Abstract
Leprosy remains a significant public health issue, particularly due to its neuropathic consequences, which affect sensory, motor, and autonomic functions, leading to severe disabilities. HIV/AIDS, another major public health concern, overlaps geographically with leprosy and is also associated with peripheral neuropathies, complicating the management of co-infected patients. Understanding how Nerve Growth Factor (NGF) is regulated in leprosy and HIV-leprosy co-infection may contribute to immunomodulatory treatments and neuroimmune response control. A cross-sectional study evaluated NGF tissue expression using immunohistochemistry in 47 HIV/leprosy co-infected patients and 61 leprosy-only patients. The co-infected group had a higher incidence of neuritis (40.4%) and a prevalence of exclusively reversal reactions. However, the occurrence of neuritis was not associated with higher expression of NGF in the tissue. Leprosy reactions were more prevalent in non-co-infected patients with multibacillary forms (50%). Multibacillary forms in both groups of patients showed higher cellular expression of NGF, with a greater tendency for higher NGF expression in non-co-infected multibacillary patients (p = 0.0021), suggesting impairment in the immune response involved in the tissue expression of neurotrophins in the co-infected group. Overall, co-infection with HIV did not influence the increase in NGF in the lesions of leprosy patients compared with patients with leprosy alone.
1. Introduction
Hansen’s disease, also known as leprosy, is a disease caused by the Mycobacterium leprae complex (M. leprae and M. lepromatosis), both obligate intracellular parasites, incapable of being cultured in artificial media, which have a slow multiplication rate, with a tropism for cells of the skin and peripheral nervous system, and cause the same clinical disease [1,2,3,4]. Transmissibility occurs through the release of the bacillus through the respiratory tract of individuals with a high bacillary load; however, leprosy is not a highly contagious disease [4]. The infected host, through the immune system, has the ability to respond in different ways to the presence of the bacillus, which gives the disease a variety of clinical and histological manifestations [1,2,3], in addition to genetic predispositions that can make the host more susceptible to the appearance of reactive episodes that aggravate the disease [5,6]. Even with great advances in studies and developments in the management of the disease and effective treatment, leprosy continues to be a serious global public health problem, with high detection rates in countries such as India, Brazil, and Indonesia, being classified as a neglected tropical disease [3,4].
Neuropathy caused by the action of the bacillus has long-term consequences, especially related to the loss of nerve function in the face, hands, and feet, and early diagnosis and treatment are necessary to avoid irreversible neural damage. The involvement encompasses the three modalities of the peripheral nervous system: sensory, motor, and autonomic functions [7], and can generate serious sequelae, ranging from loss of protective sensitivity to motor changes. Sensory/motor involvement can lead to ulcerations, bone resorption, and muscle paralysis [8,9].
Acquired immunodeficiency syndrome (AIDS), caused by the human immunodeficiency virus (HIV), is also a disease that has a major impact on public health due to the significant number of new cases. In 2023, 1.3 million people became newly infected with HIV, and in total, by the end of 2023, there were around 39.9 million people living with HIV in the world [10,11]. HIV infection can also lead to peripheral sensory neuropathies, which are the most common neurological complications of this infection, affecting between 10 and 15% of patients with HIV and up to 44% of patients with AIDS, which poses challenges in the management of these conditions. However, other forms of neuropathies are also possible in these patients, such as Inflammatory Demyelinating Polyradicoloneuropathy, Dideoxynucleoside Toxic Neuropathy, Mononeuropathy Multiplex and Autonomic Neuropathy [12,13,14].
Understanding the co-infection of these two diseases is relevant in areas where the two diseases overlap, since it has already been mentioned that leprosy can behave as a disease of immune restoration associated with the immunological improvement of patients upon initiation of highly active antiretroviral treatment (HAART), which impacts the prevalence of leprosy disease in patients living with HIV, increasing the prevalence of paucibacillary forms and even type I reaction episodes [15,16,17]. Furthermore, its symptomatic manifestation may occur as part of the Immune Reconstitution Inflammatory Syndrome (IRIS), which is an inappropriate inflammatory response to an infection, usually occurring in the first 6 months of HAART introduction [18,19,20,21], leading to neurological changes that may add to the changes already common to HIV infection, such as sensory changes, decreased strength, and peripheral neuropathies [22,23].
Previous studies have shown that patients co-infected with leprosy and HIV are more likely to develop early neural damage at the onset of the disease when associated with the period of immunological restoration when compared with patients with isolated leprosy [21] and co-infection between these diseases may represent a factor of worse prognosis of muscle function when compared with patients with one or the other disease in isolation [24].
One way to monitor nerve functions during the course of diseases is through biomarkers that signal the production of the myelin sheath by Schwann cells, such as nerve growth factor (NGF), a neurotrophin first described by Rita Levi-Montalcini, in 1953 [25]. This neurotrophin acts in the maintenance and preservation of sympathetic and sensory neurons and also participates in modulating the sensitivity of peripheral fibers to pain and heat [25,26,27]. Studies indicate NGF [28,29,30], as well as the presence of its direct autoantibody (anti-NGF) [31], as possible indicators of neural damage in leprosy according to its expression. Understanding the regulation of NGF levels in different conditions of the disease and working on anti-NGF levels may be relevant for immunomodulatory treatment and for controlling neuroimmune reactions in leprosy [32,33,34,35].
This study aimed to quantify the expression of NGF in skin lesions of patients with leprosy, with and without HIV co-infection, and to establish relationships with the different variables that characterize the nerve damage caused by the disease.
2. Materials and Methods
2.1. Study Design
This is a comparative cross-sectional study of two groups of patients diagnosed with leprosy, one without HIV and the other co-infected with HIV, and followed at the dermatology outpatient clinic of the Nucleus of Tropical Medicine of the Federal University of Pará (NMT/UFPA), located in the city of Belém, capital of the state of Pará, Brazil.
2.2. Inclusion Criteria
Patients aged 18 to 70 years diagnosed with leprosy were included. To constitute group 1, patients diagnosed with leprosy according to signs and symptoms recommended by the Ministry of Health [4] and complemented by additional tests (slit-skin-smear bacilloscopy and histopathology).
To constitute group 2 of this research, cases of co-infection were considered, with individuals previously diagnosed as HIV positive through serological screening (ELISA) and confirmatory (Western Blot) tests, according to the Brazilian Ministry of Health [36], whether or not they were treated with HAART.
2.3. Study Procedures
2.3.1. Data Collection
Sociodemographic and clinical data were collected to characterize the sample. Gender, age, occurrence of neuritis, occurrence of leprosy reaction, type of leprosy reaction presented, and number of nerve trunks affected by palpation were identified according to the Simplified Neurological Assessment, following the standards of the Brazilian Ministry of Health [4]. The Simplified Neurological Assessment is an instrument for assessing neural damage and classifying the Degree of Physical Disability, in which clinical complaints, signs and symptoms, nerve palpation, strength assessment, and sensory inspection and assessment are evaluated.
The clinical classification of leprosy followed the classification criteria of Ridley and Jopling (1966) [1,2], later grouped according to the WHO Operational Classification [7], including the leprosy clinical forms Primary Neural (NP), Indeterminate (I), Tuberculoid (TT), and Borderline Tuberculoid (BT), classified as Paucibacillary Forms (PB); and the forms Borderline Borderline (BB), Borderline Lepromatous (BL), and Lepromatous (LL), classified as Multibacillary Forms (MB) [1,2].
From the group with leprosy/HIV, specific clinical and laboratory data on the follow-up of HIV disease were also collected, including use of HAART, the temporal relationship between the use of HAART and the diagnosis of leprosy, the quantification of CD4+ T lymphocytes per cubic millimeter of peripheral blood at the time of leprosy diagnosis, and if patients had more or less than 350 CD4+ T lymphocytes per cubic millimeter of peripheral blood [36].
