Microbial Keratinolysis: Eco-Friendly Valorisation of Keratinous Waste into Functional Peptides
Abstract
1. Introduction
2. Keratinous Waste Biomass in the Environment
3. Recycling Keratinous Biomass Using Sustainable Technology
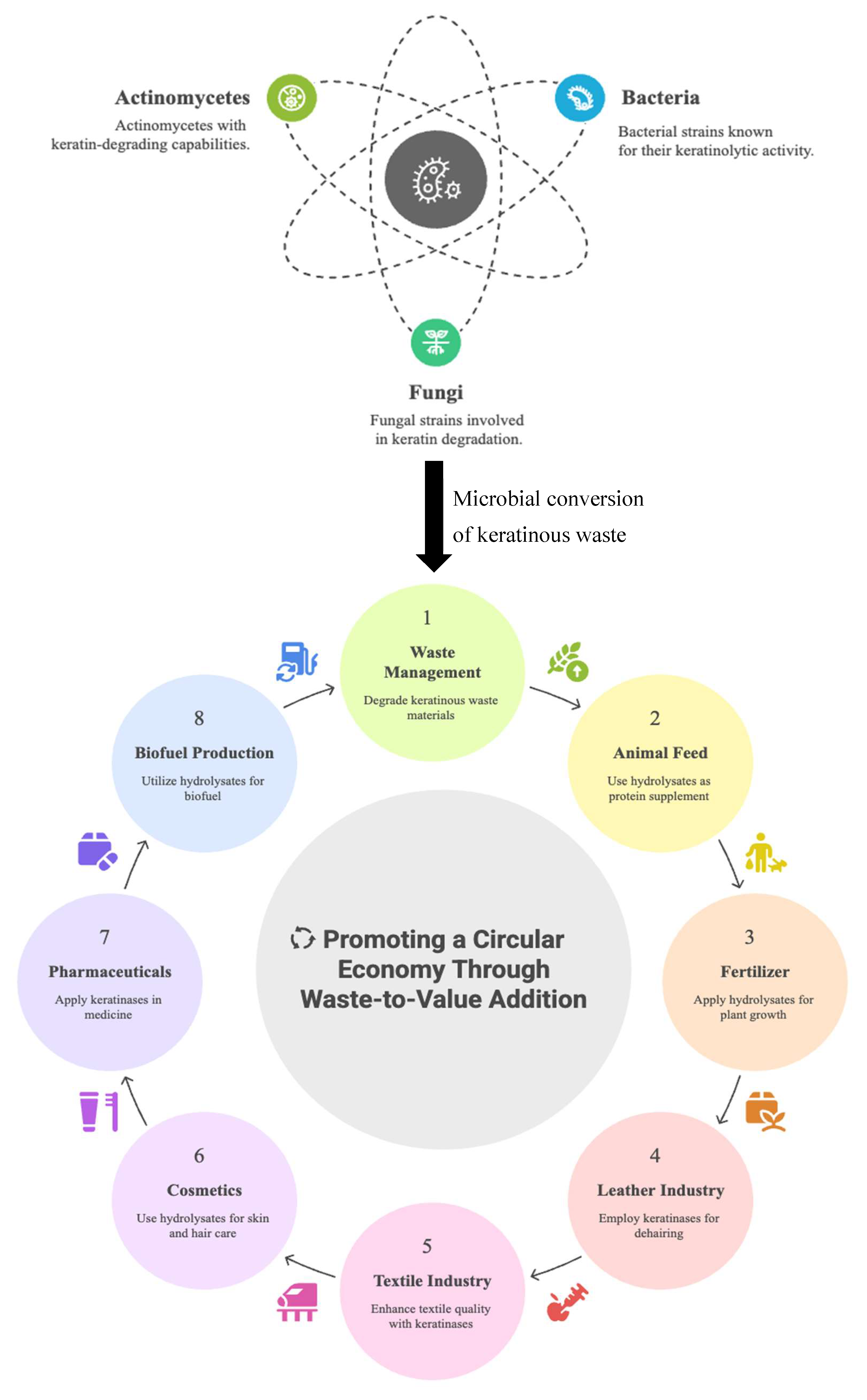
4. Sources of Microbial Keratinases
4.1. Bacteria as Sources of Keratin-Degrading Enzymes
4.2. Fungi as Sources of Keratin-Degrading Enzymes
4.3. Actinomycetes as Sources of Keratin-Degrading Enzymes
4.4. Comparative Analysis of Keratinase Production Among the Keratinolytic Microbial Strains
| Keratinolytic Strain | Domain | Enzyme Type | Optimum pH | Optimum Temperature (°C) | Molecular Weight (kDa) | Reference |
|---|---|---|---|---|---|---|
| Chryseobacterium sp. Kr6 | Bacteria | Metallo | 8.5 | 50 | 64 | [107] |
| Clostridium sporogenes | Bacteria | - | 8 | 55 | 28.7 | [108] |
| Bacillus licheniformis PWD-1 | Bacteria | Serine | 7.5 | 50 | 33 | [109] |
| Bacillus cereus DCUW | Bacteria | Serine | 8.5 | 50 | 50 | [110] |
| Bacillus licheniformis FK14 | Bacteria | Serine | 8.5 | 60 | 35 | [111] |
| Bacillus licheniformis K-508 | Bacteria | Thiol | 8.5 | 52 | 42 | [112] |
| Bacillus licheniformis RPk | Bacteria | Serine | 9 | 60 | 32 | [113] |
| Bacillus subtilis MTCC (9102) | Bacteria | Metallo | 6 | 49 | 69 | [114] |
| Streptomyces albidoflavus | Actinomycetes | Serine | 6.0–9.5 | 40–70 | 18 | [68] |
| Streptomyces pactum | Actinomycetes | Serine | 7–10 | 40–75 | 30 | [95] |
| Streptomyces thermoviolaceus | Actinomycetes | - | 8 | 55 | 40 | [98] |
| Aspergillus fumigatus | Fungi | Serine | 6.5–9 | 45 | - | [115] |
| Aspergillus oryzae | Fungi | Metallo | 8 | 50 | 60 | [116] |
| Myrothecium verrucaria | Fungi | Serine | 8.3 | 37 | 22 | [89] |
| Paecilomyces marquandii | Fungi | Serine | 8 | 60–65 | 33 | [85] |
| Scopulariopsis brevicaulis | Fungi | Serine | 8 | 40 | 36–39 | [117] |
| Trichophyton schoenleinii | Fungi | - | 5.5 | 50 | 38 | [118] |
| Trichophyton vanbreuseghemii | Fungi | Serine | 8 | - | 37 | [119] |
5. Isolation of Keratinolytic Microorganisms and Production of Keratinases for Prospective Applications
5.1. Optimizing pH Conditions for Efficient Keratinase Production
5.2. Optimizing Temperature for Efficient Keratinase Production
5.3. Optimizing Keratinous Substrate for Enhanced Keratinase Production
5.4. Optimizing Metal Ions for Enhanced Keratinase Production
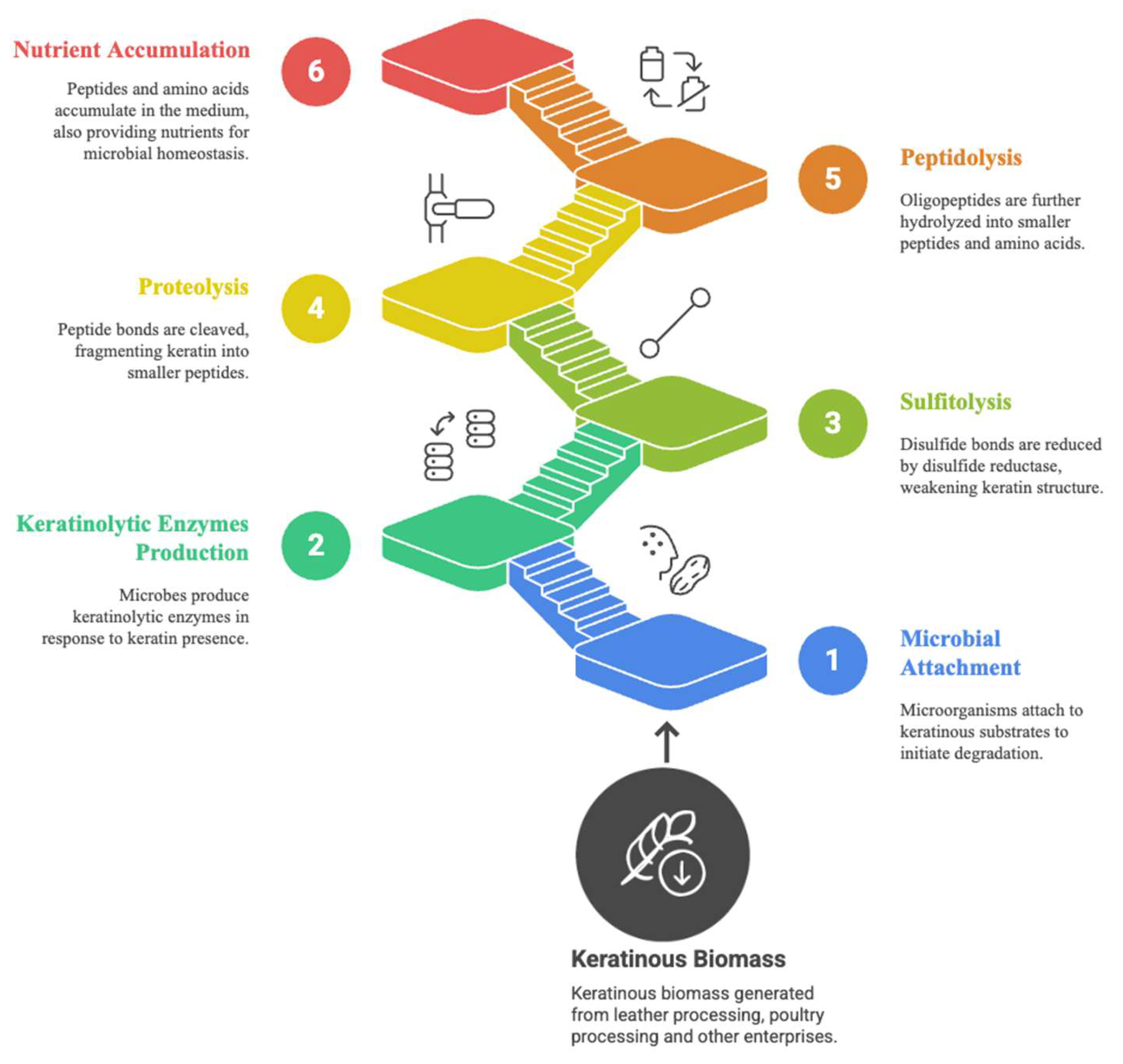
6. Transformation of Keratinous Waste Through Microbial Keratinolysis: Application Prospects
6.1. Keratinases in Waste Management and Recycling
6.2. Keratinases in the Agricultural Sector
6.3. Keratinases in Cosmetics Production
6.4. Keratinases in Detergent Formulation
6.5. Keratinases in Leather and Textile Production
6.6. Keratinases in Medicine and Pharmaceuticals
7. Keratinolytic Enzyme-Keratinous Waste-Product Relationships
| Source of Keratin Hydrolysate | Biotreatment Agent | Product Identification Method | Identified Products | Potential Uses | References |
|---|---|---|---|---|---|
| Animal hair | Brevibacterium luteolum MTCC 5982 | HPLC | Amino acids (Asp, Glu, Cys, Ser, His, Gly, Thr, Arg, Ala, Tyr, Met, Val, Phe, Ile, Leu, Lys) | - | [171] |
| Chicken feathers | ICSE coupled with keratinase | HPLC-MS/MS Amino acid auto-analyzer | Peptides (500 Da, <3 kDa) Amino acids (Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, His, Lys, Arg, Pro) | Antimicrobial | [183] |
| Feathers | Bacillus subtilis S1-4 | RP-FPLC, MALDI-TOF/TOF-MS/MS | Peptide (Sequence: Ser-Asn-Leu-Cys-Arg-Pro-Cys-Gly) | Antioxidant | [182] |
| Chicken feathers | Bacillus velezensis NCIM 5802 | NMR, ESI-MS | Amino acids (Thr, Pro, Val, Asn, Leu, Ile, Ser, Asp, Glu, Gln, Lys, Arg, His, Phe, Tyr, Met, Cys, Trp) | - | [184] |
| Sheep wool | Recombinant Bacillus subtilis | Amino acid analyzer | Amino acids (Asp, Thr, Ser, Glu, Pro, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, His, Lys, Arg) | - | [185] |
| Chicken feathers | Chryseobacterium sediminis RCM-SSR-7 | HPLC | Amino acids (Asp, Glu, Ser, His, Gly, Thr, Arg, Ala, Tyr, Met, Val, Phe, Ile, Leu, Lys) | Feed supplement, Organic fertilizer | [186] |
| Chicken feathers | Recombinant Bacillus subtilis WB600 | Amino acid analyzer | Amino acids (Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, His, Lys, Arg, Pro) | - | [187] |
| Chicken feathers | Keratinolytic bacteria, keratinase | FTIR | Peptides (<10 kDa) | Antioxidant, Antityrosinase | [181] |
| Chicken feathers | Ketatinolytic enzyme | - | Peptides (<3 kDa) | Antioxidant | [188] |
| Chicken feathers | Keratinolytic Rhodococcus erythropolis | RP-HPLC, MALDI-TOF, FTIR | Peptides (3861 Da) | Antibacterial, Antibiofilm | [189] |
| Chicken feathers | Chryseobacterium sp. kr6 | LC-MS/MS | Peptides (1155.641 Da) | Antioxidant | [190] |
| Feathers | Keratinase | UPLC/Q-TOF-MS | Peptides | ACE inhibitor, DPP IV inhibitor | [191] |
| Chicken feathers | Bacillus licheniformis WHU, Keratinase | LC-MS | Peptides Amino acids (Trp, Tyr, Asp, Thr, Ser, Glu, Ala, Val, Met, Ile, Leu, Phe, His, Lys, Arg, Pro) | Antioxidant Feed supplement | [192] |
8. Techno-Economic Considerations for Industrial-Scale Keratinase Applications
9. Limitations and Future Research Needs
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangali, S.; Brandelli, A. Feather keratin hydrolysis by a Vibrio sp. strain kr2. J. Appl. Microbiol. 2000, 89, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhang, J.; Du, G.; Chen, J. Keratin waste recycling based on microbial degradation: Mechanisms and prospects. ACS Sustain. Chem. Eng. 2019, 7, 9727–9736. [Google Scholar] [CrossRef]
- Reddy, C.C.; Khilji, I.A.; Gupta, A.; Bhuyar, P.; Mahmood, S.; AL-Japairai, K.A.; Chua, G.K. Valorization of keratin waste biomass and its potential applications. JWPE 2021, 40, 101707. [Google Scholar]
- Brandelli, A. Bacterial keratinases: Useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 2008, 1, 105–116. [Google Scholar] [CrossRef]
- Jin, H.S.; Park, S.Y.; Kim, K.; Lee, Y.J.; Nam, G.W.; Kang, N.J.; Lee, D.W. Development of a keratinase activity assay using recombinant chicken feather keratin substrates. PLoS ONE 2017, 12, e0172712. [Google Scholar] [CrossRef]
- Li, Q. Progress in microbial degradation of feather waste. Front. Microbiol. 2019, 10, 2717. [Google Scholar] [CrossRef]
