Microbial Diversity in the Rhizosphere Soils of Three Different Populations of Paphiopedilum helenae, a Critically Endangered Wild Orchid
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection and Processing
2.2. Analytical Methods
2.3. Quality Control and Analysis of Microbiome Sequencing Data
2.4. Analysis of Rhizosphere Soil Microbial Composition
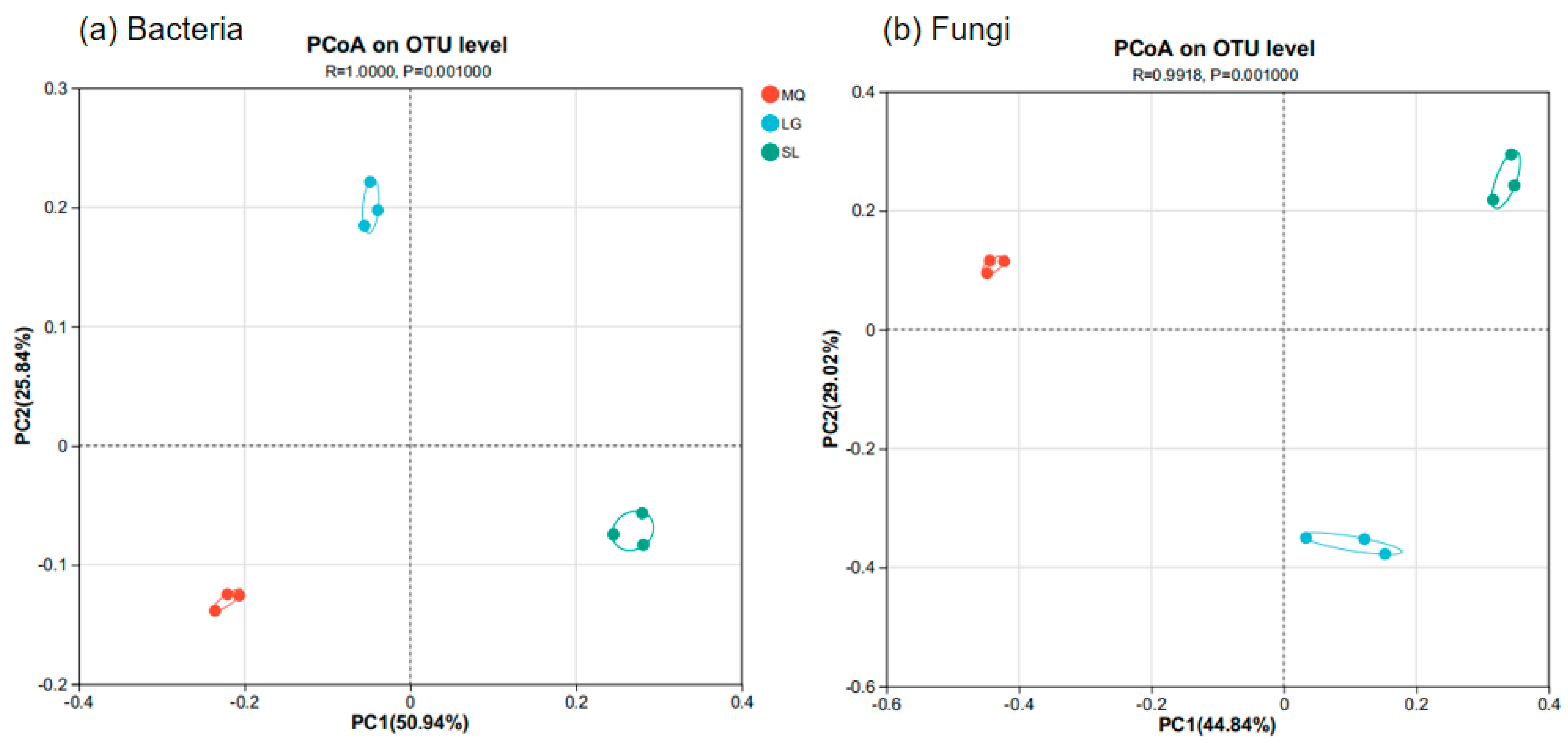
2.5. Analysis of Rhizosphere Soil Microbial Community Structure Differences
2.6. Data Processing
3. Results
3.1. Physicochemical Properties of Paphiopedilum helenae Rhizosphere Soil
3.2. Rhizosphere Soil Microbial Community Composition of Paphiopedilum helenae
3.2.1. Bacterial Community Composition Analysis
3.2.2. Fungal Community Composition Analysis
3.3. α-Diversity of Rhizosphere Soil Microorganisms in Paphiopedilum helenae
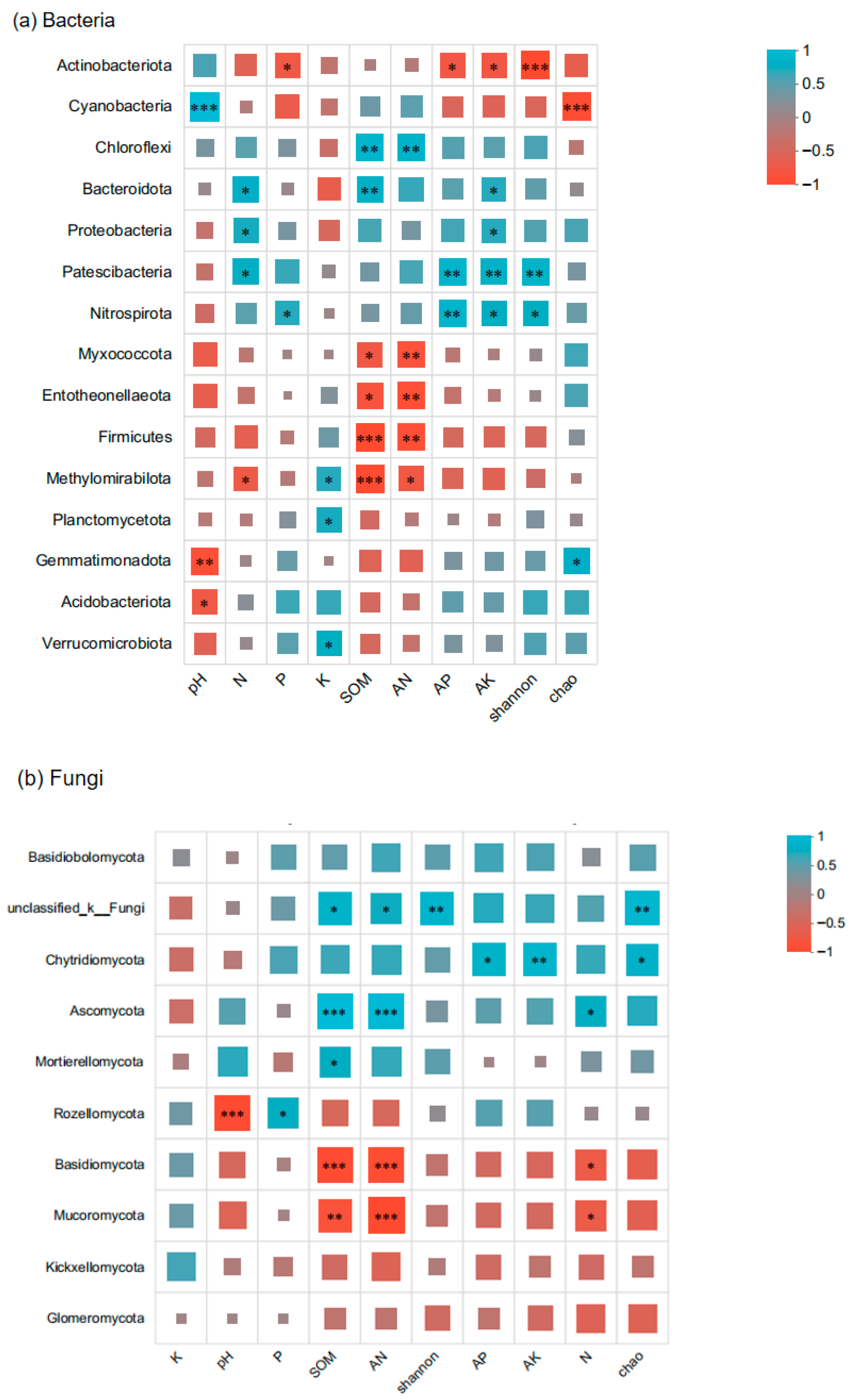
3.4. Correlation Analysis of Rhizosphere Soil Microbial Communities in Paphiopedilum helenae
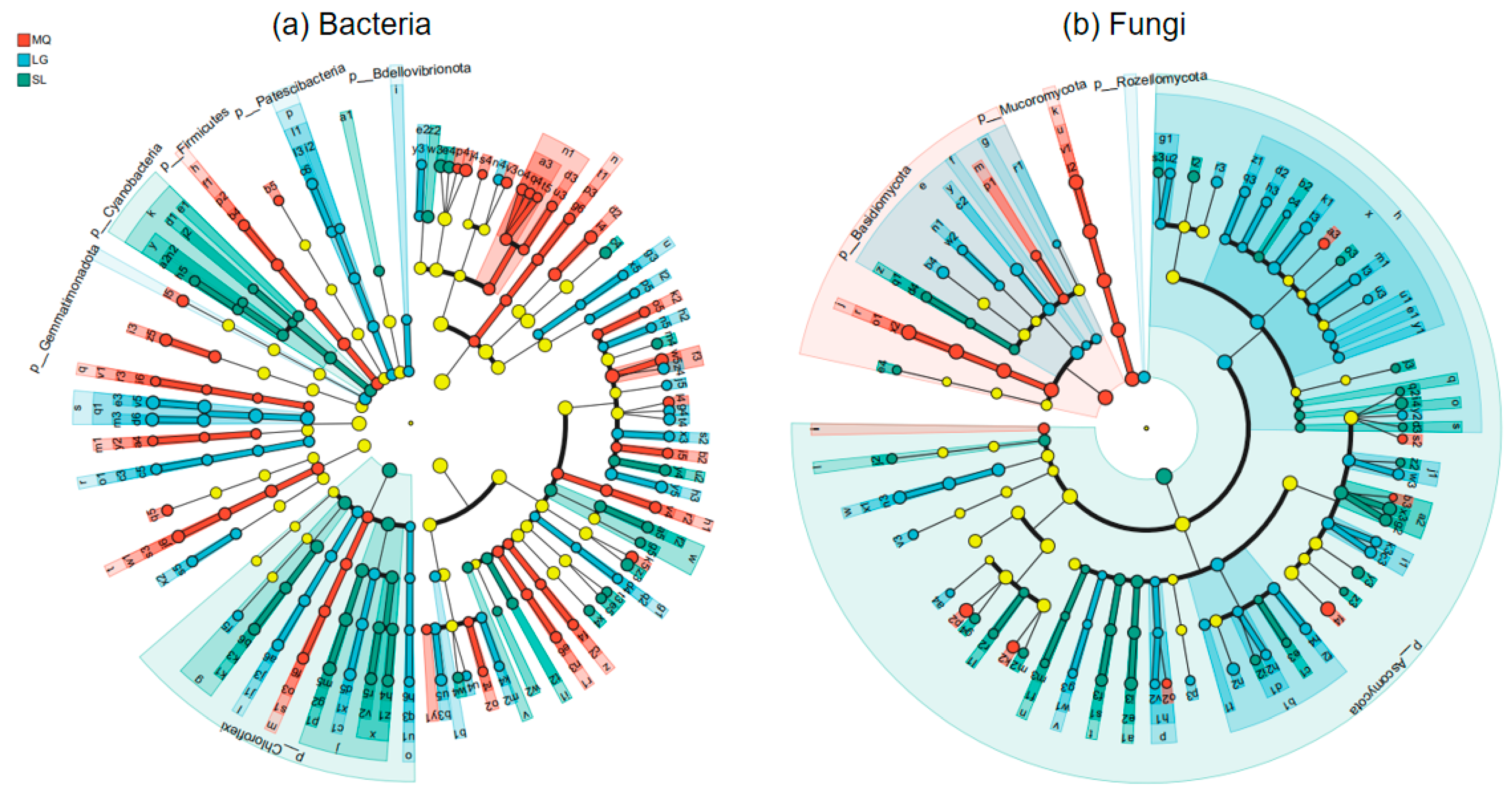
3.5. Analysis of Microbial Species Differences in Paphiopedilum helenae Rhizosphere Soil
3.6. Relationship Between Environmental Factors and Microbial Community Composition
3.7. Correlations Between Dominant Microorganisms and Ecological Factors
4. Discussion
4.1. Microbial Community Composition in the Rhizosphere Soil of P. helenae
4.2. Functional Analysis of Key Microbial Groups and Their Ecological Adaptability
4.3. Relationships Between Microbial Communities and Soil Factors
4.4. Implications of the Conservation Strategies for P. helenae
4.5. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Averyanov, L. Paphiopedilum helenae (Orchidaceae)-new slipper orchid from the North Vietnam. Bot. J. 1996, 81, 105–110. [Google Scholar]
- Huang, Y.; Xue, Y. Conservation status of Paphiopedilum helenae Aver., a newly recorded orchid in China. Acta Phytotaxon. Sin. 2007, 45, 333–336. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, Z.; Wu, K.; Duan, J. Study on introduction and cultivation of Paphiopedilum distributed in China. Chin. Wild Plant Resour. 2010, 29, 53–58. [Google Scholar]
- Yetgin, A. The dynamic interplay of root exudates and rhizosphere microbiome. Soil Stud. 2023, 12, 111–120. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.; Mendes, R.; Raaijmakers, J. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 2015, 90, 635–644. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 544–563. [Google Scholar] [CrossRef]
