Long-Term Sewage Survey of SARS-CoV-2, Influenza A and Respiratory Syncytial Virus (RSV), and Correlation to Human Cases in a City with One Million Inhabitants
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Sampling
2.2. DNA/RNA Extraction and RT-PCR Screening of SARS-CoV-2, Influenza A/B Viruses, and RSV for Wastewater Samples
2.3. DNA/RNA Extraction and qPCR Diagnosis of SARS-CoV-2, Influenza A/B Viruses, and RSV for Clinical Samples
2.4. Comparison of Wastewater and Clinical Data
2.5. Statistical Analysis
3. Results
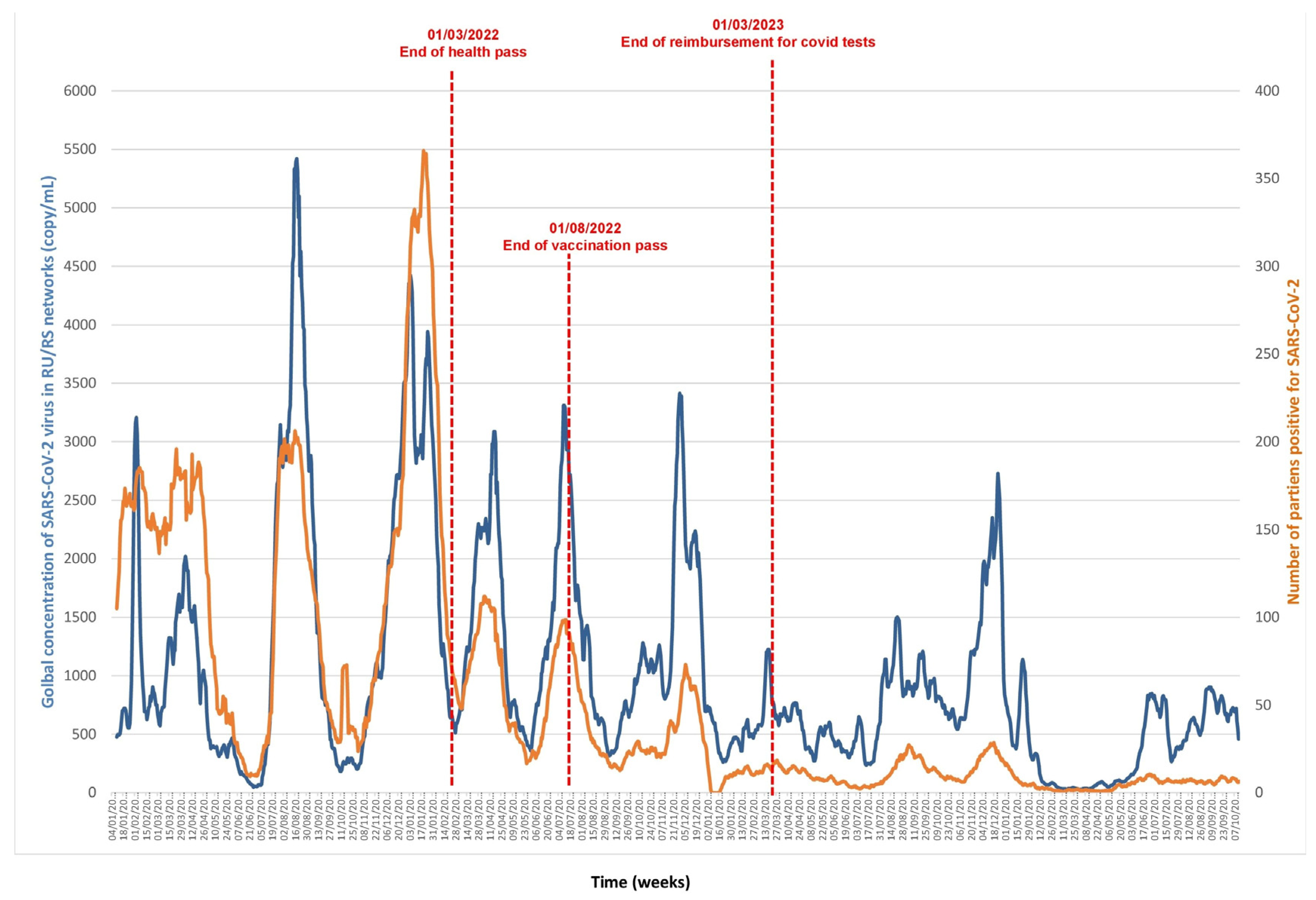
3.1. SARS-CoV2 (Figure 1 and Figure 2, Supplementary Data)
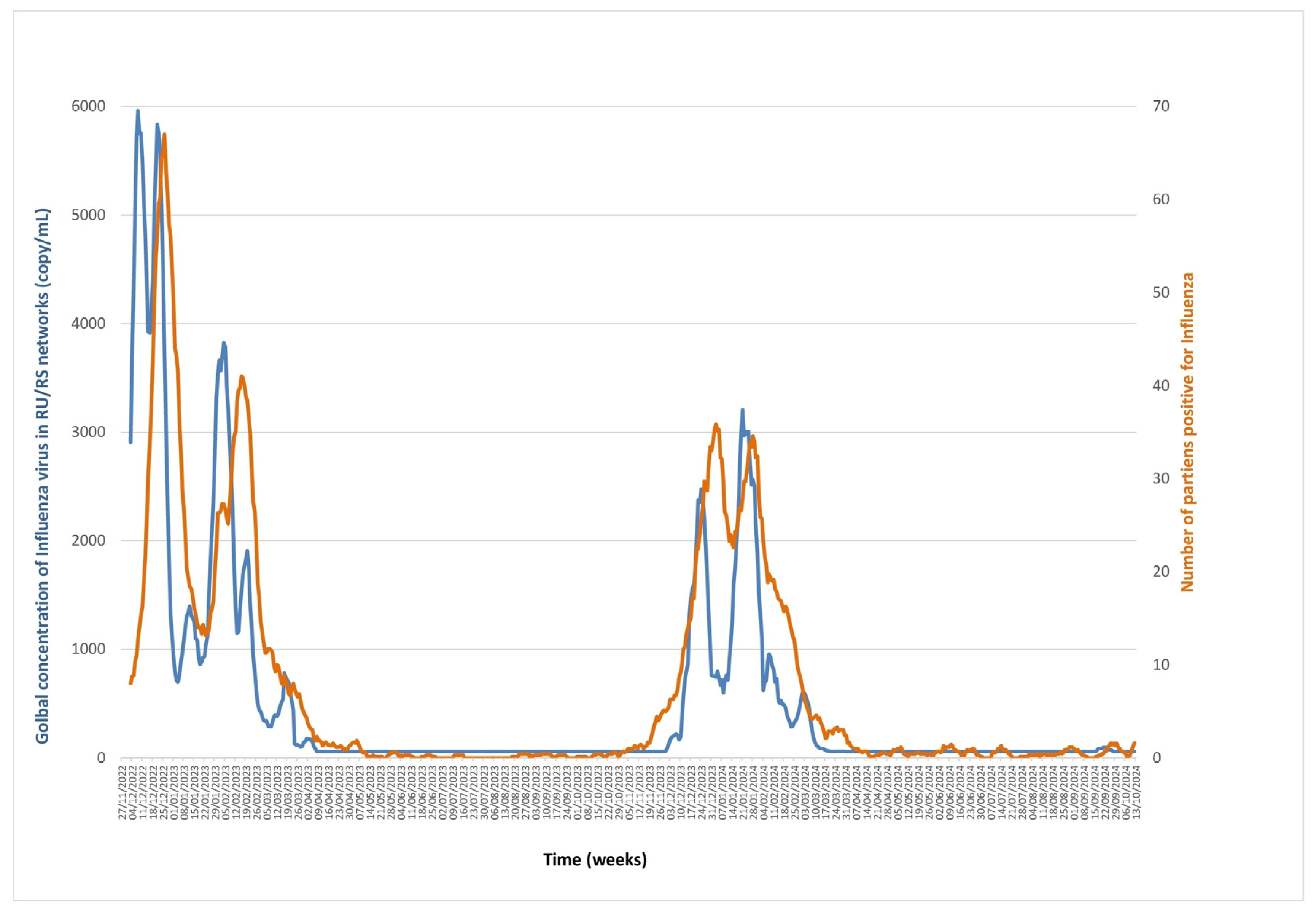
3.2. Influenza A/B Viruses (Figure 3, Supplementary Data)
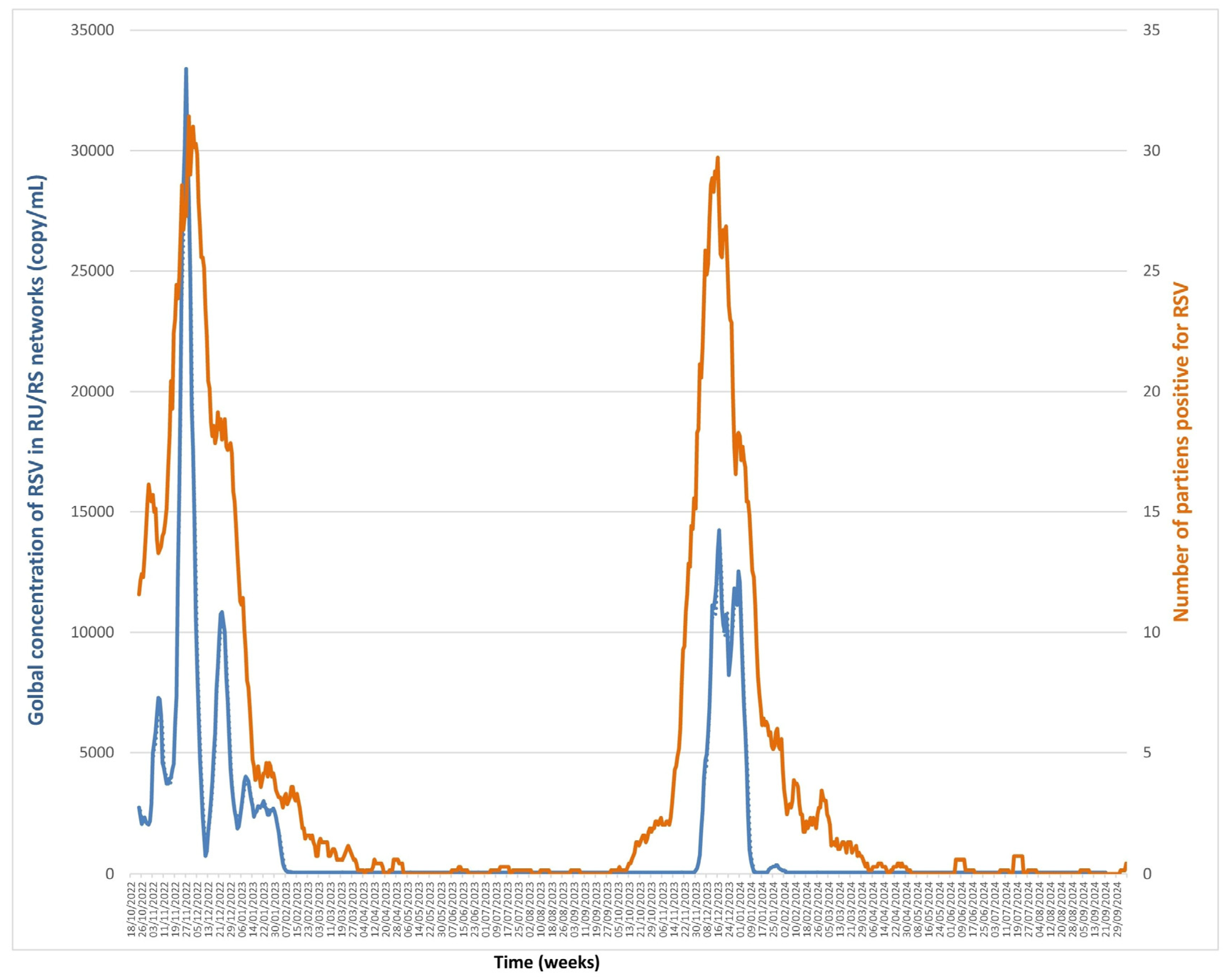
3.3. RSV (Figure 4, Supplementary Data)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Oran, D.P.; Topol, E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic. Ann. Intern. Med. 2021, 174, 1344–1345. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Zulli, A.; Brackney, D.E.; Grubaugh, N.D.; Kaplan, E.H.; Casanovas-Massana, A.; Ko, A.I.; Malik, A.A.; Wang, D.; Wang, M.; et al. Measurement of SARS-CoV-2 RNA in Wastewater Tracks Community Infection Dynamics. Nat. Biotechnol. 2020, 38, 1164–1167. [Google Scholar] [CrossRef]
- Wu, F.; Xiao, A.; Zhang, J.; Moniz, K.; Endo, N.; Armas, F.; Bonneau, R.; Brown, M.A.; Bushman, M.; Chai, P.R.; et al. SARS-CoV-2 RNA Concentrations in Wastewater Foreshadow Dynamics and Clinical Presentation of New COVID-19 Cases. Sci. Total Environ. 2022, 805, 150121. [Google Scholar] [CrossRef]
- Bi, C.; Ramos-Mandujano, G.; Tian, Y.; Hala, S.; Xu, J.; Mfarrej, S.; Esteban, C.R.; Delicado, E.N.; Alofi, F.S.; Khogeer, A.; et al. Simultaneous Detection and Mutation Surveillance of SARS-CoV-2 and Multiple Respiratory Viruses by Rapid Field-Deployable Sequencing. Med 2021, 2, 689–700.e4. [Google Scholar] [CrossRef]
- Zulli, A.; Chan, E.M.G.; Boehm, A.B. Detection of Hepatovirus A (HAV) in Wastewater Indicates Widespread National Distribution and Association with Socioeconomic Indicators of Vulnerability. mSphere 2024, 9, e0064524. [Google Scholar] [CrossRef]
