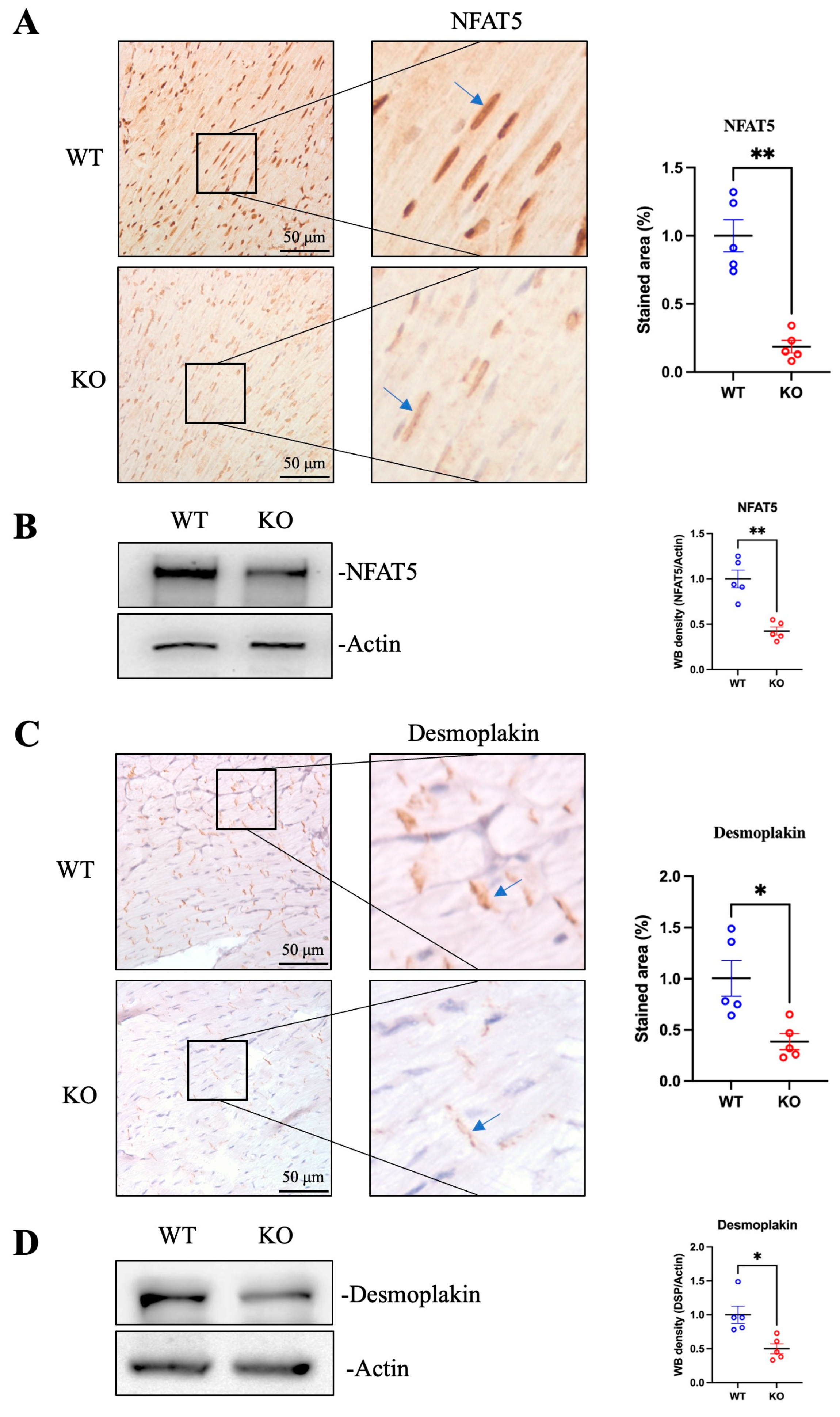
3.1. Deficiency of NFAT5 Destructs Desmoplakin and Desmosome Complex in Mouse Hearts
3.2. Desmoplakin Is a Direct Transcriptional Target of NFAT5
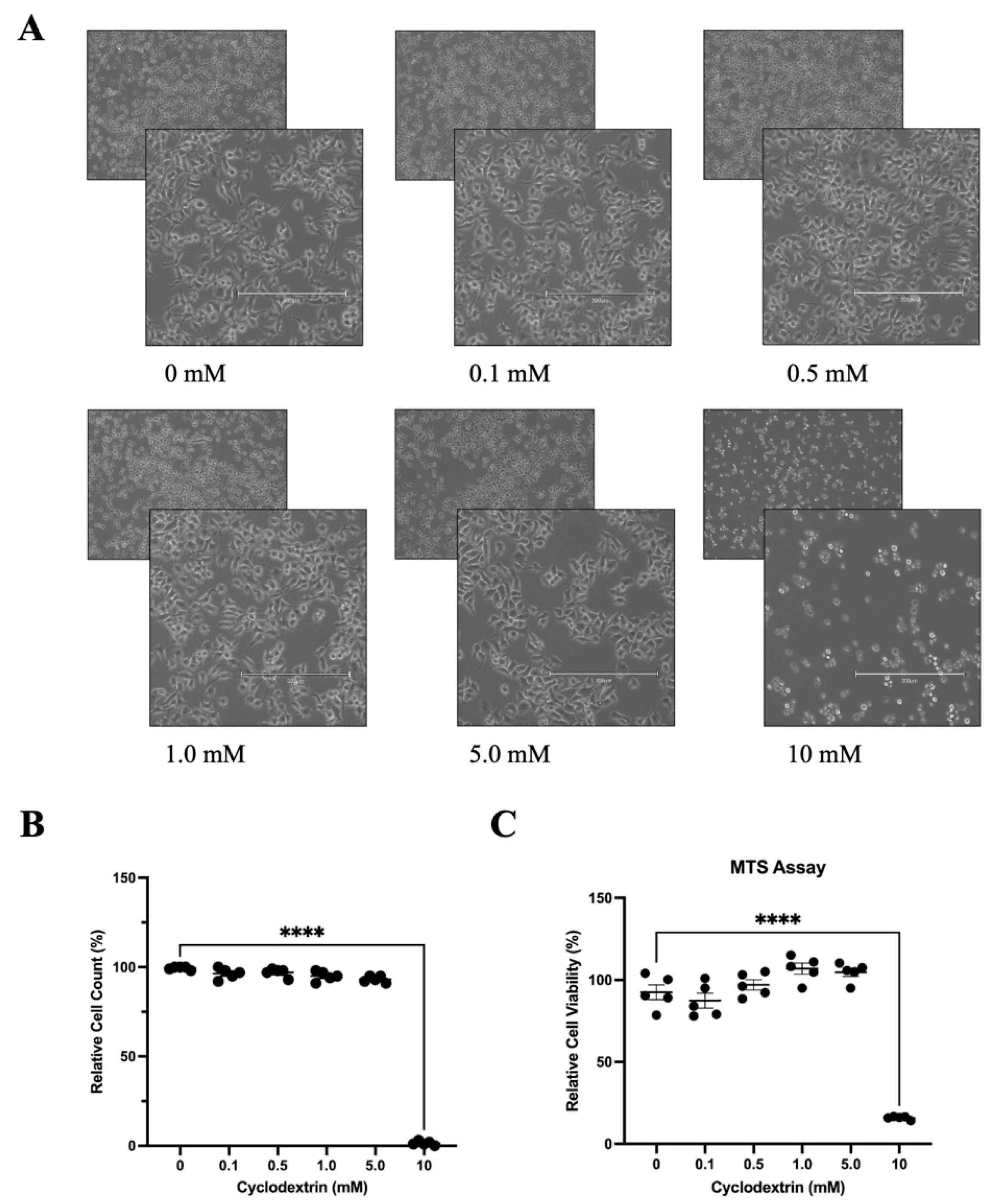
3.3. Determination of Optimal Cyclodextrin Concentration in HeLa Cells for Antiviral Evaluation
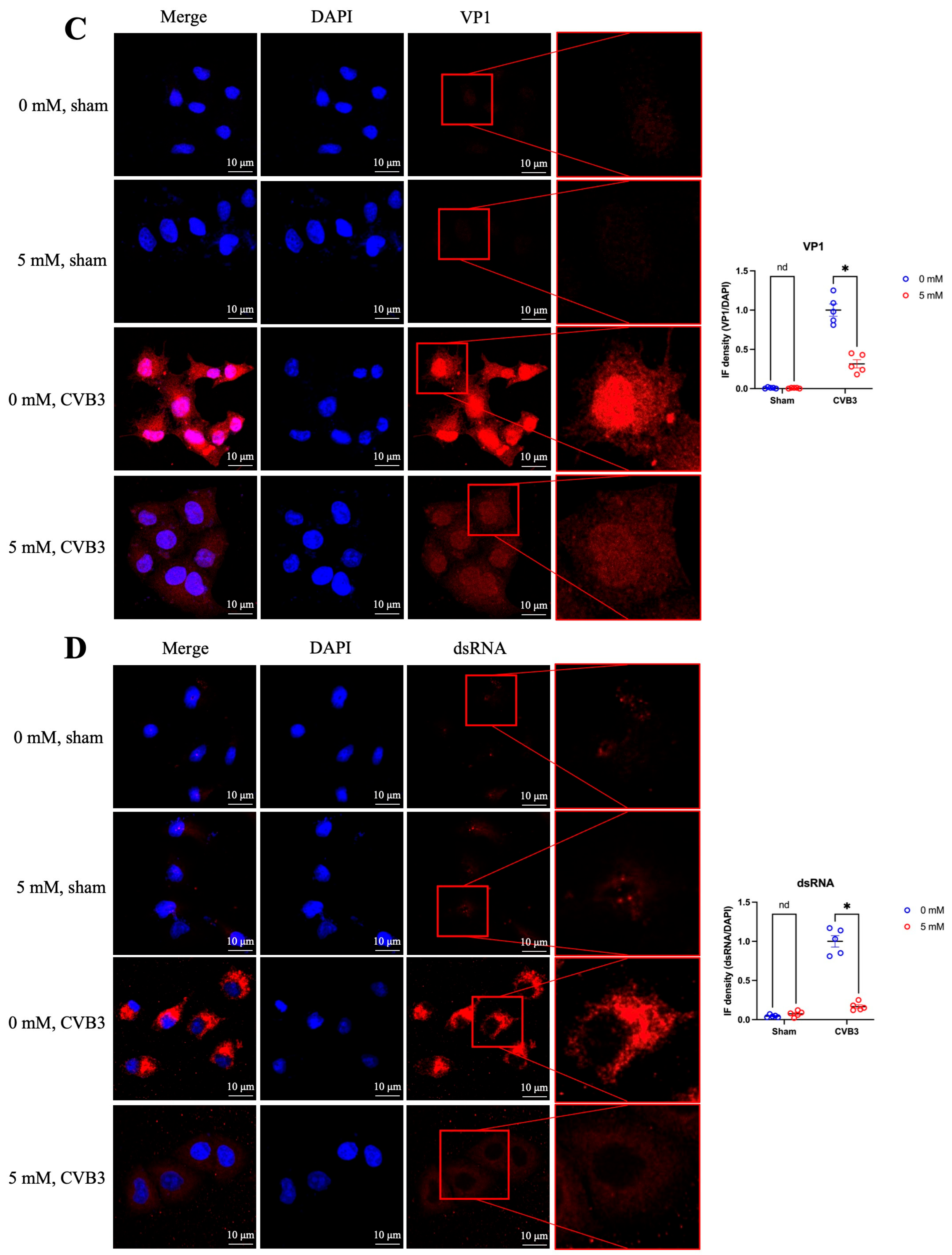
3.4. Antiviral Effect of Cyclodextrin Is Associated with NFAT5 Upregulation
3.5. Cyclodextrin Treatment Restores Desmoplakin Levels Reduced by CVB3-Caused NFAT5 Deficiency
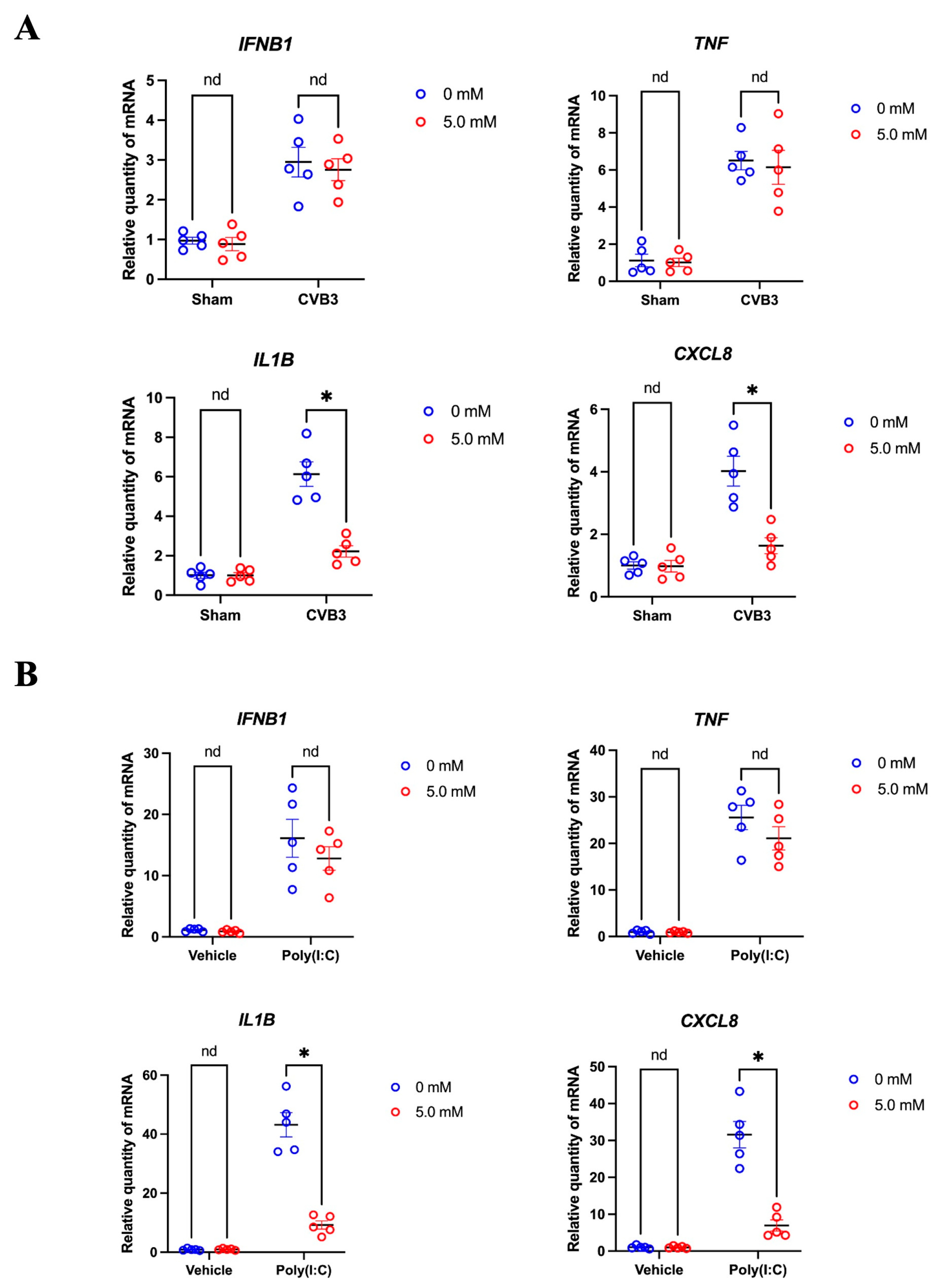
3.6. Cyclodextrin Supresses the Production of Selected Inflammatory Cytokines in CVB3 Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, G.; Qiu, Y.; Zhang, H.M.; Yang, D. Intercalated discs: Cellular adhesion and signaling in heart health and diseases. Heart Fail. Rev. 2019, 24, 115–132. [Google Scholar] [CrossRef]
- Saffitz, J.E. Desmosome mutations in arrhythmogenic right ventricular cardiomyopathy: Important insight but only part of the picture. Circ. Cardiovasc. Genet. 2009, 2, 415–417. [Google Scholar] [CrossRef]
- Chua, C.J.; Morrissette-McAlmon, J.; Tung, L.; Boheler, K.R. Understanding Arrhythmogenic Cardiomyopathy: Advances through the Use of Human Pluripotent Stem Cell Models. Genes 2023, 14, 1864. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Lahrouchi, N.; Priori, S.G. Genetics of sudden cardiac death. Circ. Res. 2015, 116, 1919–1936. [Google Scholar] [CrossRef] [PubMed]