- Hossain, M.S.; Azad, A.K.; Sayem, S.A.; Mostafa, G.; Hoq, M.M. Production and partial characterization of feather-degrading keratinolytic serine protease from Bacillus licheniformis MZK-3. J. Biol. Sci. 2007, 7, 599–606. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Udenigwe, C.C.; Okoh, A.I.; Nwodo, U.U. Microbial keratinase: Next generation green catalyst and prospective applications. Front. Microbiol. 2020, 11, 580164. [Google Scholar] [CrossRef]
- Kalaikumari, S.S.; Vennila, T.; Monika, V.; Chandraraj, K.; Gunasekaran, P.; Rajendhran, J. Bioutilization of poultry feather for keratinase production and its application in leather industry. J. Clean. Prod. 2019, 208, 44–53. [Google Scholar] [CrossRef]
- Chojnacka, K.; Górecka, H.; Michalak, I.; Górecki, H. A review: Valorization of keratinous materials. Waste Biomass Valori. 2011, 2, 317–321. [Google Scholar] [CrossRef]
- Gessesse, A.; Hatti-Kaul, R.; Gashe, B.A.; Mattiasson, B.O. Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enzyme Microb. Technol. 2003, 32, 519–524. [Google Scholar] [CrossRef]
- Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S.; Ningthoujam, D.S. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar]
- Paul, T.; Jana, A.; Mandal, A.K.; Mandal, A.; Mohpatra, P.K.; Mondal, K.C. Bacterial keratinolytic protease, imminent starter for NextGen leather and detergent industries. Sustain. Chem. Pharm. 2016, 3, 8–22. [Google Scholar] [CrossRef]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal. Agric. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Xia, Y.; Massé, D.I.; McAllister, T.A.; Beaulieu, C.; Ungerfeld, E. Anaerobic digestion of chicken feather with swine manure or slaughterhouse sludge for biogas production. Waste Manag. 2012, 32, 404–409. [Google Scholar]
- Grazziotin, A.; Pimentel, F.A.; De Jong, E.V.; Brandelli, A. Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol. 2006, 126, 135–144. [Google Scholar] [CrossRef]
- Yamamura, S.; Morita, Y.; Hasan, Q.; Yokoyama, K.; Tamiya, E. Keratin degradation: A cooperative action of two enzymes from Stenotrophomonas sp. Biochem. Biophys. Res. Commun. 2002, 294, 1138–1143. [Google Scholar]
- Sapkota, A.R.; Lefferts, L.Y.; McKenzie, S.; Walker, P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ. Health Perspect. 2007, 115, 663–670. [Google Scholar] [CrossRef]
- Akinsemolu, A.A. The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 2018, 182, 139–155. [Google Scholar] [CrossRef]
- Stiborova, H.; Branska, B.; Vesela, T.; Lovecka, P.; Stranska, M.; Hajslova, J.; Jiru, M.; Patakova, P.; Demnerova, K. Transformation of raw feather waste into digestible peptides and amino acids. J. Chem. Technol. Biotechnol. 2016, 91, 1629–1637. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Sharifi, S.D.; Tabandeh, F.; Honarbakhsh, S.; Ghazanfari, S. Bioconversion of chicken feather wastes by keratinolytic bacteria. Process Saf. Environ. Prot. 2020, 135, 171–178. [Google Scholar] [CrossRef]
- He, Z.; Sun, R.; Tang, Z.; Bu, T.; Wu, Q.; Li, C.; Chen, H. Biodegradation of feather waste keratin by the keratin-degrading strain Bacillus subtilis 8. J. Microbiol. Biotechnol. 2018, 28, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Nnolim, N.E.; Okoh, A.I.; Nwodo, U.U. Proteolytic bacteria isolated from agro-waste dumpsites produced keratinolytic enzymes. Biotechnol. Rep. 2020, 27, e00483. [Google Scholar] [CrossRef] [PubMed]
- Sohnle, P.G.; Wagner, D.K. Fungal infections, cutaneous. In Encyclopedia of Microbiology, 2nd ed.; Academic: San Diego, CA, USA, 2000; pp. 451–459. [Google Scholar]
- Wang, L.; Qian, Y.; Cao, Y.; Huang, Y.; Chang, Z.; Huang, H. Production and characterization of keratinolytic proteases by a chicken feather-degrading thermophilic strain, Thermoactinomyces sp. YT06. J. Microbiol. Biotechnol. 2017, 27, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J.; Korniłłowicz-Kowalska, T.; Kitowski, I.; Ciesielska, A. Degradation of chicken feathers by Aphanoascus keratinophilus and Chrysosporium tropicum strains from pellets of predatory birds and its practical aspect. Int. Biodeterior. Biodegrad. 2020, 151, 104968. [Google Scholar]
- Ignatova, Z.; Gousterova, A.; Spassov, G.; Nedkov, P. Isolation and partial characterization of extracellular keratinase from a wool degrading thermophilic actinomycete strain Thermoactinomyces candidus. Can. J. Microbiol. 1999, 45, 217–222. [Google Scholar] [CrossRef]
- Pettett, L.M.; Kurtböke, D.I. Development of an environmentally friendly biofertilizer with keratin degrading and antibiotic producing actinomycetes. Actinomycetologica 2004, 18, 34–42. [Google Scholar] [CrossRef]
- De Toni, C.H.; Richter, M.F.; Chagas, J.R.; Henriques, J.A.; Termignoni, C. Purification and characterization of an alkaline serine endopeptidase from a feather-degrading Xanthomonas maltophilia strain. Can. J. Microbiol. 2002, 48, 342–348. [Google Scholar] [CrossRef]
- Gupta, R.; Ramnani, P. Microbial keratinases and their prospective applications: An overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [CrossRef]
- Onifade, A.A.; Al-Sane, N.A.; Al-Musallam, A.A.; Al-Zarban, S. A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 1998, 66, 1–11. [Google Scholar] [CrossRef]
- Gerber, P.; Opio, C.; Steinfeld, H. Poultry production and the environment—A review. In Proceedings of the International Conference Poultry in the Twenty-First Century: Avian Influenza and Beyond, Bangkok, Thailand, 5–7 November 2007. [Google Scholar]
- Syed, M.; Saleem, T.; Shuja-ur-Rehman; Iqbal, M.A.; Javed, F.; Khan, M.B.; Sadiq, K. Effects of leather industry on health and recommendations for improving the situation in Pakistan. Arch. Environ. Occup. Health 2010, 65, 163–172. [Google Scholar] [CrossRef]
- Kumar, S.; Bhattacharyya, J.K.; Vaidya, A.N.; Chakrabarti, T.; Devotta, S.; Akolkar, A.B. Assessment of the status of municipal solid waste management in metro cities, state capitals, class I cities, and class II towns in India: An insight. Waste Manag. 2009, 29, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Human hair “waste” and its utilization: Gaps and possibilities. J. Waste Manag. 2014, 2014, 498018. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D. Valorisation of chicken feathers: A review on recycling and recovery route—Current status and future prospects. Clean. Technol. Environ. Policy 2017, 19, 2363–2378. [Google Scholar] [CrossRef]
- Kumawat, T.K.; Sharma, A.; Bhadauria, S. Influence of liquid culture media, temperature and hydrogen ion concentration on the growth of mycelium and sporulation of Arthroderma multifidum. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 136–141. [Google Scholar]