- Wang, J.; Bao, J.; Su, J.; Li, X.; Chen, G.; Ma, X. Impact of inorganic nitrogen additions on microbes in biological soil crusts. Soil Biol. Biochem. 2015, 88, 303–313. [Google Scholar] [CrossRef]
- Li, H.; Dong, T.; Wang, M. Effects of biochar on microbial communities and metabolic activity in rhizospheric soil of banana seedlings. J. Microbiol. 2016, 36, 42–48. [Google Scholar]
- Quan, Q.; Yang, Y.; Liang, J.; Yang, L.; Wu, C.; Xue, B. Soil Microflora change during integrated protection cultivation of wheat-maize rotation. Chin. Agric. Sci. Bull. 2016, 32, 132–138. [Google Scholar]
- Tian, L.; An, M.; Liu, F.; Zhang, Y. Fungal community characteristics of the last remaining habitat of three Paphiopedilum species in China. Sci. Rep. 2024, 14, 24737. [Google Scholar] [CrossRef]
- Tian, L.; An, M.; Wu, M.; Liu, F.; Zhang, Y. Habitat ecological characteristics and soil fungal community structure of Paphiopedilum subgenus Brachypetalum Hallier (Orchidaceae) plants in Southwest China. Plant Signal. Behav. 2023, 18, 2227365. [Google Scholar] [CrossRef]
- Wu, M. Rhizosphere Soil Microbial Diversity of Paphiopedilum Subgen. Brachypetalum Plants in China. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar] [CrossRef]
- Zhang, Z. Study on the Endangered Mechanism of Key Microorganisms in the Root-Endophytic and Rhizosphere of Four Extremely Small Populations of Paphiopedilum Plant Populations. Master’s Thesis, Henan University of Science and Technology, Luoyang, China, 2023. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, Y.; Huang, W.; Chen, X.; Xue, X.; Wu, B.; Peng, J.; Mo, J.; Zhang, Q. Bacterial and fungal diversity in the old tea plant ecosystem of Camellia sinensis ‘Fujian Shuixian’ cultivated in Gujing. Acta Microbiol. Sin. 2024, 64, 1110–1126. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zhou, M.; Li, J.; Gao, L. Diversity and ecological function of root-associated fungi in three Cypripedium species. Microbiol. China 2019, 46, 2134–2145. [Google Scholar] [CrossRef]
- Wen, D.; Yang, N.; Yang, M. Effects of re-vegetation on soil microbial functional diversity in purple soils at different vegetation stages on sloping-land in Hengyang, Hunan Province, China. Chin. J. Appl. Ecol. 2016, 27, 2645–2654. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Zhou, Q.; Yin, Y.; Qi, J.; Zhou, Y. Endangered plant Diplandrorchis of the growth of soil microorganisms. North. Hortic. 2010, 21, 100–101. [Google Scholar]
- Zhou, Q.; Sun, D.; Li, H.; Yue, J.; Chen, X.; Qu, B.; Zhang, L. Study on bacterial diversity in rhizosphere soil of rare and endangered species Diplandrorchis sinica. J. Shenyang Agric. Univ. 2020, 51, 721–726. [Google Scholar]