2.3.2. Collection of Material for Immunohistochemistry
A skin biopsy was performed, collected from the leprosy lesion at the time of diagnosis, using a number 4 punch after antisepsis and local anesthesia with 2% lidocaine. The material obtained was stored in transparent glass vials with 10% buffered formalin, embedded in paraffin, and sent to the Laboratory of Pathology of Infectious Diseases (LIM50) of the Medical School of the University of São Paulo (FMUSP).
2.3.3. Immunohistochemistry Technique
The stored material was subjected to 4 µm sections and fixed on slides. For immunostaining of the NGF neurotrophin, the Polymer Conjugated Method with Secondary Antibodies immunohistochemical method was used, following the protocol of Hsu et al. (1981) [37], with a methodology partially modified according to Quaresma et al. (2006) [38].
Histological sections were deparaffinized in xylene for 20 min and subsequently hydrated in alcohol baths in decreasing concentrations (twice in absolute alcohol and once in 70% alcohol) for 3 min each. Endogenous peroxidase was blocked with 10 washes of 3 min each with 10% hydrogen peroxide. Next, antigen retrieval was performed in a steam cooker at 96 °C for 30 min, using citrate buffer pH6. After antigen retrieval, the slides were cooled to room temperature until a temperature of 55 °C was reached.
Immediately after, nonspecific sites were blocked. To do this, the slides were incubated in an oven at 37 °C with a 6% skim milk solution in PBS for 30 min. Then, the primary antibody (Anti-NGF antibody ab6199—Abcam, Cambridge, MA, USA) was diluted in 1% bovine albumin in PBS (1:250), pipetted onto the slides, and incubated in a humid chamber for 18 h.
Subsequently, the slides were washed with PBS Tween 0.05% 3 times for 5 min and then incubated in a humid chamber at 37 °C with the post-primary antibody (NOVOLINK TM polymer detection systems—RE7280-K Leica Biosystems, Newcastle, UK) for 30 min. After the incubation time, the slides were washed again with PBS Tween 0.05% 3 times for 5 min and then incubated with the polymer from the same NOVOLINK kit mentioned above for 30 min. After this step, the slides were washed again with PBS Tween 0.05% 3 times for 5 min and then incubated with the chromogenic substrate DAB+H2O2 (diaminobenzidine with hydrogen peroxide— K3468 —AgilentDako, Santa Clara, CA, USA) for five minutes for antigen-antibody binding visualization. Then, the slides were washed in distilled water and counterstained with Harris hematoxylin.
Finally, the slides were dehydrated in baths with increasing concentrations of alcohol (once in 70% alcohol and twice in absolute alcohol) for 2 min each and mounted with coverslips and synthetic resin.
2.3.4. Quantitative Analysis of Immunostained Cells
The immunostained sections were analyzed, and quantification of immunostained cells was performed using AxioVision 4.8.2 software (Zeiss, San Diego, CA, USA) with a 40x A-plan objective lens. The AXIO IMAGER Z1-ZEISS system (Zeiss, Oberkochen, Germany), consisting of a binocular microscope and an automatic photography system with an attached Axiocam camera, was used to document and obtain photomicrographs. Five to eight different fields were randomly selected in the area of the dermal inflammatory infiltrate of the histological lesions. Once the number of stained cells was determined, the average number of cells in the different fields was obtained, with the results expressed in cells per field. The density of positive cells was then calculated, which is the result of the average number of immunostained cells per field divided by the area of the photomicrograph (0.0356412 mm2), with the result expressed in the density of stained cells per square millimeter.
2.4. Statistical Analysis
The results obtained during the experiments were stored in electronic spreadsheets using Microsoft Office Excel 2007 and analyzed using Biostat 5.0 and GraphPad Prism 10.2. Numerical variables were analyzed by obtaining measures of central tendency such as mean and median, as well as measures of variability such as standard deviation and variance. To verify the statistical significance of the frequency presented by the groups, according to the variable under analysis, the chi-square test of adherence and the g-test were used. To compare the means of NGF expressions between the groups and clinical variables, the t-test was used. To verify the correlation between the expressed values of NGF and TCD4+ lymphocytes, the Spearman correlation coefficient was used. The images were created using GraphPad Prism 10.2 and InkScape 1.2.
2.5. Ethical Aspects
This study was developed in accordance with the precepts of the standards for research involving human beings, established by Resolution 466/12 of the National Health Council and Law 14.874/2024 of Brazilian legislation. All individuals participating in this research were evaluated after being informed about the research and signing the informed consent form.
This research is linked to the research project entitled “Contributions to the Knowledge of the Immunopathology of HIV/Leprosy Co-infection and HIV-Leishmaniasis Co-infection”, approved by the Research Ethics Committee of the Nucleus of Tropical Medicine of the Federal University of Pará with CAAE 10935419.5.0000.5172 and Opinion Number 3.285.553. This study follows the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
3. Results
A total of 108 participants were selected and divided into two groups: (1) Co-infection Group, consisting of 47 patients with a mean age of 40.04 (±11.35) years, predominantly male (n = 33/70.2%) and with paucibacillary forms of the disease (n = 30/63.8%); and (2) Non-Co-infection Group, consisting of 61 patients with a mean age of 43.75 (±15.87) years, predominantly male (n = 37/60.7%), and with multibacillary forms of the disease (n = 40/65.6%).
At the time of diagnosis, the co-infected group had a higher incidence of neuritis, both for the paucibacillary and multibacillary forms. In general, type I and II leprosy reactions were more prevalent in patients with multibacillary forms in the group not co-infected with HIV. The group of co-infected patients, in turn, presented exclusively type I reactions. Upon nerve palpation, it was observed that, regardless of the clinical form or co-infection, most patients had up to 3 nerve trunks affected (Table 1).

Table 1.
Clinical characteristics of patients in the study population.