- Garmaroudi, F.S.; Marchant, D.; Hendry, R.; Luo, H.; Yang, D.; Ye, X.; Shi, J.; McManus, B.M. Coxsackievirus B3 replication and pathogenesis. Future Microbiol. 2015, 10, 629–653. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, H.M.; Qiu, Y.; Hanson, P.J.; Hemida, M.G.; Wei, W.; Hoodless, P.A.; Chu, F.; Yang, D. Coxsackievirus-induced miR-21 disrupts cardiomyocyte interactions via the downregulation of intercalated disk components. PLoS Pathog. 2014, 10, e1004070. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H.M.; Qiu, Y.; Ye, X.; Yang, D. Cleavage of Desmosomal Cadherins Promotes gamma-Catenin Degradation and Benefits Wnt Signaling in Coxsackievirus B3-Induced Destruction of Cardiomyocytes. Front. Microbiol. 2020, 11, 767. [Google Scholar]
- Zhao, G.; Aghakeshmiri, S.; Chen, Y.T.; Zhang, H.M.; Yip, F.; Yang, D. NFAT5-Mediated Signalling Pathways in Viral Infection and Cardiovascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 4872. [Google Scholar] [CrossRef]
- Qiu, Y.; Ye, X.; Zhang, H.M.; Hanson, P.; Zhao, G.; Tong, L.; Xie, R.; Yang, D. Cleavage of osmosensitive transcriptional factor NFAT5 by Coxsackieviral protease 2A promotes viral replication. PLoS Pathog. 2017, 13, e1006744. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H.M.; Nasseri, A.R.; Yip, F.; Telkar, N.; Chen, Y.T.; Aghakeshmiri, S.; Küper, C.; Lam, W.; Yang, W.; et al. Heart-specific NFAT5 knockout suppresses type I interferon signaling and aggravates coxsackievirus-induced myocarditis. Basic Res. Cardiol. 2024, 119, 1075–1092. [Google Scholar] [CrossRef]