- Tan, X.; Yang, X.; Sun, X.; Zhou, Y.; Hu, S.; Yuan, C.; Shi, Z. Analysis of fungal communities in roots and root-associated soil of Nervilia fordii from karst areas of Guangxi. Guihaia 2023, 43, 405–414. [Google Scholar]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Teregulova, G.; Sineva, O.; Markelova, N.; Sadikova, V.; Uvarov, G.; Kovalenko, M.; Manucharova, N. Evaluation of chitinolytic and antibiotic activity of Streptomyces avidinii Ina 01467 and Micromonospora aurantiaca INA 01468. Eurasian Soil Sci. 2023, 56 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, D.; Singh, S.K.; Singh, V.K.; Singh, A.V.; Kumar, A. Role of actinomycetes in bioactive and nanoparticle synthesis. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–182. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Lundberg, D.S.; Lazarovits, G.; Reis, V.M.; Raizada, M.N. Bacterial populations in juvenile maize rhizospheres originate from both seed and soil. Plant Soil 2016, 405, 337–355. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F. Effects of earthworms and arbuscular mycorrhizal fungi on improvement of fertility and microbial communities of soils heavily polluted by cadmium. Chemosphere 2022, 286, 131567. [Google Scholar] [CrossRef]
- Kielak, A.M.; Cipriano, M.A.P.; Kuramae, E.E. Acidobacteria strains from subdivision 1 act as plant growth-promoting bacteria. Arch. Microbiol. 2016, 198, 987–993. [Google Scholar] [CrossRef]
- Bastida, F.; Hernández, T.; Albaladejo, J.; García, C. Phylogenetic and functional changes in the microbial community of long-term restored soils under semiarid climate. Soil Biol. Biochem. 2013, 65, 12–21. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; López-García, Á.; Domínguez, M.T.; Kjøller, R.; Navarro-Fernández, C.M.; Rosendahl, S.; Marañón, T. Soil fungal diversity and functionality are driven by plant species used in phytoremediation. Soil Biol. Biochem. 2021, 153, 108102. [Google Scholar] [CrossRef]
- Rei, K.; Hiroyuki, N.; Kazuhikosan, N. Composition for Promoting Plant Growth and Application Thereof. CN Patent 201980016523.6, 13 July 2021. [Google Scholar]
- Chen, Y. Effects of Different Nitrogen Fertilizers Combined with Trichoderma on Growth and Nutrient Utilization of Cucumis Melo. Master’s Thesis, Heilongjiang Bayi Agricultural University, Daqing, China, 2019. [Google Scholar]