- Wang, X.; Parsons, C.M. Effect of processing systems on protein quality of feather meals and hog hair meals. Poult. Sci. 1997, 76, 491–496. [Google Scholar] [CrossRef]
- Ritter, W.F.; Chirnside, A.E. Impact of dead bird disposal pits on ground-water quality on the Delmarva Peninsula. Bioresour. Technol. 1995, 53, 105–111. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A.; Clare, B.W. Bacterial protease inhibitors. Med. Res. Rev. 2002, 22, 329–372. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsujimoto, Y.; Matsui, H.; Watanabe, K. Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. J. Biosci. Bioeng. 2006, 102, 73–81. [Google Scholar] [CrossRef]
- Marchisio, V.F. Keratinophilic fungi: Their role in nature and degradation of keratinic substrates. Biol. Dermatophytes Other Keratinophilic Fungi 2000, 17, 86–92. [Google Scholar]
- Noval, J.J.; Nickerson, W.J. Decomposition of native keratin by Streptomyces fradiae. J. Bacteriol. 1959, 77, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Richter, C.S.; MacKenzie, J.M., Jr.; Shih, J.C. Isolation, identification, and characterization of a feather-degrading bacterium. Appl. Environ. Microbiol. 1990, 56, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rajak, R.C. Keratinophilic Fungi: Nature’s keratin degrading machines! their isolation, identification and ecological role. Resonance 2003, 8, 28–40. [Google Scholar]
- Sinoy, S.; Bhausaheb, T.C.; Rajendra, P.P. Isolation and identification of feather degradable microorganism. VSRD-TNTJ 2011, 2, 128–136. [Google Scholar]
- Călin, M.; Constantinescu-Aruxandei, D.; Alexandrescu, E.; Răut, I.; Doni, M.B.; Arsene, M.L.; Oancea, F.; Jecu, L.; Lazăr, V. Degradation of keratin substrates by keratinolytic fungi. Electron. J. Biotechnol. 2017, 28, 101–112. [Google Scholar] [CrossRef]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial enzymes catalyzing keratin degradation: Classification, structure, function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef]
- Anbesaw, M.S. Bioconversion of keratin wastes using keratinolytic microorganisms to generate value-added products. Int. J. Biomater. 2022, 2022, 2048031. [Google Scholar] [CrossRef]
- Lucas, F.S.; Broennimann, O.; Febbraro, I.; Heeb, P. High diversity among feather-degrading bacteria from a dry meadow soil. Microb. Ecol. 2003, 45, 282–290. [Google Scholar] [CrossRef]
- Riffel, A.; Brandelli, A. Keratinolytic bacteria isolated from feather waste. Braz. J. Microbiol. 2006, 37, 395–399. [Google Scholar] [CrossRef]
- Manczinger, L.; Rozs, M.; Vágvölgyi, C.; Kevei, F. Isolation and characterization of a new keratinolytic Bacillus licheniformis strain. World J. Microbiol. Biotechnol. 2003, 19, 35–39. [Google Scholar] [CrossRef]
- Ramnani, P.; Singh, R.; Gupta, R. Keratinolytic potential of Bacillus licheniformis RG1: Structural and biochemical mechanism of feather degradation. Can. J. Microbiol. 2005, 51, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Tuysuz, E.; Ozkan, H.; Arslan, N.P.; Adiguzel, A.; Baltaci, M.O.; Taskin, M. Bioconversion of waste sheep wool to microbial peptone by Bacillus licheniformis EY2. Biofuel Bioprod. Bior. 2021, 15, 1372–1384. [Google Scholar] [CrossRef]
- Esawy, M.A. Isolation and partial characterization of extracellular keratinase from a novel mesophilic Streptomyces albus AZA. Res. J. Agric. Biol. Sci. 2007, 3, 808–817. [Google Scholar]
- Kansoh, A.L.; Hossiny, E.N.; Abd EL-Hameed, E.K. Keratinase production from feathers wastes using some local Streptomyces isolates. Aust. J. Basic. Appl. Sci. 2009, 3, 561–571. [Google Scholar]
- Ningthoujam, D.S.; Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S. Keratinaceous wastes and their valorization through keratinolytic microorganisms. In Keratin; IntechOpen: London, UK, 2018. [Google Scholar]
- Nam, G.W.; Lee, D.W.; Lee, H.S.; Lee, N.J.; Kim, B.C.; Choe, E.A.; Hwang, J.K.; Suhartono, M.T.; Pyun, Y.R. Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch. Microbiol. 2002, 178, 538–547. [Google Scholar] [CrossRef]
- Vigneshwaran, C.; Shanmugam, S.; Kumar, T.S. Screening and characterization of keratinase from Bacillus licheniformis isolated from Namakkal poultry farm. Researcher 2010, 2, 89–96. [Google Scholar]
- Chitte, R.R.; Nalawade, V.K.; Dey, S. Keratinolytic activity from the broth of a feather-degrading thermophilic Streptomyces thermoviolaceus strain SD8. Lett. Appl. Microbiol. 1999, 28, 131–136. [Google Scholar]
- Macedo, A.J.; da Silva, W.O.B.; Gava, R.; Driemeier, D.; Henriques, J.A.P.; Termignoni, C. Novel keratinase from Bacillus subtilis S14 showing remarkable dehairing capabilities. Appl. Environ. Microbiol. 2005, 71, 594–596. [Google Scholar]
- Tiwary, E.; Gupta, R. Medium optimization for a novel 58ákDa dimeric keratinase from Bacillus licheniformis ER-15: Biochemical characterization and application in feather degradation and dehairing of hides. Bioresour. Technol. 2010, 101, 6103–6110. [Google Scholar] [CrossRef]
- Thys, R.C.; Guzzon, S.O.; Cladera-Olivera, F.; Brandelli, A. Optimization of protease production by Microbacterium sp. in feather meal using response surface methodology. Process Biochem. 2006, 41, 67–73. [Google Scholar] [CrossRef]
- Herzog, B.; Overy, D.P.; Haltli, B.; Kerr, R.G. Discovery of keratinases using bacteria isolated from marine environments. Syst. Appl. Microbiol. 2016, 39, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Hsu, W.T.; Liang, T.W.; Yen, Y.H.; Wang, C.L. Purification and characterization of three novel keratinolytic metalloproteases produced by Chryseobacterium indologenes TKU014 in a shrimp shell powder medium. Bioresour. Technol. 2008, 99, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Bressollier, P.; Letourneau, F.; Urdaci, M.; Verneuil, B. Purification and characterization of a keratinolytic serine proteinase from Streptomyces albidoflavus. Appl. Environ. Microbiol. 1999, 65, 2570–2576. [Google Scholar] [CrossRef]
- Bokveld, A.; Nnolim, N.E.; Nwodo, U.U. Chryseobacterium aquifrigidense FANN1 produced detergent-stable metallokeratinase and amino acids through the abasement of chicken feathers. Front. Bioeng. Biotechnol. 2021, 9, 720176. [Google Scholar] [CrossRef]
- Riffel, A.; Lucas, F.; Heeb, P.; Brandelli, A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch. Microbiol. 2003, 179, 258–265. [Google Scholar] [CrossRef]
- Barman, N.C.; Zohora, F.T.; Das, K.C.; Mowla, M.G.; Banu, N.A.; Salimullah, M.; Hashem, A. Production, partial optimization and characterization of keratinase enzyme by Arthrobacter sp. NFH5 isolated from soil samples. Amb Express 2017, 7, 181. [Google Scholar] [CrossRef]