- Verani, M.; Pagani, A.; Federigi, I.; Lauretani, G.; Atomsa, N.T.; Rossi, V.; Viviani, L.; Carducci, A. Wastewater-Based Epidemiology for Viral Surveillance from an Endemic Perspective: Evidence and Challenges. Viruses 2024, 16, 482. [Google Scholar] [CrossRef]
- Markt, R.; Stillebacher, F.; Nägele, F.; Kammerer, A.; Peer, N.; Payr, M.; Scheffknecht, C.; Dria, S.; Draxl-Weiskopf, S.; Mayr, M.; et al. Expanding the Pathogen Panel in Wastewater Epidemiology to Influenza and Norovirus. Viruses 2023, 15, 263. [Google Scholar] [CrossRef]
- Wolfe, M.K.; Duong, D.; Bakker, K.M.; Ammerman, M.; Mortenson, L.; Hughes, B.; Arts, P.; Lauring, A.S.; Fitzsimmons, W.J.; Bendall, E.; et al. Wastewater-Based Detection of Two Influenza Outbreaks. Environ. Sci. Technol. Lett. 2022, 9, 687–692. [Google Scholar] [CrossRef]
- Wolken, M.; Sun, T.; McCall, C.; Schneider, R.; Caton, K.; Hundley, C.; Hopkins, L.; Ensor, K.; Domakonda, K.; Kalvapalle, P.; et al. Wastewater Surveillance of SARS-CoV-2 and Influenza in preK-12 Schools Shows School, Community, and Citywide Infections. Water Res. 2023, 231, 119648. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, K.; Xu, X.; Deng, Y.; Leung, K.; Wu, J.T.; Leung, G.M.; Peiris, M.; Poon, L.L.M.; Zhang, T. Development and Application of Influenza Virus Wastewater Surveillance in Hong Kong. Water Res. 2023, 245, 120594. [Google Scholar] [CrossRef]
- Boehm, A.B.; Hughes, B.; Duong, D.; Chan-Herur, V.; Buchman, A.; Wolfe, M.K.; White, B.J. Wastewater Concentrations of Human Influenza, Metapneumovirus, Parainfluenza, Respiratory Syncytial Virus, Rhinovirus, and Seasonal Coronavirus Nucleic-Acids during the COVID-19 Pandemic: A Surveillance Study. Lancet Microbe 2023, 4, e340–e348. [Google Scholar] [CrossRef]
- Allen, D.M.; Reyne, M.I.; Allingham, P.; Levickas, A.; Bell, S.H.; Lock, J.; Coey, J.D.; Carson, S.; Lee, A.J.; McSparron, C.; et al. Genomic Analysis and Surveillance of Respiratory Syncytial Virus Using Wastewater-Based Epidemiology. J. Infect. Dis. 2024, 230, e895–e904. [Google Scholar] [CrossRef]