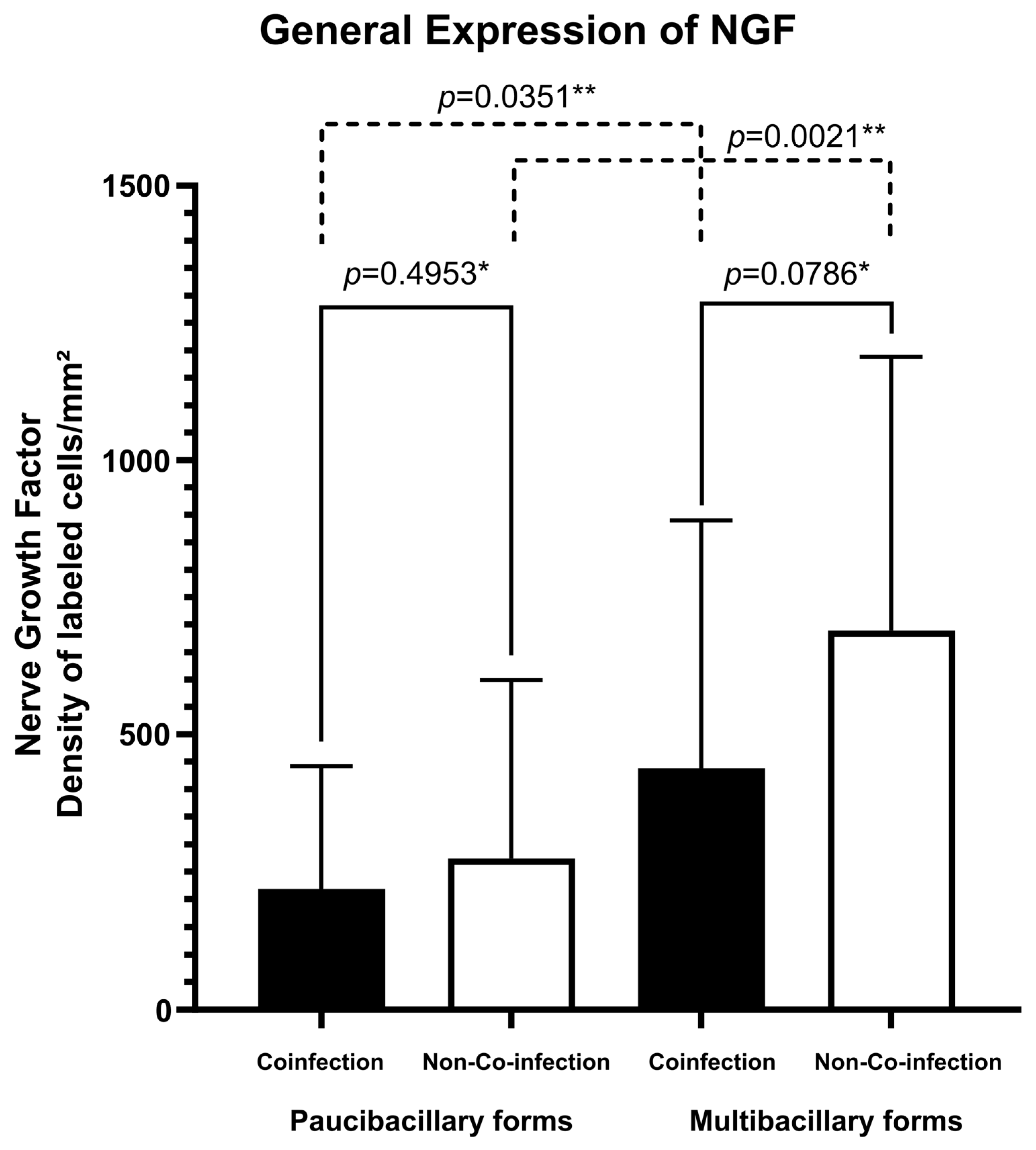
Regarding the expression of the NFG neurotrophin, multibacillary forms of both groups of patients presented greater cellular expression (273.77 ± 325.16 labeled cells per square millimeter for co-infected patients and 689.22 ± 498.54 labeled cells per square millimeter for non-co-infected patients) (Figure 1B,D) compared to paucibacillary forms of each group (218.21 ± 222.73 labeled cells per square millimeter for co-infected patients and 437.23 ± 452.30 labeled cells per square millimeter for non-co-infected patients) (Figure 1A,C). The fact of being co-infected with HIV did not change this significant expression (Figure 2). No difference was observed in the spatial distribution of cells immunostained with NGF when comparing co-infected patients with non-co-infected patients.
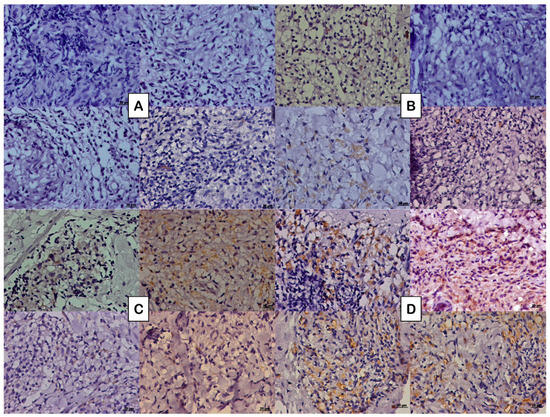
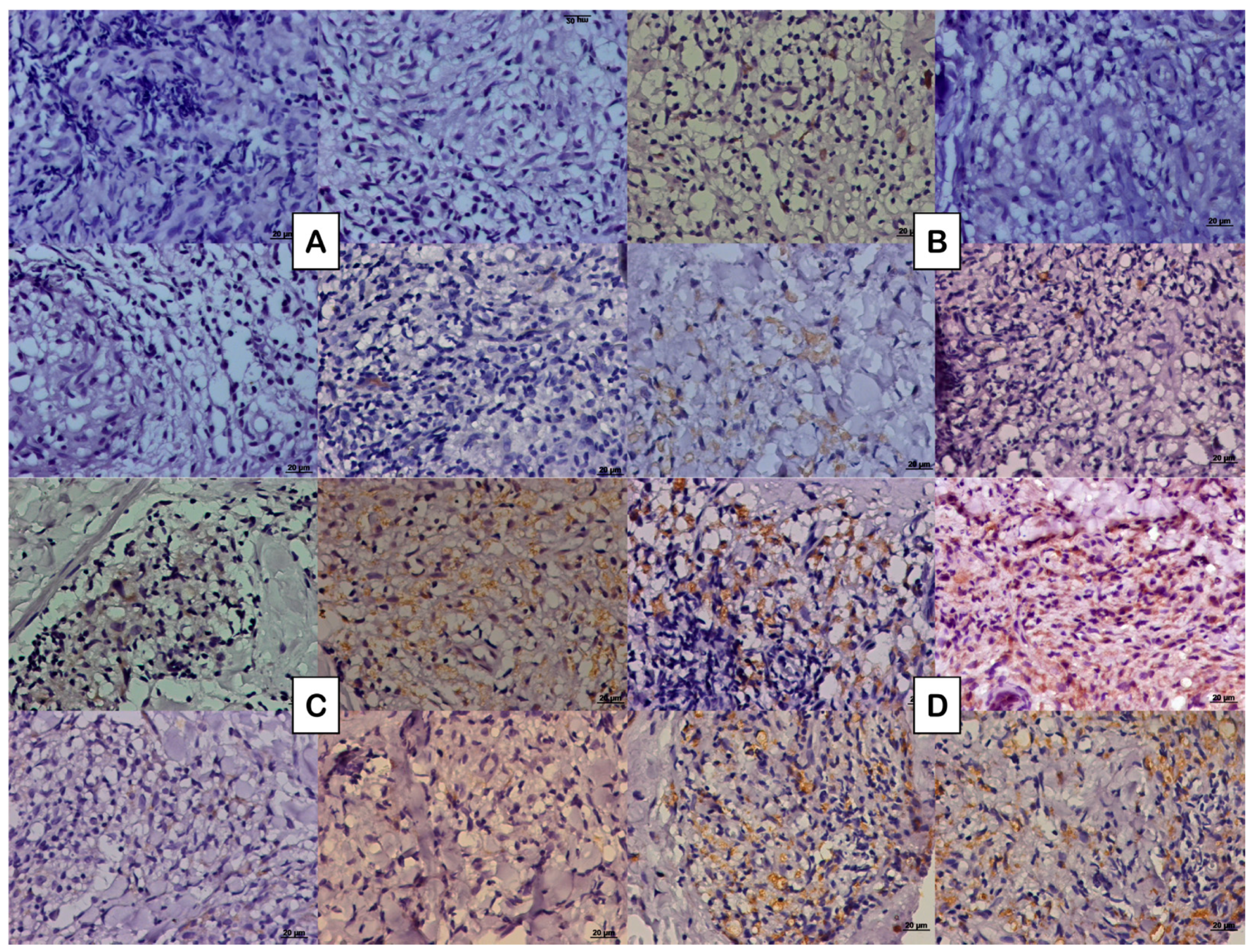
Figure 1.
Photomicrograph of the tissue expression of NGF represented by the DAB brownish coloration observed in the cytoplasm of some cells in (A) paucibacillary coinfected patients, (B) paucibacillary non-coinfected patients, (C) multibacillary coinfected patients, and (D) multibacillary non-coinfected patients.