- Qin, L.; Chen, Y.; Xie, L.; Zhang, Y.; Nong, Q.; Long, Y.; Zeng, F. Cladophialophora sp. ms2 and Its Application. CN Patent 202011239027.8, 14 March 2023. [Google Scholar]
- Gao, L.; Huang, Y.; Liu, Y.; Mohamed, O.A.A.; Fan, X.; Wang, L.; Li, L.; Ma, J. Bacterial Community Structure and Potential Microbial Coexistence Mechanism Associated with Three Halophytes Adapting to the Extremely Hypersaline Environment. Microorganisms 2022, 10, 1124. [Google Scholar] [CrossRef]
- Tiwari, P.; Bose, S.K.; Park, K.-I.; Dufossé, L.; Fouillaud, M. Plant-Microbe Interactions under the Extreme Habitats and Their Potential Applications. Microorganisms 2024, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.R.; Singh, J.S.; Kumar, A.; Prasad, S.M. The plant endomicrobiome: Structure and strategies to produce stress resilient future crop. Curr. Res. Microb. Sci. 2024, 6, 100236. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.D.J.; Neufeld, J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015, 13, 217–229. [Google Scholar] [CrossRef]
- Herrmann, M.; Wegner, C.-E.; Taubert, M.; Geesink, P.; Lehmann, K.; Yan, L.; Lehmann, R.; Totsche, K.U.; Küsel, K. Predominance of Cand. Patescibacteria in Groundwater Is Caused by Their Preferential Mobilization from Soils and Flourishing Under Oligotrophic Conditions. Front. Microbiol. 2019, 10, 1407. [Google Scholar] [CrossRef]
- Yuan, Z.; Sun, H. Montagnulaceae Bacteria in Plants and Their Uses. CN Patent 103849571B, 30 September 2015. [Google Scholar]
- Li, X.; He, X.; Hou, L.; Ren, Y.; Wang, S.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 7896. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, B.; Shen, J.; Xu, F.; Li, N.; Jia, P.; Jia, Y.; An, S.; Amoah, I.D.; Huang, Y. Shifts in C-degradation genes and microbial metabolic activity with vegetation types affected the surface soil organic carbon pool. Soil Biol. Biochem. 2024, 192, 109371. [Google Scholar] [CrossRef]
- Deng, M. Screening for Antimicrobial Endophytes and Optimization of Its Ferment Factor. Master’s Thesis, Chongqing University, Chongqing, China, 2007. [Google Scholar]
- Ai, Y.; Xie, T.; Liu, J.; Lan, S.; Peng, D.; Zhang, Q. Community structure and biological function of the root symbiotic fungi of Arundina graminifolia. Mycosystema 2019, 38, 1631–1642. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Li, H.; Zhu, S.; Sun, X.; Wu, K.; Shui, W. The characterization of microbial communities and associations in karst tiankeng. Front. Microbiol. 2022, 13, 1002198. [Google Scholar] [CrossRef]