- Pramanik, R.; Nannaware, K.; Malik, V.; Shah, P.; Sangewar, P.; Gogate, N.; Shashidhara, L.S.; Boargaonkar, R.; Patil, D.; Kale, S.; et al. Monitoring Influenza A (H1N1, H3N2), RSV, and SARS-CoV-2 Using Wastewater-Based Epidemiology: A 2-Year Longitudinal Study in an Indian Megacity Covering Omicron and Post-Omicron Phases. Food Environ. Virol. 2024, 17, 3. [Google Scholar] [CrossRef]
- Zulli, A.; Varkila, M.R.J.; Parsonnet, J.; Wolfe, M.K.; Boehm, A.B. Observations of Respiratory Syncytial Virus (RSV) Nucleic Acids in Wastewater Solids Across the United States in the 2022-2023 Season: Relationships with RSV Infection Positivity and Hospitalization Rates. ACS ES T Water 2024, 4, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, C.; Colson, P.; Lesage, L.; Stoupan, D.; Chaudet, H.; Morand, A.; La Scola, B.; Boschi, C. Coinfections and Iterative Detection of Respiratory Viruses among 17,689 Patients between March 2021 and December 2022 in Southern France. J. Clin. Virol. 2024, 175, 105744. [Google Scholar] [CrossRef] [PubMed]
- Developing a Wastewater Surveillance Sampling Strategy; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Ahmed, W.; Bivins, A.; Bertsch, P.M.; Bibby, K.; Gyawali, P.; Sherchan, S.P.; Simpson, S.L.; Thomas, K.V.; Verhagen, R.; Kitajima, M.; et al. Intraday Variability of Indicator and Pathogenic Viruses in 1-h and 24-h Composite Wastewater Samples: Implications for Wastewater-Based Epidemiology. Environ. Res. 2021, 193, 110531. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, N.; Lacoste, A.; Jardot, P.; Delache, A.; Fontaine, X.; Verlande, M.; Annessi, A.; Giraud-Gatineau, A.; Chaudet, H.; Fournier, P.-E.; et al. Viral RNA in City Wastewater as a Key Indicator of COVID-19 Recrudescence and Containment Measures Effectiveness. Front. Microbiol. 2021, 12, 664477. [Google Scholar] [CrossRef]
- Pesaran, M.H.; Shin, Y.; Smith, R.J. Bounds Testing Approaches to the Analysis of Level Relationships. J. Appl. Econom. 2001, 16, 289–326. [Google Scholar] [CrossRef]
- Rogawski McQuade, E.T.; Blake, I.M.; Brennhofer, S.A.; Islam, M.O.; Sony, S.S.S.; Rahman, T.; Bhuiyan, M.H.; Resha, S.K.; Wettstone, E.G.; Hughlett, L.; et al. Real-Time Sewage Surveillance for SARS-CoV-2 in Dhaka, Bangladesh versus Clinical COVID-19 Surveillance: A Longitudinal Environmental Surveillance Study (December, 2019-December, 2021). Lancet Microbe 2023, 4, e442–e451. [Google Scholar] [CrossRef]