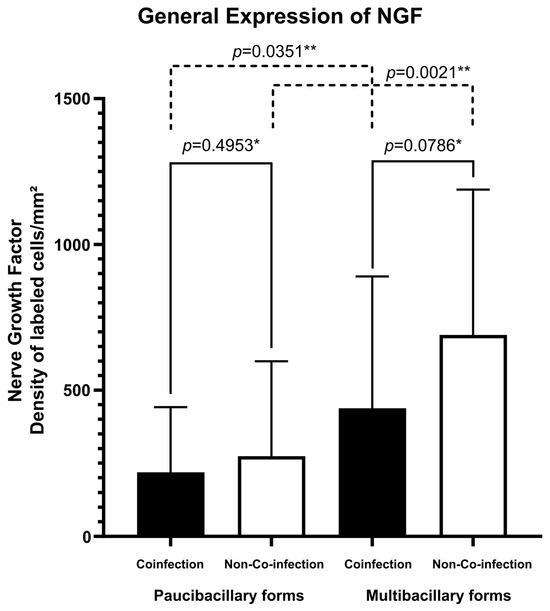
Figure 2.
Tissue expression of NGF according to the presence of co-infection and clinical classification. * p-value according to the t-test in an intragroup analysis. ** p-value according to the t-test in an intergroup analysis.
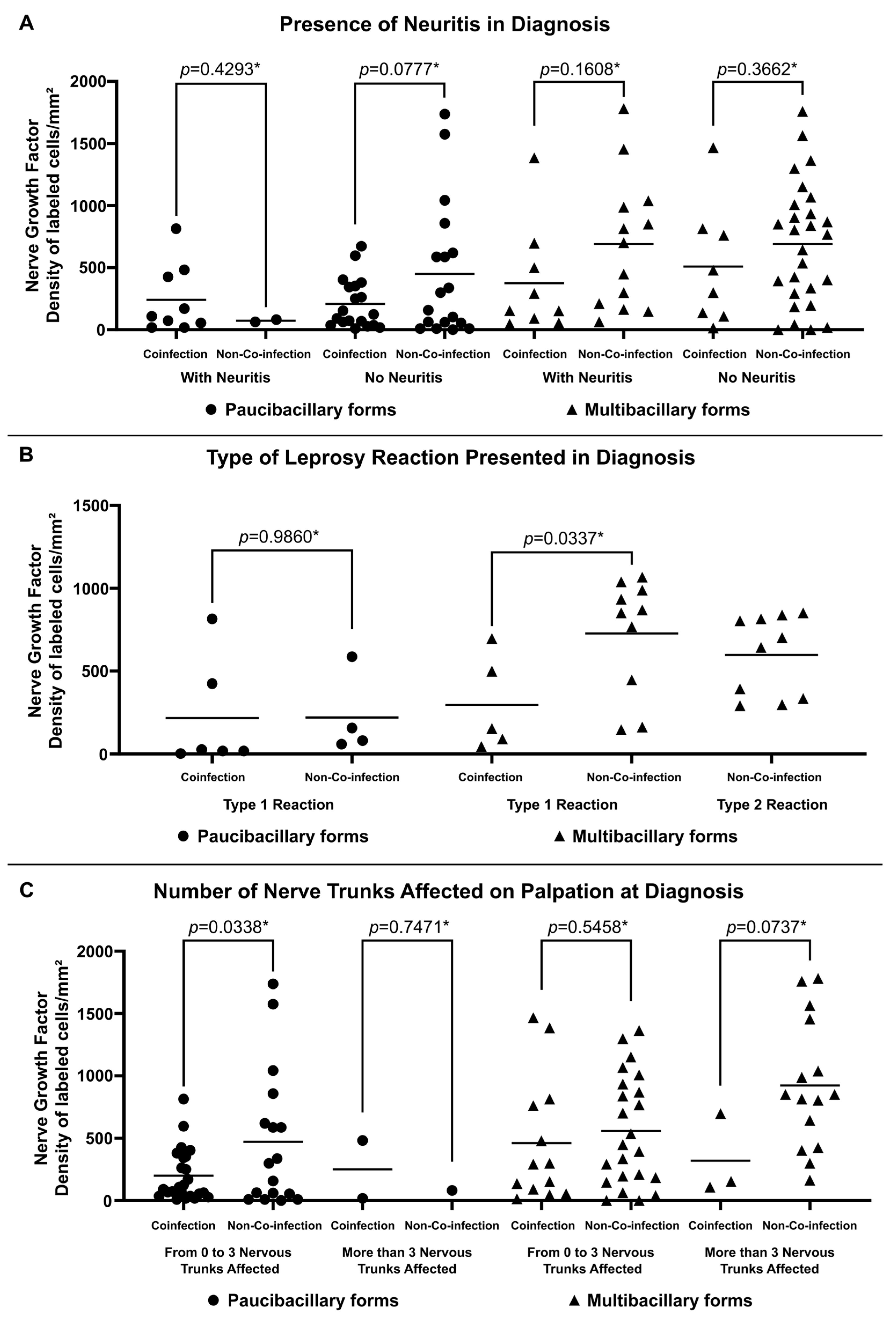
Higher expression of NGF was observed in non-co-infected multibacillary patients regardless of the occurrence of neuritis (688.38 ± 534.82 labeled cells per square millimeter for patients with neuritis and 689.62 ± 490.71 labeled cells per square millimeter for patients without neuritis) (Figure 3A). Similarly, multibacillary patients not co-infected with HIV present higher expression of NGF considering the presence of reactions (726.20 ± 348.45 labeled cells per square millimeter for Type 1 Reactions and 596.40 ± 239.97 labeled cells per square millimeter for Type 2 Reactions) (Figure 3B) and the number of altered nerve trunks on palpation (558.05 ± 439.07 labeled cells per square millimeter for patients with 0 to 3 nervous trunks affected and 921.90 ± 517.29 labeled cells per square millimeter for patients with more than 3 nervous trunks affected) (Figure 3C).
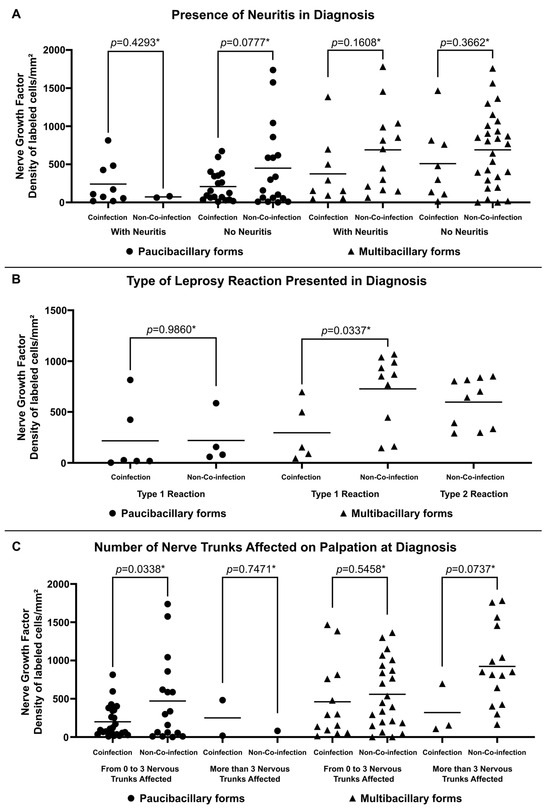
Figure 3.
Tissue expression of NGF at diagnosis according to (A) Presence of Neuritis in Diagnosis, (B) Type of Leprosy Reaction in Diagnosis and (C) Number of Nerve Trunks Affected on Palpation at Diagnosis. * p-value according to the t-test in an intragroup analysis.