- Sa Couto, A.R.; Ryzhakov, A.; Loftsson, T. 2-Hydroxypropyl-beta-Cyclodextrin Aggregates: Identification and Development of Analytical Techniques. Materials 2018, 11, 1971. [Google Scholar] [CrossRef]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409, Erratum in Pharmaceutics 2022, 14, 499. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Martina, K.; Cravotto, G. Cyclodextrins in the antiviral therapy. J. Drug Deliv. Sci. Technol. 2021, 64, 102589. [Google Scholar] [CrossRef]
- Lucia Appleton, S.; Navarro-Orcajada, S.; Martínez-Navarro, F.J.; Caldera, F.; López-Nicolás, J.M.; Trotta, F.; Matencio, A. Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules 2021, 11, 1384. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnurch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Albulescu, L.; Wubbolts, R.; Kuppeveld, F.J.M.; Strating, J.R.P.M. Cholesterol shuttling is important for RNA replication of coxsackievirus B3 and encephalomyocarditis virus. Cell. Microbiol. 2015, 17, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Siljamäki, E.; Rintanen, N.; Kirsi, M.; Upla, P.; Wang, W.; Karjalainen, M.; Ikonen, E.; Marjomäki, V. Cholesterol dependence of collagen and echovirus 1 trafficking along the novel α2β1 integrin internalization pathway. PLoS ONE 2013, 8, e55465. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, S.; Briol, A.; Hoessli, D.C. CD44 selectively associates with active Src family protein tyrosine kinases Lck and Fyn in glycosphingolipid-rich plasma membrane domains of human peripheral blood lymphocytes. Blood 1998, 91, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Coyne, C.B.; Bergelson, J.M. Dynamin- and lipid raft-dependent entry of decay-accelerating factor (DAF)-binding and non-DAF-binding coxsackieviruses into nonpolarized cells. J. Virol. 2009, 83, 11064–11077. [Google Scholar] [CrossRef]