- Lamba, S.; Ganesan, S.; Daroch, N.; Paul, K.; Joshi, S.G.; Sreenivas, D.; Nataraj, A.; Srikantaiah, V.; Mishra, R.; Ramakrishnan, U.; et al. SARS-CoV-2 Infection Dynamics and Genomic Surveillance to Detect Variants in Wastewater—A Longitudinal Study in Bengaluru, India. Lancet Reg. Health Southeast Asia 2023, 11, 100151. [Google Scholar] [CrossRef]
- Pang, X.; Lee, B.E.; Gao, T.; Rosychuk, R.J.; Immaraj, L.; Qiu, J.Y.; Wen, J.; Zelyas, N.; Howden, K.; Wallace, J.; et al. Early Warning COVID-19 Outbreak in Long-Term Care Facilities Using Wastewater Surveillance: Correlation, Prediction, and Interaction with Clinical and Serological Statuses. Lancet Microbe 2024, 5, 100894. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, N.; Boussier, M.; Souville, L.; Penant, G.; Lacoste, A.; Colson, P.; La Scola, B.; Aherfi, S. Simple Wastewater Preparation Protocol Applied to Monitor the Emergence of the Omicron 21L/BA.2 Variant by Genome Sequencing. Viruses 2023, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, N.; Revol, O.; Jardot, P.; Giraud-Gatineau, A.; Houhamdi, L.; Soumagnac, C.; Annessi, A.; Lacoste, A.; Colson, P.; Aherfi, S.; et al. Monitoring the Circulation of SARS-CoV-2 Variants by Genomic Analysis of Wastewater in Marseille, South-East France. Pathogens 2021, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Mercier, E.; D’Aoust, P.M.; Thakali, O.; Hegazy, N.; Jia, J.-J.; Zhang, Z.; Eid, W.; Plaza-Diaz, J.; Kabir, M.P.; Fang, W.; et al. Municipal and Neighbourhood Level Wastewater Surveillance and Subtyping of an Influenza Virus Outbreak. Sci. Rep. 2022, 12, 15777. [Google Scholar] [CrossRef]
- Faherty, E.A.G.; Yuce, D.; Korban, C.; Bemis, K.; Kowalski, R.; Gretsch, S.; Ramirez, E.; Poretsky, R.; Packman, A.; Leisman, K.P.; et al. Correlation of Wastewater Surveillance Data with Traditional Influenza Surveillance Measures in Cook County, Illinois, October 2022-April 2023. Sci. Total Environ. 2024, 912, 169551. [Google Scholar] [CrossRef]
- Zambrana, W.; Huang, C.; Solis, D.; Sahoo, M.K.; Pinsky, B.A.; Boehm, A.B. Spatial and Temporal Variation in Respiratory Syncytial Virus (RSV) Subtype RNA in Wastewater and Relation to Clinical Specimens. mSphere 2024, 9, e0022424. [Google Scholar] [CrossRef]