In an analysis of the leprosy and HIV co-infection group, it was found that, regardless of the clinical form, the majority were using HAART at the time of diagnosis and had a CD4+ T lymphocyte count of less than 350 cells per cubic millimeter. When the time of use of antiretroviral therapy and the diagnosis of leprosy were correlated, it was found that most leprosy diagnoses occurred within the first six months of starting antiretroviral therapy (Table 2).

Table 2.
Clinical characteristics related to HIV in patients in the Co-infection Group.
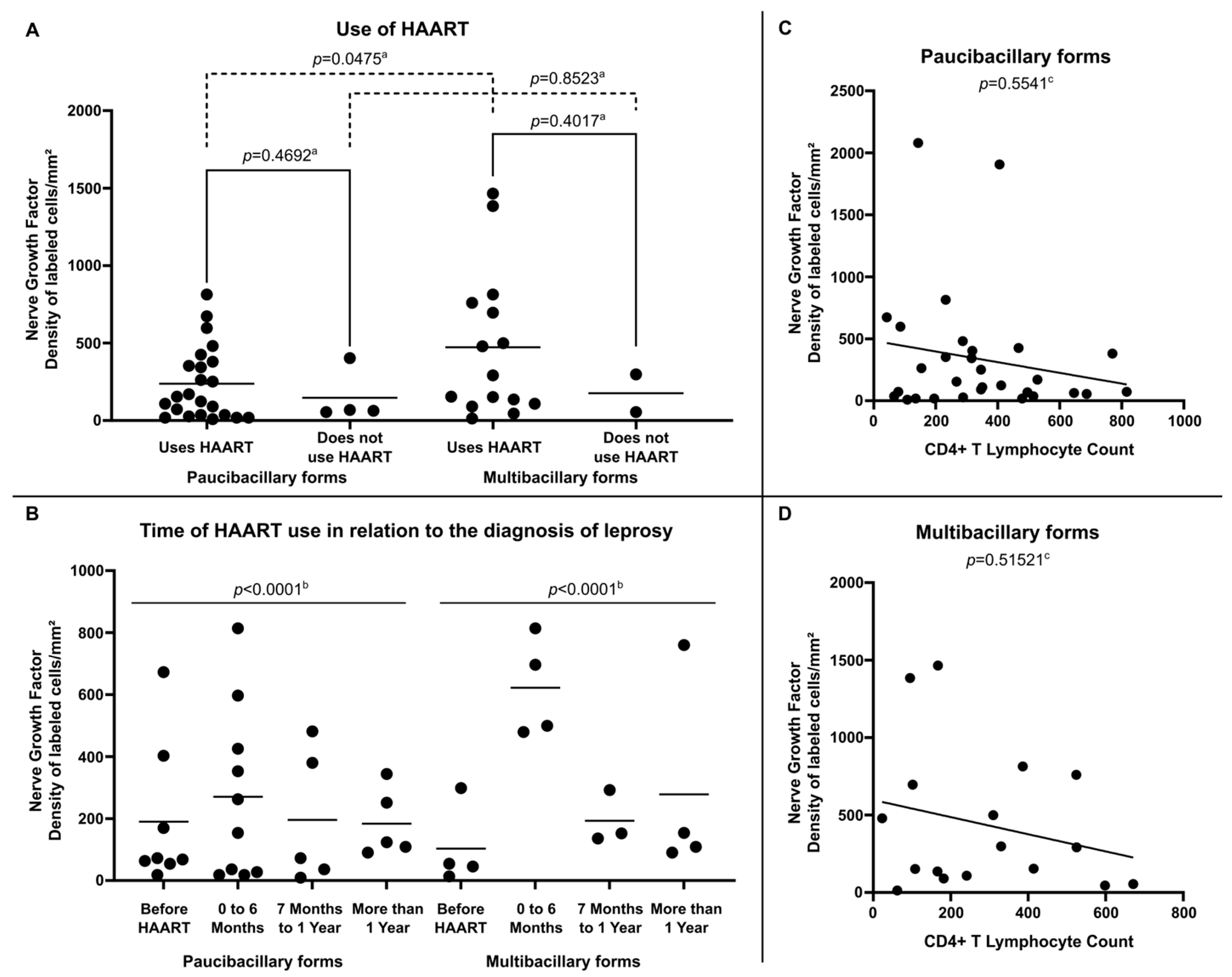
Internal analysis in the group of HIV-positive leprosy patients demonstrated that NGF expression was more relevant in multibacillary clinical forms, especially among those using HAART (472.91 ± 469.69 labeled cells per square millimeter) (Figure 4A) and who were diagnosed with leprosy in the period suggestive of immune restoration (in the first six months after starting therapy) (622.30 ± 161.06 labeled cells per square millimeter) (Figure 4B). Regarding the analysis of NGF expression related to the number of CD4+ T lymphocytes in peripheral blood, no significant correlation was observed between these variables in either paucibacillary (Figure 4C) or multibacillary patients (Figure 4D).
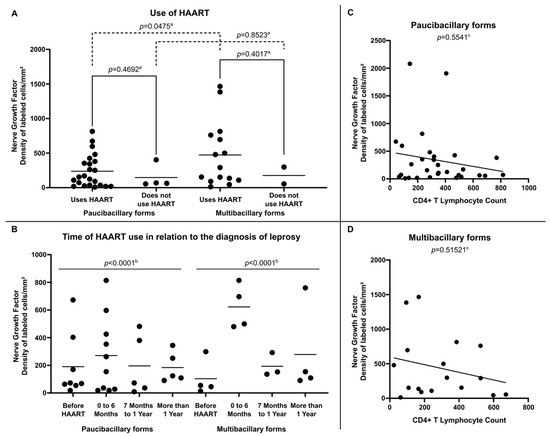
Figure 4.
(A) Tissue expression of NGF at diagnosis according to Use of HAART, (B) Tissue expression of NGF at diagnosis according to Time of HAART use in relation to the diagnosis of leprosy, (C) Correlation between NGF tissue expression and CD4+ T Lymphocyte count per cubic millimeter of peripheral blood in Paucibacilary Forms and (D) Correlation between NGF expression and CD4+ T Lymphocyte count per cubic millimeter of peripheral blood in Multibacilary Forms. a p-value achieved according to the t-test. b p-value according to the Chi-square test of adherence. c p-value achieved according to the Spearman Correlation Coefficient.
4. Discussion
Co-infection of leprosy with HIV is a challenge in several aspects [39]. Xavier [15], in 2006, described a series of cases in Brazil in which 31 cases were followed and a prevalence of paucibacillary forms and the occurrence of neuritis were observed during the period of immunological restoration associated with the initiation of HAART [40]. Mouchard and collaborators [21] described, in 2022, a series of cases of co-infected patients, pointing out similar findings. Furthermore, the same authors conducted a systematic review of the literature in which they analyzed 73 patients, predominantly men, with paucibacillary clinical forms and who presented a prevalence of type I leprosy reactions. The observation of two comparative cohorts of leprosy patients coinfected with HIV and another without co-infection, demonstrated similar results and incidence of type 1 leprosy reaction with neuritis [16]. These results reaffirmed data previously suggested in the literature that HIV did not change the polarization of the disease to its malignant pole and presents itself as a disease associated with immunological restoration after the start of HAART [41,42,43,44]. The clinical characteristics of the two groups analyzed here at the time of leprosy diagnosis resemble characteristics already reported in the literature.