- Kosmala, W.; Przewlocka-Kosmala, M.; Mazurek, W. Proinflammatory cytokines and myocardial viability in patients after acute myocardial infarction. Int. J. Cardiol. 2005, 101, 449–456. [Google Scholar] [CrossRef]
- Gui, J.; Yue, Y.; Chen, R.; Xu, W.; Xiong, S. A20 (TNFAIP3) alleviates CVB3-induced myocarditis via inhibiting NF-kappaB signaling. PLoS ONE 2012, 7, e46515. [Google Scholar] [CrossRef] [PubMed]
- Heim, A.; Zeuke, S.; Weiss, S.; Ruschewski, W.; Grumbach, I.M. Transient induction of cytokine production in human myocardial fibroblasts by coxsackievirus B3. Circ. Res. 2000, 86, 753–759. [Google Scholar] [CrossRef]
- Koestner, W.; Spanier, J.; Klause, T.; Tegtmeyer, P.-K.; Becker, J.; Herder, V.; Borst, K.; Todt, D.; Lienenklaus, S.; Gerhauser, I.; et al. Interferon-beta expression and type I interferon receptor signaling of hepatocytes prevent hepatic necrosis and virus dissemination in Coxsackievirus B3-infected mice. PLoS Pathog. 2018, 14, e1007235. [Google Scholar] [CrossRef]
- Deonarain, R.; Cerullo, D.; Fuse, K.; Liu, P.P.; Fish, E.N. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation 2004, 110, 3540–3543. [Google Scholar] [CrossRef]
- Huber, S. Tumor necrosis factor-alpha promotes myocarditis in female mice infected with coxsackievirus B3 through upregulation of CD1d on hematopoietic cells. Viral Immunol. 2010, 23, 79–86. [Google Scholar] [CrossRef]