- Hughes, B.; Duong, D.; White, B.J.; Wigginton, K.R.; Chan, E.M.G.; Wolfe, M.K.; Boehm, A.B. Respiratory Syncytial Virus (RSV) RNA in Wastewater Settled Solids Reflects RSV Clinical Positivity Rates. Environ. Sci. Technol. Lett. 2022, 9, 173–178. [Google Scholar] [CrossRef]
- Ye, Y.; Ellenberg, R.M.; Graham, K.E.; Wigginton, K.R. Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater. Environ. Sci. Technol. 2016, 50, 5077–5085. [Google Scholar] [CrossRef]
- Forés, E.; Bofill-Mas, S.; Itarte, M.; Martínez-Puchol, S.; Hundesa, A.; Calvo, M.; Borrego, C.M.; Corominas, L.L.; Girones, R.; Rusiñol, M. Evaluation of Two Rapid Ultrafiltration-Based Methods for SARS-CoV-2 Concentration from Wastewater. Sci. Total Environ. 2021, 768, 144786. [Google Scholar] [CrossRef]
- Roldan-Hernandez, L.; Boehm, A.B. Adsorption of Respiratory Syncytial Virus, Rhinovirus, SARS-CoV-2, and F+ Bacteriophage MS2 RNA onto Wastewater Solids from Raw Wastewater. Environ. Sci. Technol. 2023, 57, 13346–13355. [Google Scholar] [CrossRef]
- Juel, M.A.I.; Stark, N.; Nicolosi, B.; Lontai, J.; Lambirth, K.; Schlueter, J.; Gibas, C.; Munir, M. Performance Evaluation of Virus Concentration Methods for Implementing SARS-CoV-2 Wastewater Based Epidemiology Emphasizing Quick Data Turnaround. Sci. Total Environ. 2021, 801, 149656. [Google Scholar] [CrossRef]
- Zhan, Q.; Babler, K.M.; Sharkey, M.E.; Amirali, A.; Beaver, C.C.; Boone, M.M.; Comerford, S.; Cooper, D.; Cortizas, E.M.; Currall, B.B.; et al. Relationships between SARS-CoV-2 in Wastewater and COVID-19 Clinical Cases and Hospitalizations, with and without Normalization against Indicators of Human Waste. ACS ES T Water 2022, 2, 1992–2003. [Google Scholar] [CrossRef]
- Malla, B.; Shrestha, S.; Sthapit, N.; Hirai, S.; Raya, S.; Rahmani, A.F.; Angga, M.S.; Siri, Y.; Ruti, A.A.; Haramoto, E. Beyond COVID-19: Wastewater-Based Epidemiology for Multipathogen Surveillance and Normalization Strategies. Sci. Total Environ. 2024, 946, 174419. [Google Scholar] [CrossRef] [PubMed]
- Savvides, C.; Siegel, R. Asymptomatic and Presymptomatic Transmission of SARS-CoV-2: A Systematic Review. medRxiv 2020. [Google Scholar] [CrossRef]