The main objective of this study was to analyze the expression of NGF in leprosy lesions and observe possible differences between patients coinfected with HIV, thus assuming that the presence of HIV could increase neural damage, affecting the expression of NGF. Nerve growth factor is a widely studied neurotrophin with defined roles in the development, differentiation, maturation, and preservation of nervous tissue, in addition to its role in inflammatory processes, in which it is increased and can act in a pro-inflammatory and anti-inflammatory way depending on the target tissue [27]. In a study comparing tissue levels of NGF in the skin and peripheral nervous tissues of patients with borderline-virchowian leprosy and healthy individuals, Anand et al. [28], in 1994, found lower levels of this neurotrophin in leprosy patients. The authors suggest decreased levels of NGF as a relevant factor in nervous involvement, such as the loss of sensitivity characteristic of leprosy. Another study [45] also detected lower expression of NGF in leprosy patients compared to healthy individuals.
Regarding the antibody to NGF, there are few studies. Anti-NGF antibodies were found in the serum of patients with all forms of leprosy, with a significant decrease after the use of oral Cyclosporine for the treatment of type II reaction, suggesting the possibility of the contribution of neurotrophin in the pathophysiology of peripheral neuritis observed in leprosy disease [31]. The authors theorized that the high level of the antibody in leprosy patients could explain the decrease in NGF. High levels of anti-NGF antibodies have been detected in inflammatory diseases that produce nerve damage, such as lupus, thyroiditis, and rheumatoid arthritis. However, the relationship between NGF expression and anti-NGF antibodies still appears unclear, and, therefore, studies are still needed to clarify these relationships in diseases of immunological origin [27].
HIV/leprosy co-infection has been shown to be a factor that increases sensory and motor neural damage. Novais et al. [24] analyzed motor alterations in three groups of patients—(1) patients with HIV, (2) patients co-infected with HIV and leprosy, and (3) patients with leprosy without any other comorbidity—and observed a reduction in bilateral strength levels in the co-infected group in relation to the groups of patients with isolated diseases. The sum of sensory and motor damage could be justified by alterations in neurotrophin.
Anand [46] suggested that the decrease in NGF levels in leprosy patients would be an early change in the course of the disease and could be related to the loss of pain sensitivity even with intense tissue inflammatory activity, which could be related to the modulatory activity of neurotrophin on sensitivity in peripheral fibers to pain and heat [26].
The results of this study do not allow comparing leprosy patients co-infected with HIV and non-co-infected with healthy controls. However, by establishing similarities and the occurrence of greater neural damage at the time of diagnosis in leprosy patients also carrying HIV, due to the inflammatory state and sum of nerve damage [13,20], it was expected to find greater expression of NGF in co-infected patients than in patients not co-infected with HIV. However, the results presented here suggest that being a carrier of HIV did not significantly change the expression of NGF. This fact can be justified by the higher prevalence of multibacillary forms in non-co-infected patients and of paucibacillary forms in co-infected patients, according to the paradox of leprosy in patients living with HIV [15,39], and, since, as reported in previous studies, multibacillary forms present higher expression of this neurotrophin [35,47]. The multibacillary clinical form was the factor associated with the greater expression of this neurotrophin in both groups. The results found in this study, indicating a higher expression of NGF in lesions of leprosy patients with multibacillary forms, corroborate studies that compared the expression of NGF among leprosy patients with different clinical forms of the disease, with a higher expression of neurotrophin in patients with multibacillary forms [35,47], indicating an attempt to promote greater neuroprotection by increasing neurotrophin expression and consequent tissue remodeling and regeneration [27,48,49]. This pattern was also observed in other cytokines, such as TGF-β [35,50]. This could indicate a relationship between NGF expression and a Th2 inflammatory response pattern, which involves the expression of cytokines such as IL-4 and IL-10, greater nervous involvement, and greater inflammatory activity, characteristic of multibacillary forms of the disease [51].
The analysis of possible differences between neural damage in patients co-infected and non-co-infected with HIV was carried out by Xavier et al. [22] in 2018. A higher incidence of neuritis was observed in patients co-infected at the time of diagnosis, associated with the period of immunological restoration, with a slightly higher probability of developing sensory damage, especially in patients with multibacillary forms of the disease. However, the group of co-infected leprosy patients showed a significantly faster improvement in symptoms during treatment with MDT when compared to the group of leprosy patients without HIV. This would be justified by the fact that the non-co-infected group had a higher prevalence of multibacillary forms, with reactions with more prolonged neuritis, indicating that the neural damage in the co-infected group, despite being early and suggesting some influence from possible damage associated with HIV, tends to be reversible and follow, after immune restoration, the natural course of neural involvement caused by the leprosy bacillus [22].
Several studies have evaluated the role of neurotrophins, especially NGF, and suggest that they have a notable role in nerve damage, with expression in both neural and non-neural tissues, and have confirmed their function and that of their receptors as a marker of nerve injury, an adjuvant in regeneration, and in the activation/sensitization function of nociception [52]. It is postulated that the increase in expression in multibacillary forms in relation to other forms is due to the intensity of inflammatory reactions, immunological reactions towards the Th2 pole, and high bacillary load with a greater number of lesions and affected nerves, culminating in more prominent sensory alterations. The results found here showed a greater tendency for greater expression of NGF in non-co-infected multibacillary forms, considering the occurrence of neuritis, occurrence of reactions, and a greater number of affected nerve trunks, even when compared to co-infected multibacillary forms. Considering that patients with HIV, even during the period of immunological restoration, present intense immunological modifications with alterations in CD4+ lymphocytes, quantitatively and qualitatively, what would justify the difference in the inflammatory response and the difference in neurotrophin expression, and could it be one of the reasons for the non-elevation of NGF as expected [53,54,55].
Analyzing the expression of NGF only in the group of leprosy patients with HIV, it was observed that this was more relevant in multibacillary clinical forms, especially among those who used HAART and who were diagnosed with leprosy in the period suggestive of immune restoration (in the first six months after starting therapy), which corroborates the assumption that immune restoration favors neurotrophin production [53,56].
Levels of peripheral CD4+ lymphocytes did not correlate with the expression of NGF in the tissue. It is known that the tissue response is compartmentalized, and other studies have already demonstrated that levels of peripheral CD4+ lymphocytes are not related to the profile of CD4+ lymphocytes and tissue cytokines [15,57].
The main limitation of the study is the number of patients analyzed, since leprosy has a wide range of clinical manifestations within the pauci-multibacillary spectrum. However, in the context of leprosy-HIV co-infection, there is still a shortage of patients reported in the literature, and these are restricted to areas of significant geographic overlap, such as the northern region of Brazil. Considering that co-infection of leprosy with HIV is still prevalent in areas of high endemicity for both diseases, investigations should be conducted to better understand the immunopathology of nerve damage caused by this co-infection.
The importance of this study is due to its contribution to the immunopathological knowledge of leprosy and leprosy-HIV co-infection. Therefore, in regions with geographic overlap of these diseases, where there may be a sum of neural damage caused by each of them [22], it is important that possible biological markers, such as NGF, be studied so that they can be used as predictors of early neural damage, thus enabling the establishment of protective measures and measures to minimize this damage in patients. Further studies should be carried out with this marker so that this discussion can evolve.
The clinical and immunopathological characteristics of peripheral nerve damage in patients with concomitant HIV infection still have many gaps. It is necessary to consider that paucibacillary forms differ clinically and immunologically, and analyses should be made from this perspective. In addition, based on this study, other immunostainings should be performed to better understand the immunopathology of leprosy and leprosy-HIV co-infection.