- Yang, J.I.; Kuo, J.M.; Chen, W.M.; Ke, H.J.; Chou, Y.J. Feather keratin hydrolysis by an aquatic bacterium Meiothermus I40 from hot spring water. Int. J. Food Eng. 2011, 7, 17. [Google Scholar] [CrossRef]
- El-Bondkly, A.M.; El-Gendy, M.M. Keratinolytic activity from new recombinant fusant AYA2000, derived from endophytic Micromonospora strains. Can. J. Microbiol. 2010, 56, 748–760. [Google Scholar] [CrossRef]
- Cai, S.B.; Huang, Z.H.; Zhang, X.Q.; Cao, Z.J.; Zhou, M.H.; Hong, F. Identification of a keratinase-producing bacterial strain and enzymatic study for its improvement on shrink resistance and tensile strength of wool-and polyester-blended fabric. Appl. Biochem. Biotechnol. 2011, 163, 112–126. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.H.; Kim, H.K.; Lee, J.S. Production and characterization of keratinase from Paracoccus sp. WJ-98. Biotechnol. Bioprocess. Eng. 2004, 9, 17–22. [Google Scholar] [CrossRef]
- Allpress, J.D.; Mountain, G.; Gowland, P.C. Production, purification and characterization of an extracellular keratinase from Lysobacter NCIMB 9497. Lett. Appl. Microbiol. 2002, 34, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Riessen, S.; Antranikian, G. Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles 2001, 5, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Vanbreuseghem, R. Technique biologique pour l’isolement des dermatophytes du sol. Ann. Soc. Belge Med. Trop. 1952, 32, 173–178. [Google Scholar]
- Hassan, M.A.; Abol-Fotouh, D.; Omer, A.M.; Tamer, T.M.; Abbas, E. Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review. Int. J. Biol. Macromol. 2020, 154, 567–583. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Liu, B.; Du, G.; Chen, J. Biochemical characterization of three keratinolytic enzymes from Stenotrophomonas maltophilia BBE11-1 for biodegrading keratin wastes. Int. Biodeterior. Biodegrad. 2013, 82, 166–172. [Google Scholar] [CrossRef]
- Jaouadi, N.Z.; Rekik, H.; Badis, A.; Trabelsi, S.; Belhoul, M.; Yahiaoui, A.B.; Aicha, H.B.; Toumi, A.; Bejar, S.; Jaouadi, B. Biochemical and molecular characterization of a serine keratinase from Brevibacillus brevis US575 with promising keratin-biodegradation and hide-dehairing activities. PLoS ONE 2013, 8, e76722. [Google Scholar] [CrossRef]
- Falco, F.C.; Espersen, R.; Svensson, B.; Gernaey, K.V.; Lantz, A.E. An integrated strategy for the effective production of bristle protein hydrolysate by the keratinolytic filamentous bacterium Amycolatopsis keratiniphila D2. Waste Manag. 2019, 89, 94–102. [Google Scholar] [CrossRef]
- Günyar, O.A.; Kıraç, S.; Aldı, B.; Ergin, Ç. Isolation and identification of keratinophilic fungi in soil samples from excavation area of ancient city of Stratonikeia, Turkey and determination of its enzyme potentials py. J. Environ. Biol. 2020, 41, 1521–1525. [Google Scholar]
- Köhler, J.R.; Casadevall, A.; Perfect, J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2015, 5, a019273. [Google Scholar] [CrossRef]
- Gradisar, H.; Friedrich, J.; Krizaj, I.; Jerala, R. Similarities and specificities of fungal keratinolytic proteases: Comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Appl. Environ. Microbiol. 2005, 71, 3420–3426. [Google Scholar] [CrossRef]
- Huang, Y.; Busk, P.K.; Herbst, F.A.; Lange, L. Genome and secretome analyses provide insights into keratin decomposition by novel proteases from the non-pathogenic fungus Onygena corvina. Appl. Environ. Microbiol. 2015, 99, 9635–9649. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, W.; Hennrich, N.; Klockow, M.; Metz, H.; Orth, H.D.; Lang, H. Proteinase K from Tritirachium album limber. Eur. J. Biochem. 1974, 47, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Anitha, T.S.; Palanivelu, P. Purification and characterization of an extracellular keratinolytic protease from a new isolate of Aspergillus parasiticus. Protein Expr. Purif. 2013, 88, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Gasparin, F.G.; de Souza, C.G.M.; Costa, A.M.; Alexandrino, A.M.; Bracht, C.K.; Boer, C.G.; Peralta, R.M. Purification and characterization of an efficient poultry feather degrading-protease from Myrothecium verrucaria. Biodegradation 2009, 20, 727–736. [Google Scholar] [CrossRef]
- Kansoh, A.L.; Nagieb, Z.A. Xylanase and mannanase enzymes from Streptomyces galbus NR and their use in biobleaching of softwood kraft pulp. Antonie Van Leeuwenhoek 2004, 85, 103–114. [Google Scholar] [CrossRef]
- Gousterova, A.; Braikova, D.; Goshev, I.; Christov, P.; Tishinov, K.; Vasileva-Tonkova, E.; Haertle, T.; Nedkov, P. Degradation of keratin and collagen containing wastes by newly isolated Thermoactinomycetes or by alkaline hydrolysis. Lett. Appl. Microbiol. 2005, 40, 335–340. [Google Scholar] [CrossRef]
- Li, Z.W.; Liang, S.; Ke, Y.; Deng, J.J.; Zhang, M.S.; Lu, D.L.; Li, J.Z.; Luo, X.C. The feather degradation mechanisms of a new Streptomyces sp. isolate SCUT-3. Commun. Biol. 2020, 3, 191. [Google Scholar] [CrossRef]
- Johnson, P.; Smillie, L.B. The amino acid sequence and predicted structure of Streptomyces griseus protease A. FEBS Lett. 1972, 47, 1–6. [Google Scholar] [CrossRef]
- Kitadokoro, K.; Tsuzuki, H.; Nakamura, E.; Sato, T.; Teraoka, H. Purification, characterization, primary structure, crystallization and preliminary crystallographic study of a serine proteinase from Streptomyces fradiae ATCC 14544. Eur. J. Biochem. 1994, 220, 55–61. [Google Scholar] [CrossRef]
- Böckle, B.; Galunsky, B. Characterization of a keratinolytic serine proteinase from Streptomyces pactum DSM 40530. Appl. Environ. Microbiol. 1995, 61, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Benedek, A.; Szabó, I.M.; Barabas, G.Y. Feather degradation with a thermotolerant Streptomyces graminofaciens strain. World J. Microbiol. Biotechnol. 2000, 16, 253–255. [Google Scholar] [CrossRef]
- Habbeche, A.; Saoudi, B.; Jaouadi, B.; Haberra, S.; Kerouaz, B.; Boudelaa, M.; Badis, A.; Ladjama, A. Purification and biochemical characterization of a detergent-stable keratinase from a newly thermophilic actinomycete Actinomadura keratinilytica strain Cpt29 isolated from poultry compost. J. Biosci. Bioeng. 2014, 117, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Cairo, J.; Coello, N. Purification and characterization of a novel exocellular keratinase from Kocuria rosea. Enzyme Microb. Technol. 2006, 38, 49–54. [Google Scholar] [CrossRef]