- Chen, J.; Yang, F.; Shi, S.; Liu, X.; Qin, F.; Wei, X.; Huang, Y.; Liang, W.; Miao, L. The Severity of CVB3-Induced Myocarditis Can Be Improved by Blocking the Orchestration of NLRP3 and Th17 in Balb/c Mice. Mediat. Inflamm. 2021, 2021, 5551578. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ko, B.C. NFAT5 in cellular adaptation to hypertonic stress—Regulations and functional significance. J. Mol. Signal. 2013, 8, 5. [Google Scholar] [CrossRef]
- Yang, Z.; Bowles, N.E.; Scherer, S.E.; Taylor, M.D.; Kearney, D.L.; Ge, S.; Nadvoretskiy, V.V.; DeFreitas, G.; Carabello, B.; Brandon, L.I.; et al. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ. Res. 2006, 99, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Raimondi, F.; Piriou, N.; Infirri, L.S.; Mohiddin, S.A.; Mazzanti, A.; Shenoy, C.; Cavallari, U.A.; Imazio, M.; Aquaro, G.D.; et al. Acute Myocarditis Associated With Desmosomal Gene Variants. JACC Heart Fail. 2022, 10, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Varadi, J.; Hermenean, A.; Gesztelyi, R.; Jeney, V.; Balogh, E.; Majoros, L.; Malanga, M.; Fenyvesi, É.; Szente, L.; Bácskay, I.; et al. Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin. Biomolecules 2019, 9, 509. [Google Scholar] [CrossRef]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Tresoldi, M.; Dagna, L. Myocarditis: An Interleukin-1-Mediated Disease? Front. Immunol. 2018, 9, 1335. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Morosky, S.A.; Delorme-Axford, E.; Dybdahl-Sissoko, N.; Oberste, M.S.; Wang, T.; Coyne, C.B. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011, 7, e1001311. [Google Scholar] [CrossRef] [PubMed]