- Yanardağ, I.H.; Zornoza, R.; Bastida, F.; Büyükkiliç-Yanardağ, A.; García, C.; Faz, A.; Mermut, A.R. Native soil organic matter conditions the response of microbial communities to organic inputs with different stability. Geoderma 2017, 295, 1–9. [Google Scholar] [CrossRef]
- Manici, L.M.; Caputo, F.; Fornasier, F.; Paletto, A.; Ceotto, E.; De Meo, I. Ascomycota and Basidiomycota fungal phyla as indicators of land use efficiency for soil organic carbon accrual with woody plantations. Ecol. Indic. 2024, 160, 111796. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Shen, Y.; Chen, X.; He, G.; He, X.; Wang, G.; He, H.; Lv, Z. The response of nutrient cycle, microbial community abundance and metabolic function to nitrogen fertilizer in rhizosphere soil of Phellodendron chinense Schneid seedlings. Front. Microbiol. 2023, 14, 1302775. [Google Scholar] [CrossRef]
- Thiel, V.; Fukushima, S.-I.; Kanno, N.; Hanada, S. Chloroflexi. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Lyu, H.; Sawada, K.; Zhong, R.; Kilasara, M.; Hartono, A.; Dahlgren, R.A.; Funakawa, S.; Watanabe, T. Disentangling divergent factors controlling bacterial and fungal communities in topsoil and subsoil horizons across environmental gradients of tropical volcanic regions. CATENA 2024, 239, 107907. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.-C.; Jia, B.; Gang, S.; Li, Y.; Mou, X.M.; Kuzyakov, Y.; Li, X.G. Regulation of soil nitrogen cycling by shrubs in grasslands. Soil Biol. Biochem. 2024, 191, 109327. [Google Scholar] [CrossRef]
- Wu, J.; Qi, L.; Huang, T.; Wang, J.; Sun, Q. A short period of revegetation and fertilization increased the nutrients, enzyme activities, and bacterial community diversity in backfill soils. Appl. Soil Ecol. 2023, 189, 104959. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Sarikhani, M.R.; Khoshru, B.; Oustan, S. Efficiency of Some Bacterial Strains in Potassium Release from Mica and Phosphate Solubilization under In Vitro Conditions. Geomicrobiol. J. 2016, 33, 832–838. [Google Scholar] [CrossRef]
- Serna Posso, E.J.; Sánchez de Prager, M.; Cisneros Rojas, C.A. Organic acids production by rhizosphere microorganisms isolated from a Typic Melanudands and its effects on the inorganic phosphates solubilization. Acta Agron. 2017, 66, 234–241. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Niu, Y.; Jiang, W.; Wang, Y.; Liu, S.; Wei, W. 44-Years of Fertilization Altered Soil Microbial Community Structure by Changing Soil Physical, Chemical Properties and Enzyme Activity. J. Soil Sci. Plant Nutr. 2024, 24, 3150–3161. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Yang, T.; Chu, H. Threshold effects of soil pH on microbial co-occurrence structure in acidic and alkaline arable lands. Sci. Total Environ. 2021, 800, 149592. [Google Scholar] [CrossRef]