- Longshaw, C.M.; Wright, J.D.; Farrell, A.M.; Holland, K.T. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J. Appl. Microbiol. 2002, 93, 810–816. [Google Scholar] [CrossRef]
- Verma, A.; Singh, H.; Anwar, S.; Chattopadhyay, A.; Tiwari, K.K.; Kaur, S.; Dhilon, G.S. Microbial keratinases: Industrial enzymes with waste management potential. Crit. Rev. Biotechnol. 2017, 37, 476–491. [Google Scholar] [CrossRef]
- Mitsuiki, S.; Sakai, M.; Moriyama, Y.; Goto, M.; Furukawa, K. Purification and some properties of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Biosci. Biotechnol. Biochem. 2002, 66, 164–167. [Google Scholar] [CrossRef]
- Claverías, F.P.; Undabarrena, A.; González, M.; Seeger, M.; Cámara, B. Culturable diversity and antimicrobial activity of Actinobacteria from marine sediments in Valparaíso bay, Chile. Front. Microbiol. 2015, 6, 737. [Google Scholar] [CrossRef]
- Verma, A.; Pal, H.S.; Singh, R.; Agarwal, S. Potential of alkaline protease isolated from Thermoactinomyces sp. RM4 as an alternative to conventional chemicals in leather industry dehairing process. Int. J. Agric. Environ. Biotechnol. 2011, 4, 173–178. [Google Scholar]
- Dastager, S.G.; Li, W.J.; Agasar, D.; Sulochana, M.B.; Tang, S.K.; Tian, X.P.; Zhi, X.Y. Streptomyces gulbargensis sp. nov., isolated from soil in Karnataka, India. Antonie Van Leeuwenhoek 2007, 91, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Badis, A.; Ferradji, F.Z.; Boucherit, A.; Fodil, D.; Boutoumi, H. Characterization and biodegradation of soil humic acids and preliminary identification of decolorizing actinomycetes at Mitidja plain soils (Algeria). Afr. J. Microbiol. Res. 2009, 3, 997–1007. [Google Scholar]
- Jaouadi, B.; Abdelmalek, B.; Fodil, D.; Ferradji, F.Z.; Rekik, H.; Zaraî, N.; Bejar, S. Purification and characterization of a thermostable keratinolytic serine alkaline proteinase from Streptomyces sp. strain AB1 with high stability in organic solvents. Bioresour. Technol. 2010, 101, 8361–8369. [Google Scholar] [CrossRef] [PubMed]
- Riffel, A.; Brandelli, A.; Bellato, C.D.M.; Souza, G.H.; Eberlin, M.N.; Tavares, F.C. Purification and characterization of a keratinolytic metalloprotease from Chryseobacterium sp. kr6. J. Biotechnol. 2007, 128, 693–703. [Google Scholar] [CrossRef]
- Ionata, E.; Canganella, F.; Bianconi, G.; Benno, Y.; Sakamoto, M.; Capasso, A.; Rossi, M.; La Cara, F. A novel keratinase from Clostridium sporogenes bv. pennavorans bv. nov., a thermotolerant organism isolated from solfataric muds. Microbiol. Res. 2008, 163, 105–112. [Google Scholar] [CrossRef]
- Lin, X.; Lee, C.G.; Casale, E.S.; Shih, J.C. Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Appl. Environ. Microbiol. 1992, 58, 3271–3275. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakrabarti, K.; Chattopadhyay, D. Degradation of raw feather by a novel high molecular weight extracellular protease from newly isolated Bacillus cereus DCUW. J. Ind. Microbiol. Biotechnol. 2008, 35, 825–834. [Google Scholar] [CrossRef]
- Suntornsuk, W.; Tongjun, J.; Onnim, P.; Oyama, H.; Ratanakanokchai, K.; Kusamran, T.; Oda, K. Purification and characterization of keratinase from a thermotolerant feather-degrading bacterium. World J. Microbiol. Biotechnol. 2005, 21, 1111–1117. [Google Scholar] [CrossRef]
- Rozs, M.; Manczinger, L.; Vágvölgyi, C.; Kevei, F. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol. Lett. 2001, 205, 221–224. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Kanoun, S.; Manni, L.; Nasri, M. Production and biochemical and molecular characterization of a keratinolytic serine protease from chicken feather-degrading Bacillus licheniformis RPk. Can. J. Microbiol. 2009, 55, 427–436. [Google Scholar] [CrossRef]
- Balaji, S.; Senthil Kumar, M.; Karthikeyan, R.; Kumar, R.; Kirubanandan, S.; Sridhar, R.; Sehgal, P.K. Purification and characterization of an extracellular keratinase from a hornmeal-degrading Bacillus subtilis MTCC (9102). W. J. Microbiol. Biotechnol. 2008, 24, 2741–2745. [Google Scholar] [CrossRef]
- Santos, R.M.; Firmino, A.A.; De Sa, C.M.; Felix, C.R. Keratinolytic activity of Aspergillus fumigatus Fresenius. Curr. Microbiol. 1996, 33, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Hassan, M.A. Purification, characterization and immobilization of a keratinase from Aspergillus oryzae. Enzyme Microb. Technol. 2004, 34, 85–93. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Hilda, A.; Annadurai, G. Purification of keratinase from poultry farm isolate-Scopulariopsis brevicaulis and statistical optimization of enzyme activity. Enzyme Microb. Technol. 2005, 36, 639–647. [Google Scholar] [CrossRef]
- Qin, L.M.; Dekio, S.; Jidoi, J. Some biochemical characteristics of a partially purified extracellular keratinase from Trichophyton schoenleinii. Zentralbl Bakteriol. 1992, 277, 236–244. [Google Scholar] [CrossRef]
- Moallaei, H.; Zaini, F.; Larcher, G.; Beucher, B.; Bouchara, J.P. Partial purification and characterization of a 37 kDa extracellular proteinase from Trichophyton vanbreuseghemii. Mycopathologia 2006, 161, 369–375. [Google Scholar] [CrossRef]
- Jeevana Lakshmi, P.; Kumari Chitturi, C.M.; Lakshmi, V.V. Efficient degradation of feather by keratinase producing Bacillus sp. Int. J. Microbiol. 2013, 2013, 608321. [Google Scholar]
- Gegeckas, A.; Gudiukaitė, R.; Citavicius, D. Keratinolytic proteinase from Bacillus thuringiensis AD-12. Int. J. Biol. Macromol. 2014, 69, 46–51. [Google Scholar] [CrossRef]
- Vidmar, B.; Vodovnik, M. Microbial keratinases: Enzymes with promising biotechnological applications. Food Technol. Biotechnol. 2018, 56, 312–328. [Google Scholar] [CrossRef]
- Hendrick, Q.; Nnolim, N.E.; Nwodo, U.U. Chryseobacterium cucumeris FHN1 keratinolytic enzyme valorized chicken feathers to amino acids with polar, anionic and non-polar imino side chain characteristics. Biocatal. Agric. Biotechnol. 2021, 35, 102109. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Riffel, A. Biochemical features of microbial keratinases and their production and applications. Appl. Microbiol. Biotechnol. 2010, 85, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Jacquiod, S.; Herschend, J.; Wei, S.; Nesme, J.; Sørensen, S.J. Construction of simplified microbial consortia to degrade recalcitrant materials based on enrichment and dilution-to-extinction cultures. Front. Microbiol. 2020, 10, 3010. [Google Scholar] [CrossRef] [PubMed]