- Buscarini, E.; Manfredi, G.; Brambilla, G.; Menozzi, F.; Londoni, C.; Alicante, S.; Iiritano, E.; Romeo, S.; Pedaci, M.; Benelli, G.; et al. GI Symptoms as Early Signs of COVID-19 in Hospitalised Italian Patients. Gut 2020, 69, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.Y.H.; Morris, S.E.; Stockwell, M.S.; Bowman, N.M.; Asturias, E.; Rao, S.; Lutrick, K.; Ellingson, K.D.; Nguyen, H.Q.; Maldonado, Y.; et al. Estimating the Generation Time for Influenza Transmission Using Household Data in the United States. Epidemics 2025, 50, 100815. [Google Scholar] [CrossRef]
- Otomaru, H.; Sornillo, J.B.T.; Kamigaki, T.; Bado, S.L.P.; Okamoto, M.; Saito-Obata, M.; Inobaya, M.T.; Segubre-Mercado, E.; Alday, P.P.; Saito, M.; et al. Risk of Transmission and Viral Shedding From the Time of Infection for Respiratory Syncytial Virus in Households. Am. J. Epidemiol. 2021, 190, 2536–2543. [Google Scholar] [CrossRef]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Moreira, L.P.; Watanabe, A.S.A.; Camargo, C.N.; Melchior, T.B.; Granato, C.; Bellei, N. Respiratory Syncytial Virus Evaluation among Asymptomatic and Symptomatic Subjects in a University Hospital in Sao Paulo, Brazil, in the Period of 2009-2013. Influenza Other Respir. Viruses 2018, 12, 326–330. [Google Scholar] [CrossRef]
- Self, W.H.; Williams, D.J.; Zhu, Y.; Ampofo, K.; Pavia, A.T.; Chappell, J.D.; Hymas, W.C.; Stockmann, C.; Bramley, A.M.; Schneider, E.; et al. Respiratory Viral Detection in Children and Adults: Comparing Asymptomatic Controls and Patients With Community-Acquired Pneumonia. J. Infect. Dis. 2016, 213, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L.; Xu, C.; Ip, D.K.M.; Cowling, B.J. Review Article: The Fraction of Influenza Virus Infections That Are Asymptomatic: A Systematic Review and Meta-Analysis. Epidemiology 2015, 26, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.P.; Morris, S.E.; Rolfes, M.A.; Kittikraisak, W.; Samuels, A.M.; Biggerstaff, M.; Davis, W.W.; Reed, C.; Olsen, S.J. The Role of Asymptomatic Infections in Influenza Transmission: What Do We Really Know. Lancet Infect. Dis. 2024, 24, e394–e404. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Cox, M.; Milinovich, G.J.; Magalhaes, R.J.S.; Mackay, I.M.; Yakob, L. Heterogeneous and Dynamic Prevalence of Asymptomatic Influenza Virus Infections. Emerg. Infect. Dis. 2016, 22, 1052–1056. [Google Scholar] [CrossRef]