- Barral, P.M.; Sarkar, D.; Fisher, P.B.; Racaniello, V.R. RIG-I is cleaved during picornavirus infection. Virology 2009, 391, 171–176. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Zhang, H.M.; Zhang, G.J.; Yang, W.; Küper, C.; McManus, B.M.; Yang, D. Cyclodextrin Counteracts Coxsackievirus-Induced Cardiac Damage by Protecting Desmosome Integrity and Suppressing Proinflammatory Cytokine Expression. Microorganisms 2025, 13, 2294. https://doi.org/10.3390/microorganisms13102294
Zhao G, Zhang HM, Zhang GJ, Yang W, Küper C, McManus BM, Yang D. Cyclodextrin Counteracts Coxsackievirus-Induced Cardiac Damage by Protecting Desmosome Integrity and Suppressing Proinflammatory Cytokine Expression. Microorganisms. 2025; 13(10):2294. https://doi.org/10.3390/microorganisms13102294
Chicago/Turabian StyleZhao, Guangze, Huifang M. Zhang, Grace J. Zhang, Wenli Yang, Christoph Küper, Bruce M. McManus, and Decheng Yang. 2025. "Cyclodextrin Counteracts Coxsackievirus-Induced Cardiac Damage by Protecting Desmosome Integrity and Suppressing Proinflammatory Cytokine Expression" Microorganisms 13, no. 10: 2294. https://doi.org/10.3390/microorganisms13102294
APA StyleZhao, G., Zhang, H. M., Zhang, G. J., Yang, W., Küper, C., McManus, B. M., & Yang, D. (2025). Cyclodextrin Counteracts Coxsackievirus-Induced Cardiac Damage by Protecting Desmosome Integrity and Suppressing Proinflammatory Cytokine Expression. Microorganisms, 13(10), 2294. https://doi.org/10.3390/microorganisms13102294