- Nasipuri, P.; Herschend, J.; Brejnrod, A.D.; Madsen, J.S.; Espersen, R.; Svensson, B.; Burmølle, M.; Jacquiod, S.; Sørensen, S.J. Community-intrinsic properties enhance keratin degradation from bacterial consortia. PLoS ONE 2020, 15, e0228108. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.S.; Sequeiros, C.; Garcia, S.; Olivera, N.L. Newly isolated Bacillus sp. G51 from Patagonian wool produces an enzyme combination suitable for felt-resist treatments of organic wool. Bioprocess Biosyst. Eng. 2017, 40, 833–842. [Google Scholar] [CrossRef]
- Sittipol, D.; Rodpan, S.; Ajingi, Y.U.S.; Lohnoo, T.; Lerksuthirat, T.; Kumsang, Y.; Yingyong, W.; Khunrae, P.; Rattanarojpong, T.; Pattanapanyasat, K.; et al. Identification, overexpression, purification, and biochemical characterization of a novel hyperthermostable keratinase from Geoglobus acetivorans. 3 Biotech 2021, 11, 2. [Google Scholar] [CrossRef]
- Gradišar, H.K.S.F.; Kern, S.; Friedrich, J. Keratinase of Doratomyces microsporus. Appl. Microbiol. Biotechnol. 2000, 53, 196–200. [Google Scholar] [CrossRef]
- Bach, E.; Daroit, D.J.; Corrêa, A.P.F.; Brandelli, A. Production and properties of keratinolytic proteases from three novel Gram-negative feather-degrading bacteria isolated from Brazilian soils. Biodegradation 2011, 22, 1191–1201. [Google Scholar] [CrossRef]
- Kuo, J.M.; Yang, J.I.; Chen, W.M.; Pan, M.H.; Tsai, M.L.; Lai, Y.J.; Hwang, A.; Pan, B.S.; Lin, C.Y. Purification and characterization of a thermostable keratinase from Meiothermus sp. I40. Int. Biodeterior. Biodegrad. 2012, 70, 111–116. [Google Scholar] [CrossRef]
- Bose, A.; Pathan, S.; Pathak, K.; Keharia, H. Keratinolytic protease production by Bacillus amyloliquefaciens 6B using feather meal as substrate and application of feather hydrolysate as organic nitrogen input for agricultural soil. Waste Biomass Valori. 2014, 5, 595–605. [Google Scholar] [CrossRef]
- Akhter, M.; Wal Marzan, L.; Akter, Y.; Shimizu, K. Microbial bioremediation of feather waste for keratinase production: An outstanding solution for leather dehairing in tanneries. Microbiol. Insights 2020, 13, 1178636120913280. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A. Keratinase production by endophytic Penicillium spp. Morsy1 under solid-state fermentation using rice straw. Appl. Biochem. Biotechnol. 2010, 162, 780–794. [Google Scholar] [CrossRef]
- Purchase, D. Microbial keratinases: Characteristics, biotechnological applications, and potential. In The Handbook of Microbial Bioresources; CAB International Publishing: Wallingford, UK, 2016; pp. 634–674. [Google Scholar]
- Mitsuiki, S.; Ichikawa, M.; Oka, T.; Sakai, M.; Moriyama, Y.; Sameshima, Y.; Goto, M.; Furukawa, K. Molecular characterization of a keratinolytic enzyme from an alkaliphilic Nocardiopsis sp. TOA-1. Enzyme Microb. Technol. 2004, 34, 482–489. [Google Scholar] [CrossRef]
- Benkiar, A.; Nadia, Z.J.; Badis, A.; Rebzani, F.; Soraya, B.T.; Rekik, H.; Naili, B.; Ferradji, F.Z.; Bejar, S.; Jaouadi, B. Biochemical and molecular characterization of a thermo-and detergent-stable alkaline serine keratinolytic protease from Bacillus circulans strain DZ100 for detergent formulations and feather-biodegradation process. Int. Biodeterior. Biodegrad. 2013, 83, 129–138. [Google Scholar] [CrossRef]
- Taskin, M.; Unver, Y.; Firat, A.; Ortucu, S.; Yildiz, M. Sheep wool protein hydrolysate: A new peptone source for microorganisms. J. Chem. Technol. Biotechnol. 2016, 91, 1675–1680. [Google Scholar] [CrossRef]
- Friedrich, A.B.; Antranikian, G. Keratin degradation by Fervidobacterium pennavorans, a novel thermophilic anaerobic species of the order Thermotogales. Appl. Environ. Microbiol. 1996, 62, 2875–2882. [Google Scholar] [CrossRef]
- Intagun, W.; Kanoksilapatham, W. A review: Biodegradation and applications of keratin degrading microorganisms and keratinolytic enzymes, focusing on thermophiles and thermostable serine proteases. Am. J. Appl. Sci. 2017, 14, 1016–1023. [Google Scholar] [CrossRef]
- Wu, W.L.; Chen, M.Y.; Tu, I.F.; Lin, Y.C.; EswarKumar, N.; Chen, M.Y.; Ho, M.C.; Wu, S.H. The discovery of novel heat-stable keratinases from Meiothermus taiwanensis WR-220 and other extremophiles. Sci. Rep. 2017, 7, 4658. [Google Scholar] [CrossRef] [PubMed]
- Laba, W.; Rodziewicz, A. Biodegradation of hard keratins by two Bacillus strains. Jundishapur J. Microbiol. 2014, 7, e8896. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, I.; Imtiaz, A.; Hussain, A.; Javid, A.; Jabeen, F.; Akmal, M.; Qazi, J.I. Microbial production and industrial applications of keratinases: An overview. Int. Microbiol. 2018, 21, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Moridshahi, R.; Bahreini, M.; Sharifmoghaddam, M.; Asoodeh, A. Biochemical characterization of an alkaline surfactant-stable keratinase from a new keratinase producer, Bacillus zhangzhouensis. Extremophiles 2020, 24, 693–704. [Google Scholar] [CrossRef]
- Hamiche, S.; Mechri, S.; Khelouia, L.; Annane, R.; El Hattab, M.; Badis, A.; Jaouadi, B. Purification and biochemical characterization of two keratinases from Bacillus amyloliquefaciens S13 isolated from marine brown alga Zonaria tournefortii with potential keratin-biodegradation and hide-unhairing activities. Int. J. Biol. Macromol. 2019, 122, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Vasileva-Tonkova, E.; Gousterova, A.; Neshev, G. Ecologically safe method for improved feather wastes biodegradation. Int. Biodeterior. Biodegrad. 2009, 63, 1008–1012. [Google Scholar] [CrossRef]
- Sivakumar, T.; Balamurugan, P.; Ramasubramanian, V. Characterization and applications of keratinase enzyme by Bacillus thuringiensis TS2. Int. J. Future Biotechnol. 2013, 2, 1–8. [Google Scholar]
- Gupta, R.; Sharma, R.; Beg, Q.K. Revisiting microbial keratinases: Next generation proteases for sustainable biotechnology. Crit. Rev. Biotechnol. 2013, 33, 216–228. [Google Scholar] [CrossRef]
- Okoroma, E.A.; Garelick, H.; Abiola, O.O.; Purchase, D. Identification and characterization of a Bacillus licheniformis strain with profound keratinase activity for degradation of melanized feather. Int. Biodeterior. Biodegrad. 2012, 74, 54–60. [Google Scholar] [CrossRef]
- Gupta, R.; Rajput, R.; Sharma, R.; Gupta, N. Biotechnological applications and prospective market of microbial keratinases. Appl. Microbiol. Biotechnol. 2013, 97, 9931–9940. [Google Scholar] [CrossRef]
- Patinvoh, R.J.; Feuk-Lagerstedt, E.; Lundin, M.; Sárvári Horváth, I.; Taherzadeh, M.J. Biological pretreatment of chicken feather and biogas production from total broth. Appl. Biochem. Biotechnol. 2016, 180, 1401–1415. [Google Scholar] [CrossRef]