Author Contributions
Conceptualization: M.B.X. and C.E.P.C.; data curation: L.d.S.F. and M.G.B.d.N.; formal analysis: L.d.S.F.; funding acquisition: M.B.X.; investigation: M.B.X., L.d.S.F., M.G.B.d.N., S.R.d.P., D.P.X., L.d.S.A. and E.B.d.B.; methodology: M.B.X.; project administration: M.B.X.; resources: E.B.d.B., C.M.d.C.G. and C.E.P.C.; supervision: M.B.X. and C.E.P.C.; Validation: C.M.d.C.G. and C.E.P.C.; visualization: M.B.X. and L.d.S.F.; writing—original draft: M.B.X., L.d.S.F. and M.G.B.d.N.; writing—review and editing: C.M.d.C.G. and C.E.P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Amazon Foundation to Support Studies and Research (Fapespa), Coordination of Superior Level Staff Improvement (CAPES) and Federal University of Pará (UFPA). L.d.S.F. and D.P.X. received grants by Amazon Foundation to Support Studies and Research (Fapespa), L.d.S.F. and S.R.d.P. received grants by Federal University of Pará (UFPA) and M.G.B.d.N. received grants by Coordination of Superior Level Staff Improvement (CAPES). The research was supported by São Paulo Research Foundation (FAPESP), grant #2014/50315-0.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of the Nucleus of Tropical Medicine of the Federal University of Pará with CAAE 10935419.5.0000.5172 and Protocol Code 3.285.553 on 8 August 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Nucleus of Tropical Medicine of Federal University of Pará for the assistance in the data collection; we thank the Infectious Diseases Pathology Laboratory (LIM50) of Medical School of Sao Paulo University for the assistance in the realization on the immunohistochemistry part of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AIDS | Acquired Immunodeficiency Syndrome |
| HIV | Human Immunodeficiency Virus |
| HAART | Highly Active Antiretroviral Treatment |
| NGF | Nerve Growth Factor |
| NP | Primary Neural |
| I | Indeterminate |
| TT | Tuberculoid |
| BT | Borderline Tuberculoid |
| PB | Paucibacillary Forms |
| BB | Borderline Borderline |
| BL | Borderline Lepromatous |
| LL | Lepromatous |
| MB | Multibacillary Forms |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
References
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966, 34, 255–273. [Google Scholar] [PubMed]
- Ridley, D.S. Histological classification and the immunological spectrum of leprosy. Bull. World Health Organ. 1974, 51, 451–465. [Google Scholar] [PubMed] [PubMed Central]
- WHO. Towards Zero Leprosy. Global Leprosy (Hansen’s Disease) Strategy 2021–2023. 2021. Available online: https://www.who.int/publications/i/item/9789290228509 (accessed on 5 February 2025).
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Doenças Crônicas e Infecções Sexualmente Transmissíveis. Protocolo Clínico e Diretrizes Terapêuticas da Hanseníase. Brasília—DF. 2022. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/protocolo_clinico_diretrizes_terapeuticas_hanseniase.pdf (accessed on 6 February 2025).
- Bochud, P.Y.; Hawn, T.R.; Siddiqui, M.R.; Saunderson, P.; Britton, S.; Abraham, I.; Argaw, A.T.; Janer, M.; Zhao, L.P.; Kaplan, G.; et al. Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J. Infect. Dis. 2008, 197, 253–261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupnik, K.M.; Bair, T.B.; Maia, A.O.; Amorim, F.M.; Costa, M.R.; Keesen, T.S.; Valverde, J.G.; Queiroz Mdo, C.; Medeiros, L.L.; de Lucena, N.L.; et al. Transcriptional changes that characterize the immune reactions of leprosy. J. Infect. Dis. 2015, 211, 1658–1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rathod, S.P.; Jagati, A.; Chowdhary, P. Disabilities in leprosy: An open, retrospective analyses of institutional records. An. Bras. Dermatol. 2020, 95, 52–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO. Leprosy Management of Reactions and Prevention of Disabilities. 2020. Available online: https://www.who.int/publications/i/item/9789290227595 (accessed on 12 February 2025).
- Nogueira, M.R.S.; Amôr, N.G.; Michellin, L.B.; Cury Filho, M.; Rosa, P.S.; Latini, A.C.P.; Rodrigues, L.S.; Lemes, R.M.R.; Lara, F.A.; Pessolani, M.C.V. Effect of Mycobacterium leprae on neurotrophins expression in human Schwann cells and mouse sciatic nerves. Mem. Inst. Oswaldo. Cruz. 2020, 115, e200075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- UNAIDS. Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS Statistics—Fact Sheet. 2024. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 12 February 2025).
- WHO. Global AIDS Strategy 2021–2026 End Inequalities. End AIDS. 2021. Available online: https://www.unaids.org/en/resources/documents/2021/2021-2026-global-AIDS-strategy (accessed on 12 February 2025).
- Verma, A. Epidemiology and clinical features of HIV-1 associated neuropathies. J. Peripher. Nerv. Syst. 2001, 6, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gabbai, A.A.; Castelo, A.; Oliveira, A.S. HIV peripheral neuropathy. Handb. Clin. Neurol. 2013, 115, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, F.; Liu, S.; Da, Y.; Guo, D. Neurological manifestations, laboratory and neuroimaging features in HIV-infected patients. Neurosciences 2017, 22, 311–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, M.B. Estudo Clínico e Imunopatológico em Pacientes Coinfectados pelo Vírus da Imunodeficiência Humana. Ph.D. Thesis, Federal University of Pará, Belém, Brazil, 2006. Available online: http://www.bibcentral.ufpa.br/arquivos/145000/146600/19_146659.htm (accessed on 4 February 2025).
- Pires, C.A.; Jucá Neto, F.O.; de Albuquerque, N.C.; Macedo, G.M.; Batista Kde, N.; Xavier, M.B. Leprosy Reactions in Patients Coinfected with HIV: Clinical Aspects and Outcomes in Two Comparative Cohorts in the Amazon Region, Brazil. PLoS Negl. Trop. Dis. 2015, 9, e0003818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pires, C.A.A.; Quaresma, J.A.S.; de Souza Aarão, T.L.; de Souza, J.R.; Macedo, G.M.M.; Neto, F.O.M.J.; Xavier, M.B. Expression of interleukin-1β and interleukin-6 in leprosy reactions in patients with human immunodeficiency virus coinfection. Acta Trop. 2017, 172, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Wood, C.; Lockwood, D.N. Borderline tuberculoid leprosy: An immune reconstitution phenomenon in a human immunodeficiency virus-infected person. Clin. Infect. Dis. 2003, 36, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Deps, P.D.; Lockwood, D.N. Leprosy occurring as immune reconstitution syndrome. Trans R Soc Trop Med Hyg. 2008, 102, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Sarno, E.N.; Illarramendi, X.; Nery, J.A.; Sales, A.M.; Gutierrez-Galhardo, M.C.; Fernandes Penna, M.L.; Sampaio, E.P.; Kaplan, G. HIV-M. leprae interaction: Can HAART modify the course of leprosy? Public Health Rep. 2008, 123, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Mouchard, A.; Blaizot, R.; Graille, J.; Couppié, P.; Bertin, C. Leprosy as immune reconstitution inflammatory syndrome in patients living with HIV: Description of French Guiana’s cases over 20 years and systematic review of the literature. PLoS Negl. Trop. Dis. 2022, 16, e0010239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, M.B.; do Nascimento, M.G.B.; Batista, K.N.M.; Somensi, D.N.; Juca Neto, F.O.M.; Carneiro, T.X.; Gomes, C.M.C.; Corbett, C.E.P. Peripheral nerve abnormality in HIV leprosy patients. PLoS Negl. Trop. Dis. 2018, 12, e0006633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acácio, J.A.B. Avaliação do Dano Neural Periférico Sensitivo e Motor em Pacientes Hansenianos, com HIV/AIDS e co-Infectados Hanseníase/HIV Utilizando-se a Avaliação Neurológica Simplificada e Técnicas Complementares. Ph.D. Thesis, Federal University of Pará, Belém, Brazil, 2015. Available online: https://repositorio.ufpa.br/jspui/handle/2011/9105 (accessed on 4 February 2025).