- Shang, W.; Kang, L.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1049. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Zhang, S.; Kong, Y.; Shen, Z.; Zhang, J. Proportion of Asymptomatic Infection and Nonsevere Disease Caused by SARS-CoV-2 Omicron Variant: A Systematic Review and Analysis. J. Med. Virol. 2022, 94, 5790–5801. [Google Scholar] [CrossRef]
- Sah, P.; Fitzpatrick, M.C.; Zimmer, C.F.; Abdollahi, E.; Juden-Kelly, L.; Moghadas, S.M.; Singer, B.H.; Galvani, A.P. Asymptomatic SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109229118. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals With Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef]
- von Linstow, M.-L.; Eugen-Olsen, J.; Koch, A.; Winther, T.N.; Westh, H.; Hogh, B. Excretion Patterns of Human Metapneumovirus and Respiratory Syncytial Virus among Young Children. Eur. J. Med. Res. 2006, 11, 329–335. [Google Scholar]
- Lowry, S.A.; Wolfe, M.K.; Boehm, A.B. Respiratory Virus Concentrations in Human Excretions That Contribute to Wastewater: A Systematic Review and Meta-Analysis. J. Water Health 2023, 21, 831–848. [Google Scholar] [CrossRef]
- Wootton, S.H.; Scheifele, D.W.; Mak, A.; Petric, M.; Skowronski, D.M. Detection of Human Influenza Virus in the Stool of Children. Pediatr. Infect. Dis. J. 2006, 25, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Bénet, T.; Amour, S.; Valette, M.; Saadatian-Elahi, M.; Aho-Glélé, L.S.; Berthelot, P.; Denis, M.-A.; Grando, J.; Landelle, C.; Astruc, K.; et al. Incidence of Asymptomatic and Symptomatic Influenza Among Healthcare Workers: A Multicenter Prospective Cohort Study. Clin. Infect. Dis. 2021, 72, e311–e318. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Alsing, J. Faecal Shedding Models for SARS-CoV-2 RNA among Hospitalised Patients and Implications for Wastewater-Based Epidemiology. J. R. Stat. Soc. Ser. C Appl. Stat. 2023, 72, 330–345. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Gupta, S.; Parker, J.; Smits, S.; Underwood, J.; Dolwani, S. Persistent Viral Shedding of SARS-CoV-2 in Faeces—A Rapid Review. Color. Dis. 2020, 22, 611–620. [Google Scholar] [CrossRef]