- Jaouadi, B.; Ellouz-Chaabouni, S.; Ali, M.B.; Messaoud, E.B.; Naili, B.; Dhouib, A.; Bejar, S. Excellent laundry detergent compatibility and high dehairing ability of the Bacillus pumilus CBS alkaline proteinase (SAPB). Biotechnol. Bioprocess Eng. 2009, 14, 503–512. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Łaba, W.; Rodziewicz, A. Enhancement of pig bristles waste bioconversion by inoculum of keratinolytic bacteria during composting. Waste Manag. 2019, 84, 269–276. [Google Scholar] [CrossRef]
- Brandelli, A.; Sala, L.; Kalil, S.J. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 2015, 73, 3–12. [Google Scholar] [CrossRef]
- Adelere, I.A.; Lateef, A. Keratinases: Emerging trends in production and applications as novel multifunctional biocatalysts. Kuwait J. Sci. 2016, 43, 118–127. [Google Scholar]
- Ramakrishnan, J.; Balakrishnan, H.; Raja, S.T.; Sundararamakrishnan, N.; Renganathan, S.; Radha, V.N. Formulation of economical microbial feed using degraded chicken feathers by a novel Streptomyces sp: Mitigation of environmental pollution. Braz. J. Microbiol. 2011, 42, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Lee, C.G.; Garlich, J.D.; Shih, J.C. Evaluation of a bacterial feather fermentation product, feather-lysate, as a feed protein. Poult. Sci. 1991, 70, 85–94. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Adejumo, I.O. Efficacy of crude and immobilizedenzymes from Bacillus licheniformis for production of biodegraded feather meal and their assessment on chickens. Environ. Technol. Innov. 2018, 11, 116–124. [Google Scholar] [CrossRef]
- Tiwary, E.; Gupta, R. Rapid conversion of chicken feather to feather meal using dimeric keratinase from Bacillus licheniformis ER-15. J. Bioprocess Biotech. 2012, 2, 1000123. [Google Scholar] [CrossRef]
- Petek, B.; Marinšek Logar, R. Management of waste sheep wool as valuable organic substrate in European Union countries. J. Mater. Cycles Waste Manag. 2021, 23, 44–54. [Google Scholar] [CrossRef]
- Arasu, V.T.; Sivakumar, T.; Ramasubramanian, V.; Nalini, K.; Kiruthiga, R. The potential application of keratinase from Bacillus sp. as plant growth promoters. J. Pure Appl. Microbiol. 2009, 3, 583–590. [Google Scholar]
- Veselá, M.; Friedrich, J. Amino acid and soluble protein cocktail from waste keratin hydrolyzed by a fungal keratinase of Paecilomyces marquandii. Biotechnol. Bioprocess. Eng. 2009, 14, 84–90. [Google Scholar] [CrossRef]
- Paul, T.; Das, A.; Mandal, A.; Halder, S.K.; DasMohapatra, P.K.; Pati, B.R.; Mondal, K.C. Biochemical and structural characterization of a detergent stable alkaline serine keratinase from Paenibacillus woosongensis TKB2: A potential additive for laundry detergent. Waste Biomass Valori. 2014, 5, 563–574. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.; Arifin, M.A.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef]
- Barba, C.; Méndez, S.; Roddick-Lanzilotta, A.; Kelly, R.; Parra, J.L.; Coderch, L. Cosmetic effectiveness of topically applied hydrolyzed keratin peptides and lipids derived from wool. Skin. Res. Technol. 2008, 14, 243–248. [Google Scholar] [CrossRef]
- Tsakas, S. Use of Dual Compartment Mixing Container for Enzyme Mixtures Useful to Treat Acne. U.S. Patent 6,627,192, 3 June 1999. [Google Scholar]
- Villa, A.L.; Aragão, M.R.; Dos Santos, E.P.; Mazotto, A.M.; Zingali, R.B.; De Souza, E.P.; Vermelho, A.B. Feather keratin hydrolysates obtained from microbial keratinases: Effect on hair fiber. BMC Biotechnol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Selvam, K.; Vishnupriya, B. Biochemical and molecular characterization of microbial keratinase and its remarkable applications. Int. J. Pharm. Biol. Arch. 2012, 3, 267–275. [Google Scholar]
- Paul, T.; Das, A.; Mandal, A.; Halder, S.K.; Jana, A.; Maity, C.; DasMohapatra, P.K.; Pati, B.R.; Mondal, K.C. An efficient cloth cleaning properties of a crude keratinase combined with detergent: Towards industrial viewpoint. J. Clean. Prod. 2014, 66, 672–684. [Google Scholar] [CrossRef]
- Hammami, A.; Fakhfakh, N.; Abdelhedi, O.; Nasri, M.; Bayoudh, A. Proteolytic and amylolytic enzymes from a newly isolated Bacillus mojavensis SA: Characterization and applications as laundry detergent additive and in leather processing. Int. J. Biol. Macromol. 2018, 108, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Thankaswamy, S.R.; Sundaramoorthy, S.; Palanivel, S.; Ramudu, K.N. Improved microbial degradation of animal hair waste from leather industry using Brevibacterium luteolum (MTCC 5982). J. Clean. Prod. 2018, 189, 701–708. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Z.; Long, X.; Tian, Y.; Shi, B. High-expression keratinase by Bacillus subtilis SCK6 for enzymatic dehairing of goatskins. Int. J. Biol. Macromol. 2019, 135, 119–126. [Google Scholar] [CrossRef]
- ul Haq, I.; Akram, F. Striking applications of keratinase enzyme isolated from various natural sources: A review: Striking applications of keratinase enzyme isolated from various natural sources. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2018, 55, 1–7. [Google Scholar]
- Shen, J.; Rushforth, M.; Cavaco-Paulo, A.; Guebitz, G.; Lenting, H. Development and industrialization of enzymatic shrink-resist process based on modified proteases for wool machine washability. Enzyme Microb. Technol. 2007, 40, 1656–1661. [Google Scholar] [CrossRef]
- Nigam, P.S. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules 2013, 3, 597–611. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, R. Hydrolyzing proficiency of keratinases in feather degradation. Indian J. Microbiol. 2014, 54, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Dlume, T.; Nnolim, N.E.; Nwodo, U.U. Exiguobacterium acetylicum transformed poultry feathers into amino acids through an extracellular secretion of keratinolytic enzymes. AIMS Bioeng. 2024, 11, 489–505. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 61. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Liu, B.; Du, G.; Chen, J. Biodegradation of wool waste and keratinase production in scale-up fermenter with different strategies by Stenotrophomonas maltophilia BBE11-1. Bioresour. Technol. 2013, 140, 286–291. [Google Scholar] [CrossRef]
- Kshetri, P.; Singh, P.L.; Chanu, S.B.; Singh, T.S.; Rajiv, C.; Tamreihao, K.; Singh, H.N.; Chongtham, T.; Devi, A.K.; Sharma, S.K.; et al. Biological activity of peptides isolated from feather keratin waste through microbial and enzymatic hydrolysis. Electron. J. Biotechnol. 2022, 60, 11–18. [Google Scholar] [CrossRef]