- Novais, D.V.C.; do Nascimento, M.G.B.; Lopes, G.L.; de Brito, J.A.G.S.M.; Carneiro, T.X.; Souza, G.S.; Xavier, M.B. The Correlation between Anthropometric Variables and Muscular Strength in Patients Coinfected with Leprosy and HIV. Indian J. Dermatol. 2023, 68, 127–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996, 19, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.R.; Barde, Y.A. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996, 19, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Aarão, T.L.S.; de Sousa, J.R.; Falcão, A.S.C.; Falcão, L.F.M.; Quaresma, J.A.S. Nerve Growth Factor and Pathogenesis of Leprosy: Review and Update. Front Immunol. 2018, 9, 939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anand, P.; Pandya, S.; Ladiwala, U.; Singhal, B.; Sinicropi, D.V.; Williams-Chestnut, R.E. Depletion of nerve growth factor in leprosy. Lancet 1994, 344, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Scully, J.L.; Otten, U. NGF: Not just for neurons. Cell Biol. Int. 1995, 19, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S.; Fahnestock, M. ProNGF, but not NGF, switches from neurotrophic to apoptotic activity in response to reductions in TrkA receptor levels. Int. J. Mol. Sci. 2017, 18, 599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sena, C.B.; Salgado, C.G.; Tavares, C.M.; Da Cruz, C.A.; Xavier, M.B.; Do Nascimento, J.L. Cyclosporine A treatment of leprosy patients with chronic neuritis is associated with pain control and reduction in antibodies against nerve growth factor. Lepr. Rev. 2006, 77, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Widasmara, D.; Menaldi, S.L.; Turchan, A. Evaluation of nerve growth factor serum level for early detection of leprosy disability. Pan. Afr. Med. J. 2020, 37, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jesus, J.B.; Sena, C.B.C.; Macchi, B.M.; do Nascimento, J.L.M. Cyclosporin A as an Alternative Neuroimmune Strategy to Control Neurites and Recover Neuronal Tissues in Leprosy. Neuroimmunomodulation 2022, 29, 15–20. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.T.; Evangelista, B.G.; Venega, R.A.G.; Seminowicz, D.A.; Chacur, M. Anti-NGF treatment can reduce chronic neuropathic pain by changing peripheral mediators and brain activity in rats. Behav. Pharmacol. 2019, 30, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Aarão, T.L.; Esteves, N.R.; Esteves, N.; Soares, L.P.; da Silva Pinto, D.; Fuzii, H.T.; Quaresma, J.A.S. Relationship between growth factors and its implication in the pathogenesis of leprosy. Microb. Pathog. 2014, 77, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Manual Técnico para o Diagnóstico da Infecção pelo HIV em Adultos e Crianças. Brasília—DF. 2018. Available online: https://www.gov.br/aids/pt-br/central-de-conteudo/publicacoes/2018/manual_tecnico_hiv_27_11_2018_web.pdf (accessed on 12 February 2025).
- Hsu, S.M.; Raine, L.; Fanger, H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Barros, V.L.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.; Andrade, H.F., Jr.; Vasconcelos, P.F.; Duarte, M.I. Revisiting the liver in human yellow fever: Virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology 2006, 345, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ustianowski, A.P.; Lawn, S.D.; Lockwood, D.N. Interactions between HIV infection and leprosy: A paradox. Lancet Infect. Dis. 2006, 6, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Deps, P.; Lockwood, D.N. Leprosy presenting as immune reconstitution inflammatory syndrome: Proposed definitions and classification. Lepr. Rev. 2010, 81, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A. Leprosy and AIDS: A review of the literature and speculations on the impact of CD4+ lymphocyte depletion on immunity to Mycobacterium leprae. Int. J. Lepr. Other Mycobact. Dis. 1991, 59, 639–644. [Google Scholar] [PubMed]
- Lucas, S. Human immunodeficiency virus and leprosy. Lepr. Rev. 1993, 64, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Nery, J.A.; Sampaio, E.P.; Galhardo, M.C.; Perissé, A.R.; Vieira, L.M.; Salles, A.M.; Sarno, E.N. M. leprae-HIV co-infection: Pattern of immune response in vivo and in vitro. Indian J. Lepr. 2000, 72, 155–167. [Google Scholar] [PubMed]
- Lockwood, D.N.; Lambert, S.M. Human immunodeficiency virus and leprosy: An update. Dermatol. Clin. 2011, 29, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Facer, P.; Mann, D.; Mathur, R.; Pandya, S.; Ladiwala, U.; Singhal, B.; Hongo, J.; Sinicropi, D.V.; Terenghi, G.; Anand, P. Do nerve growth factor-related mechanisms contribute to loss of cutaneous nociception in leprosy? Pain 2000, 85, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Anand, P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog. Brain Res. 2004, 146, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Aarão, T.L.; de Sousa, J.R.; Botelho, B.S.; Fuzii, H.T.; Quaresma, J.A. Correlation between nerve growth factor and tissue expression of IL-17 in leprosy. Microb. Pathog. 2016, 90, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Michellin, L.B.; Barreto, J.A.; Marciano, L.H.; Lara, F.A.; Nogueira, M.E.; Souza, V.N.; Costa, M.R. Leprosy patients: Neurotrophic factors and axonal markers in skin lesions. Arq. Neuropsiquiatr. 2012, 70, 281–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karanth, S.S.; Springall, D.R.; Lucas, S.; Levy, D.; Ashby, P.; Levene, M.M.; Polak, J.M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 1989, 157, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Simoes Quaresma, J.A.; de Almeida, F.A.; de Souza Aarao, T.L.; de Miranda Araujo Soares, L.P.; Nunes Magno, I.M.; Fuzii, H.T.; Feio Libonati, R.M.; Xavier, M.B.; Pagliari, C.; Seixas Duarte, M.I. Transforming growth factor β and apoptosis in leprosy skin lesions: Possible relationship with the control of the tissue immune response in the Mycobacterium leprae infection. Microbes. Infect. 2012, 14, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Britton, W.J.; Lockwood, D.N. Leprosy. Lancet 2004, 363, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.S.; Hsu, E.; Hottinger, D.G.; Cohen, S.P. Anti-nerve growth factor in pain management: Current evidence. J. Pain Res. 2016, 9, 373–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bucy, R.P.; Hockett, R.D.; Derdeyn, C.A.; Saag, M.S.; Squires, K.; Sillers, M.; Mitsuyasu, R.T.; Kilby, J.M. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J. Clin. Investig. 1999, 103, 1391–1398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaplan, J.E.; Masur, H.; Holmes, K.K.; USPHS. Infectious Disease Society of America. Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm. Rep. 2002, 51, 1–52. [Google Scholar] [PubMed]
- Santambrogio, L.; Benedetti, M.; Chao, M.V.; Muzaffar, R.; Kulig, K.; Gabellini, N.; Hochwald, G. Nerve growth factor production by lymphocytes. J. Immunol. 1994, 153, 4488–4495. [Google Scholar] [CrossRef] [PubMed]
- Bichara, C.N.C.; Bichara, C.D.A.; Tostes, C.; Povoa, M.M.; Quaresma, J.A.S.; Xavier, M.B. Prevalence of autoantibodies against cellular antigens in patients with HIV and leprosy coinfection in the Amazon region. Infect. Dis. Poverty. 2017, 6, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abulafia, J.; Vignale, R.A. Leprosy: Accessory immune system as effector of infectious, metabolic, and immunologic reactions. Int. J. Dermatol. 2001, 40, 673–687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).