- Liao, L.; Wang, X.; Wang, J.; Liu, G.; Zhang, C. Nitrogen fertilization increases fungal diversity and abundance of saprotrophs while reducing nitrogen fixation potential in a semiarid grassland. Plant Soil 2021, 465, 515–532. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Jia, J.; Zhang, J.; Zhang, F. Bacterial taxa and fungal diversity are the key factors determining soil multifunctionality in different cropping systems. Land Degrad. Dev. 2021, 32, 5012–5022. [Google Scholar] [CrossRef]
- Qin, Y.; Pan, X.; Kubicek, C.; Druzhinina, I.; Chenthamara, K.; Labbé, J.; Yuan, Z. Diverse Plant-Associated Pleosporalean Fungi from Saline Areas: Ecological Tolerance and Nitrogen-Status Dependent Effects on Plant Growth. Front. Microbiol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hall, S.J.; Hu, H.; Dutta, S.; Miao, Q.; Wang, J.; Kang, H. Chronic nitrogen deposition drives microbial community change and disrupts bacterial-fungal interactions along a subtropical urbanization gradient. Soil Biol. Biochem. 2022, 169, 108676. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Luo, J.; Qin, M.; Johnson, N.C.; Öpik, M.; Vasar, M.; Chai, Y.; Zhou, X.; Mao, L.; et al. Dynamics of arbuscular mycorrhizal fungal community structure and functioning along a nitrogen enrichment gradient in an alpine meadow ecosystem. New Phytol. 2018, 220, 1222–1235. [Google Scholar] [CrossRef]
- Tomazelli, D.; Klauberg-Filho, O.; Mendes, S.D.C.; Baldissera, T.C.; Garagorry, F.C.; Tsai, S.M.; Pinto, C.E.; Mendes, L.W.; Goss-Souza, D. Pasture management intensification shifts the soil microbiome composition and ecosystem functions. Agric. Ecosyst. Environ. 2023, 346, 108355. [Google Scholar] [CrossRef]
- Zhang, H.; Degré, A.; De Clerck, C.; Li, S.; Lian, J.; Peng, Y.; Sun, T.; Luo, L.; Yue, Y.; Li, G.; et al. Changes in bacterial community structure and carbon metabolism in sandy soil under the long-term application of chitin-rich organic material and attapulgite. Appl. Soil Ecol. 2024, 194, 105161. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, J.; Zhang, M.; Guo, L.; Omidvar, N.; Xu, Z.; Hui, C.; Liu, W. The role of soil chemical properties and microbial communities on Dendrocalamus brandisii bamboo shoot quality, Yunnan Province, China. Front. Microbiol. 2025, 16, 1551638. [Google Scholar] [CrossRef]