- Wan, M.Y.; Dong, G.; Yang, B.Q.; Feng, H. Identification and characterization of a novel antioxidant peptide from feather keratin hydrolysate. Biotechnol. Lett. 2016, 38, 643–649. [Google Scholar] [CrossRef]
- Qin, X.; Xu, X.; Guo, Y.; Shen, Q.; Liu, J.; Yang, C.; Scott, E.; Bitter, H.; Zhang, C. A sustainable and efficient recycling strategy of feather waste into keratin peptides with antimicrobial activity. Waste Manag. 2022, 144, 421–430. [Google Scholar] [CrossRef]
- Sharma, I.; Pranaw, K.; Soni, H.; Rawat, H.K.; Kango, N. Parametrically optimized feather degradation by Bacillus velezensis NCIM 5802 and delineation of keratin hydrolysis by multi-scale analysis for poultry waste management. Sci. Rep. 2022, 12, 17118. [Google Scholar] [CrossRef]
- Zaghloul, T.I.; Embaby, A.M.; Elmahdy, A.R. Key determinants affecting sheep wool biodegradation directed by a keratinase-producing Bacillus subtilis recombinant strain. Biodegradation 2011, 22, 111–128. [Google Scholar] [CrossRef]
- Kshetri, P.; Roy, S.S.; Sharma, S.K.; Singh, T.S.; Ansari, M.A.; Prakash, N.; Ngachan, S.V. Transforming chicken feather waste into feather protein hydrolysate using a newly isolated multifaceted keratinolytic bacterium Chryseobacterium sediminis RCM-SSR-7. Waste Biomass Valor. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Li, K.; Li, G.; Peng, S.; Tan, M. Effective biodegradation on chicken feather by the recombinant KerJY-23 Bacillus subtilis WB600: A synergistic process coupled by disulfide reductase and keratinase. Int. J. Biol. Macromol. 2023, 253, 127194. [Google Scholar] [CrossRef]
- da Cunha, I.C.; Brandelli, A.; Braga, A.R.C.; Sala, L.; Kalil, S.J. Feather meal as a source of peptides with antioxidant activity from enzymatic hydrolysis. Waste Biomass Valor. 2023, 14, 421–430. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Nazarpour, M. Peptide recovery from chicken feather keratin and their anti-biofilm properties against methicillin-resistant Staphylococcus aureus (MRSA). World J. Microbiol. Biotechnol. 2024, 40, 123. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, R.; Daroit, D.J.; Corrêa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Yates, J.R., III; Moreira, J.C.F.; Brandelli, A. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019, 49, 71–76. [Google Scholar] [CrossRef]
- Guo, L.; Lu, L.; Yin, M.; Yang, R.; Zhang, Z.; Zhao, W. Valorization of refractory keratinous waste using a new and sustainable bio-catalysis. Chem. Eng. J. 2020, 397, 125420. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, M.; Wu, L.; Yang, Y.; Sun, Y.; Wang, Q.; Gao, X. Bioconversion of feather waste into bioactive nutrients in water by Bacillus licheniformis WHU. Appl. Microbiol. Biotechnol. 2023, 107, 7055–7070. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, B.; Khatri, M.; Singh, G.; Arya, S.K. Microbial keratinases: An overview of biochemical characterization and its eco-friendly approach for industrial applications. J. Clean. Prod. 2020, 252, 119847. [Google Scholar] [CrossRef]
- Das, S.; Das, A.; Das, N.; Nath, T.; Langthasa, M.; Pandey, P.; Kumar, V.; Choure, K.; Kumar, S.; Pandey, P. Harnessing the potential of microbial keratinases for bioconversion of keratin waste. Environ. Sci. Pollut. Res. Int. 2024, 31, 57478–57507. [Google Scholar] [CrossRef]
- Yahaya, R.S.; Normi, Y.M.; Phang, L.Y.; Ahmad, S.A.; Abdullah, J.O.; Sabri, S. Molecular strategies to increase keratinase production in heterologous expression systems for industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 3955–3969. [Google Scholar] [CrossRef]
- Catalán, E.; Komilis, D.; Sánchez, A. A life cycle assessment on the dehairing of rawhides: Chemical treatment versus enzymatic recovery through solid state fermentation. J. Ind. Ecol. 2019, 23, 361–373. [Google Scholar] [CrossRef]
- Ossai, I.C.; Hamid, F.S.; Hassan, A. Valorisation of keratinous wastes: A sustainable approach towards a circular economy. Waste Manag. 2022, 151, 81–104. [Google Scholar] [CrossRef]

| Keratinolytic Bacteria | Source | Reference |
|---|---|---|
| Bacillus licheniformis PWD-1 | Poultry waste | [45] |
| Microbacterium sp. kr10 | Decomposing feathers | [65] |
| Bacillus subtilis S14 | Soil | [63] |
| Bacillus pseudofirmus | Alkaline soda lake | [11] |
| B. pumilus AT16 | Tunicate Didemnum maculosum | [66] |
| B. subtilis DB01 | Harbour sediment | [66] |
| Chryseobacterium indologenes TKU014 | Soil | [67] |
| B. licheniformis ER-15 | Soil | [64] |
| Streptomyces albidoflavus K1-02 | Hen house soil | [68] |
| Chryseobacterium aquifrigidense FANN1 | Poultry dumpsites | [69] |
| Bacillus macroides | Dry meadow soil | [52] |
| Bacillus cereus | Dry meadow soil | [52] |
| Chryseobacterium sp. strain kr6 | Poultry waste | [70] |
| Microbacterium sp. Kr10 | Decomposing feathers | [47] |
| Arthrobacter sp. NFH5 | Soil | [71] |
| Meiothermus sp. I40 | Water from a hot spring | [72] |
| Micromonospora sp. AYA2000 | Protoplast fusion | [73] |
| Vibrio sp. Kr2 | Poultry abattoir soil | [1] |
| Pseudomonas sp. 3096-4 | Decomposing wool | [74] |
| Paracoccus sp. WJ-98 | Soil from a poultry factory | [75] |
| Lysobacter sp. NCIMB 9497 | Collection culture | [76] |
| Stenotrophomonas sp. | Deer fur | [17] |
| Thermoanaerobacter keratinophilus | Geothermal hot spring | [77] |
| Xanthomonas maltophila POA-1 | Poultry waste | [29] |
| Fervidobacterium islandicum AW-1 | Geothermal hot stream | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpaka, L.; Nnolim, N.E.; Nwodo, U.U. Microbial Keratinolysis: Eco-Friendly Valorisation of Keratinous Waste into Functional Peptides. Microorganisms 2025, 13, 2270. https://doi.org/10.3390/microorganisms13102270
Mpaka L, Nnolim NE, Nwodo UU. Microbial Keratinolysis: Eco-Friendly Valorisation of Keratinous Waste into Functional Peptides. Microorganisms. 2025; 13(10):2270. https://doi.org/10.3390/microorganisms13102270
Chicago/Turabian StyleMpaka, Lindelwa, Nonso E. Nnolim, and Uchechukwu U. Nwodo. 2025. "Microbial Keratinolysis: Eco-Friendly Valorisation of Keratinous Waste into Functional Peptides" Microorganisms 13, no. 10: 2270. https://doi.org/10.3390/microorganisms13102270
APA StyleMpaka, L., Nnolim, N. E., & Nwodo, U. U. (2025). Microbial Keratinolysis: Eco-Friendly Valorisation of Keratinous Waste into Functional Peptides. Microorganisms, 13(10), 2270. https://doi.org/10.3390/microorganisms13102270