- Deng, S.; Wu, Y.; Wu, K.; Fang, L.; Li, L.; Zeng, S. Breeding characteristics and artificial propagation of 14 species of Wild Plant with Extremely Small Populations (WPESP) in China. Biodivers. Sci. 2020, 28, 385–400. [Google Scholar] [CrossRef]
- Shi, J.; Chen, H.; An, M.; Zhang, Y.; Ye, C.; Wu, J. Analyses on distribution characteristics and protection effect of wild Paphiopedilum in Guizhou Province. Guihaia 2022, 42, 1059–1066. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, C.; Zhang, M.; Wang, X.; Zhu, L. Dynamic changes of rhizosphere soil microbial biomass and nutrition of different Cherry rootstocks. Acta Agric. Boreali-Occident. Sin. 2015, 24, 123–129. [Google Scholar]
- Akinola, S.; Ayangbenro, A.; Babalola, O. Metagenomic Insight into the Community Structure of Maize-Rhizosphere Bacteria as Predicted by Different Environmental Factors and Their Functioning within Plant Proximity. Microorganisms 2021, 9, 1419. [Google Scholar] [CrossRef]
- He, X.; Wang, D.; Jiang, Y.; Li, M.; Delgado-Baquerizo, M.; McLaughlin, C.; Marcon, C.; Guo, L.; Baer, M.; Moya, Y.A.T.; et al. Heritable microbiome variation is correlated with source environment in locally adapted maize varieties. Nat. Plants 2024, 10, 598–617. [Google Scholar] [CrossRef]
- Thiergart, T.; Paloma, D.; Ellis, T.; Vannier, N.; Hacquard, S. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nat. Ecol. Evol. 2020, 4, 122–131. [Google Scholar] [CrossRef] [PubMed]

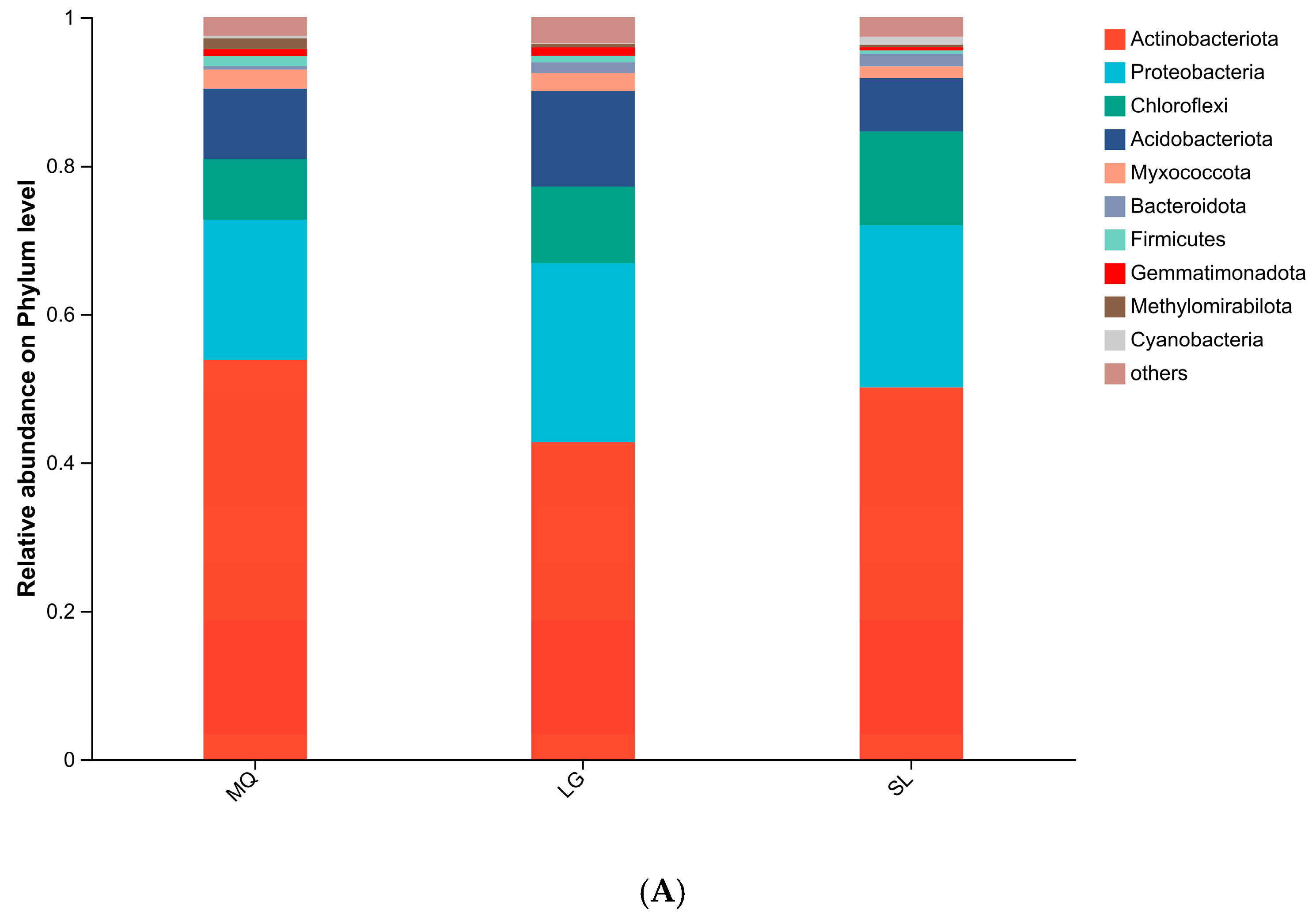
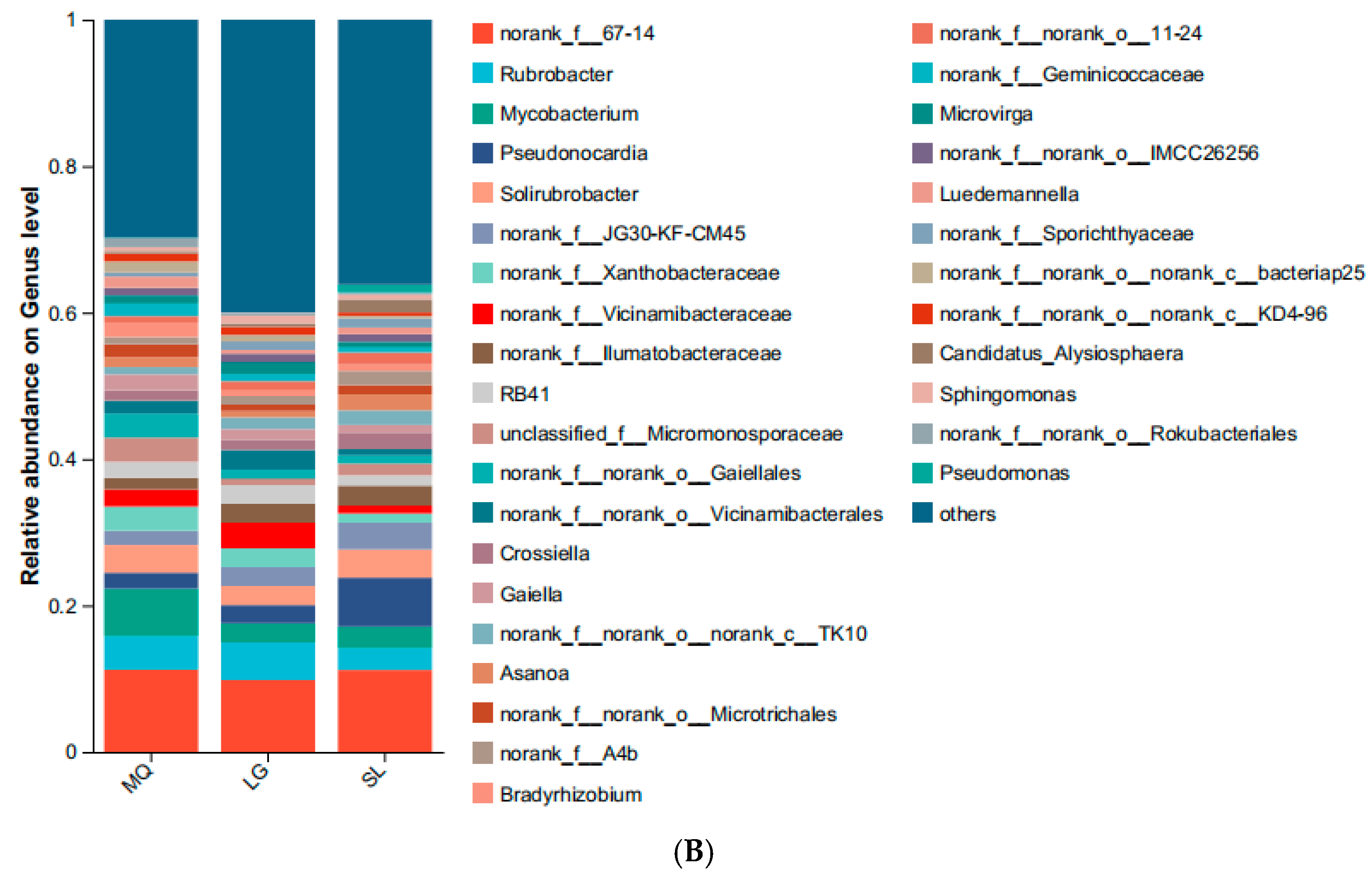
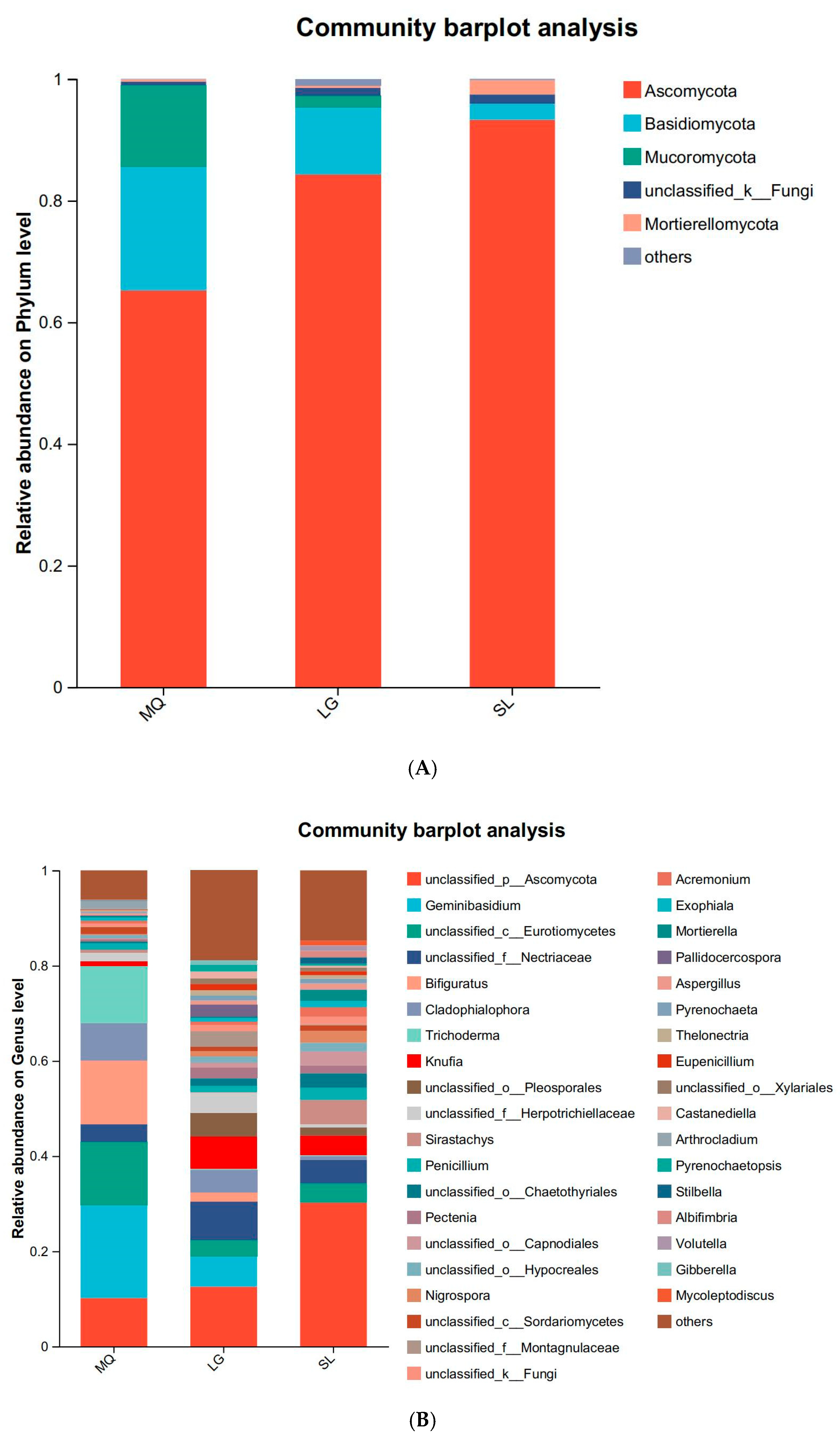
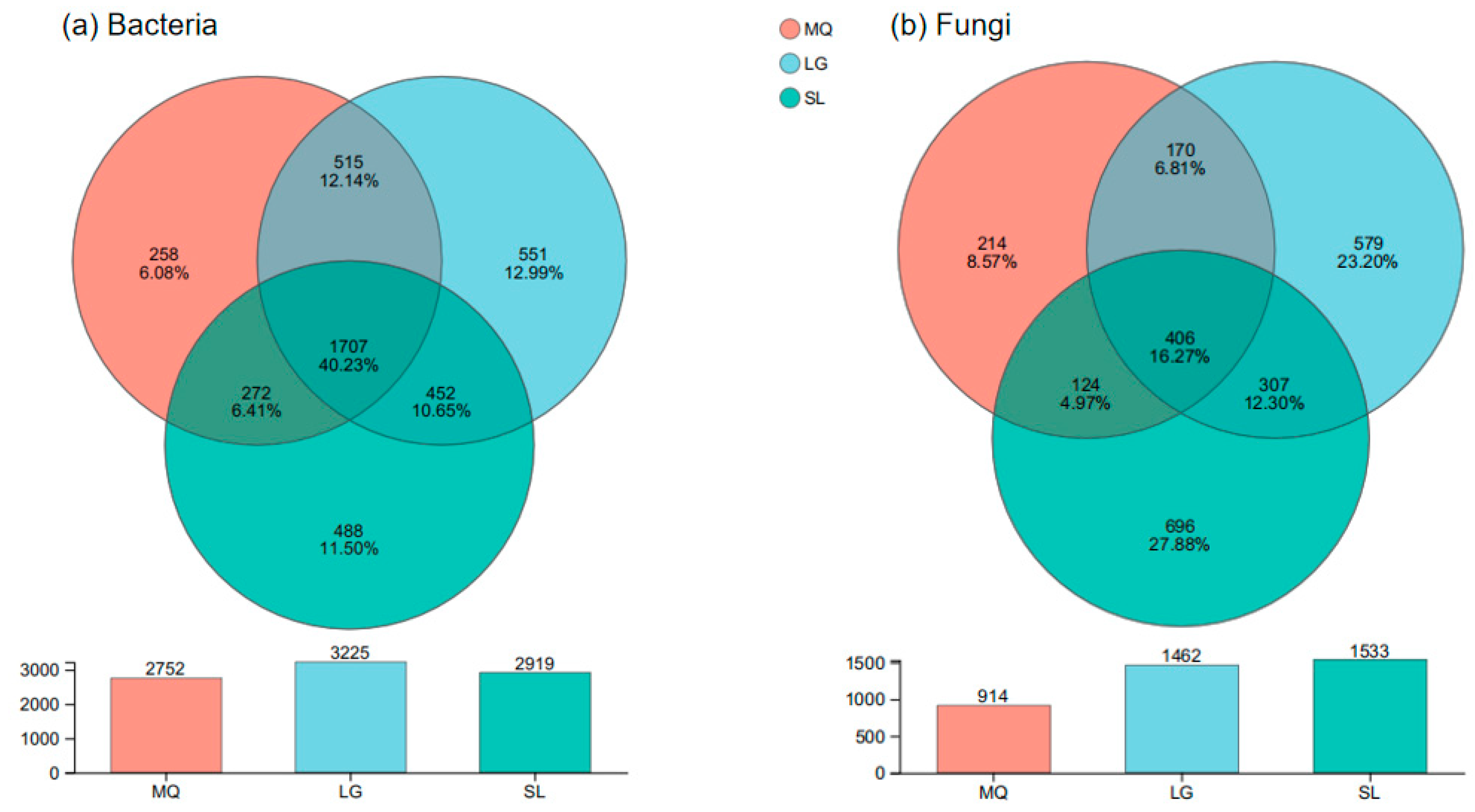


| Population Number | Collection Location | Position | Altitude (m) | Grade of Slope (°) | Aspect of Slope | Number of Plants | Growth Condition |
|---|---|---|---|---|---|---|---|
| MQ | Mingqiang village in Longzhou County | Rhizosphere | 450 | 70–80 | Southwest | 50 | The plants grew well, mainly in groups of more than 10 plants |
| LG | LongGang village in Longzhou County | Rhizosphere | 430 | 70–80 | Northwest | 50 | The plants grew well, mainly in groups of more than 10 plants |
| SL | Sanlian Village in Longzhou County | Rhizosphere | 520 | 80–90 | Northeast | 40 | The plants grew well, showing scattered small populations of 1–3 plants |
| Number | Potential of Hydrogen (pH) | Nitrogen (N) (g·kg−1) | Phosohorus (P) (g·kg−1) | Potassium (K) (g·kg−1) | Soil Organic Matter (SOM) (g·kg−1) | Ammonium Nitrogen (AN) (mg·kg−1) | Available Phosphorus (AP) (mg·kg−1) | Available Potassium (AK) (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| MQ | 7.96 ± 0.04 b | 6.60 ± 0.04 b | 0.42 ± 0.02 b | 4.80 ± 0.29 a | 126.4 ± 0.56 c | 374.58 ± 3.76 c | 8.43 ± 0.35 c | 93.59 ± 4.26 c |
| LG | 5.97 ± 0.03 c | 14.99 ± 0.15 a | 0.58 ± 0.03 a | 4.76 ± 0.26 a | 305.34 ± 14.92 b | 1002.68 ± 15.43 b | 58.84 ± 1.18 a | 220.76 ± 8.20 a |
| SL | 8.05 ± 0.01 a | 14.94 ± 0.11 a | 0.43 ± 0.02 b | 4.56 ± 0.32 a | 398.88 ± 2.81 a | 1104.50 ± 26.05 a | 14.54 ± 0.27 b | 204.15 ± 7.49 b |
| Population Number | Bacterial | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|
| Ace Index | Chao Index | Shannon Index | Simpson Index | Ace Index | Chao Index | Shannon Index | Simpson Index | |
| MQ | 2651.18 ± 89.64 b | 2643.49 ± 79.72 b | 5.89 ± 0.13 b | 0.0084 ± 0.0016 a | 712.69 ± 5101.89 b | 696.44 ± 114.26 b | 3.47 ± 0.24 b | 0.0673 ± 0.0123 a |
| LG | 2977.86 ± 137.52 a | 2912.71 ± 131.73 a | 6.40 ± 0.06 a | 0.0047 ± 0.0005 b | 1024.98 ± 104.24 a | 1015.37 ± 102.26 a | 4.67 ± 0.22 a | 0.0263 ± 0.0053 b |
| SL | 2511.45 ± 138.92 b | 2515.80 ± 120.60 b | 6.09 ± 0.14 b | 0.0074 ± 0.0012 a | 1014.26 ± 110.21 a | 1013.32 ± 107.67 a | 4.90 ± 0.04 a | 0.0182 ± 0.0018 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, K.; Sang, J.; Su, J.; Huang, N.; Wu, W.; He, J.; Liu, B.; Fu, C. Microbial Diversity in the Rhizosphere Soils of Three Different Populations of Paphiopedilum helenae, a Critically Endangered Wild Orchid. Microorganisms 2025, 13, 2282. https://doi.org/10.3390/microorganisms13102282
Xian K, Sang J, Su J, Huang N, Wu W, He J, Liu B, Fu C. Microbial Diversity in the Rhizosphere Soils of Three Different Populations of Paphiopedilum helenae, a Critically Endangered Wild Orchid. Microorganisms. 2025; 13(10):2282. https://doi.org/10.3390/microorganisms13102282
Chicago/Turabian StyleXian, Kanghua, Jinhan Sang, Jiang Su, Ningzhen Huang, Wenlong Wu, Jinxiang He, Baojun Liu, and Chuanming Fu. 2025. "Microbial Diversity in the Rhizosphere Soils of Three Different Populations of Paphiopedilum helenae, a Critically Endangered Wild Orchid" Microorganisms 13, no. 10: 2282. https://doi.org/10.3390/microorganisms13102282
APA StyleXian, K., Sang, J., Su, J., Huang, N., Wu, W., He, J., Liu, B., & Fu, C. (2025). Microbial Diversity in the Rhizosphere Soils of Three Different Populations of Paphiopedilum helenae, a Critically Endangered Wild Orchid. Microorganisms, 13(10), 2282. https://doi.org/10.3390/microorganisms13102282