- Zhang, Y.; Cen, M.; Hu, M.; Du, L.; Hu, W.; Kim, J.J.; Dai, N. Prevalence and Persistent Shedding of Fecal SARS-CoV-2 RNA in Patients With COVID-19 Infection: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2021, 12, e00343. [Google Scholar] [CrossRef]
- Jones, D.L.; Baluja, M.Q.; Graham, D.W.; Corbishley, A.; McDonald, J.E.; Malham, S.K.; Hillary, L.S.; Connor, T.R.; Gaze, W.H.; Moura, I.B.; et al. Shedding of SARS-CoV-2 in Feces and Urine and Its Potential Role in Person-to-Person Transmission and the Environment-Based Spread of COVID-19. Sci. Total Environ. 2020, 749, 141364. [Google Scholar] [CrossRef]
- Hirose, R.; Daidoji, T.; Naito, Y.; Watanabe, Y.; Arai, Y.; Oda, T.; Konishi, H.; Yamawaki, M.; Itoh, Y.; Nakaya, T. Long-Term Detection of Seasonal Influenza RNA in Faeces and Intestine. Clin. Microbiol. Infect. 2016, 22, 813.e1–813.e7. [Google Scholar] [CrossRef]
- Al Khatib, H.A.; Coyle, P.V.; Al Maslamani, M.A.; Al Thani, A.A.; Pathan, S.A.; Yassine, H.M. Molecular and Biological Characterization of Influenza A Viruses Isolated from Human Fecal Samples. Infect. Genet. Evol. 2021, 93, 104972. [Google Scholar] [CrossRef]
- Kashi, A.H.; De la Rosette, J.; Amini, E.; Abdi, H.; Fallah-Karkan, M.; Vaezjalali, M. Urinary Viral Shedding of COVID-19 and Its Clinical Associations: A Systematic Review and Meta-Analysis of Observational Studies. Urol. J. 2020, 17, 433–441. [Google Scholar] [CrossRef]
- Acer, P.T.; Kelly, L.M.; Lover, A.A.; Butler, C.S. Quantifying the Relationship between SARS-CoV-2 Wastewater Concentrations and Building-Level COVID-19 Prevalence at an Isolation Residence: A Passive Sampling Approach. Int. J. Environ. Res. Public. Health 2022, 19, 11245. [Google Scholar] [CrossRef]
- Wathuo, M.; Medley, G.F.; Nokes, D.J.; Munywoki, P.K. Quantification and Determinants of the Amount of Respiratory Syncytial Virus (RSV) Shed Using Real Time PCR Data from a Longitudinal Household Study. Wellcome Open Res. 2016, 1, 27. [Google Scholar] [CrossRef]
- Le Targa, L.; Wurtz, N.; Lacoste, A.; Penant, G.; Jardot, P.; Annessi, A.; Colson, P.; La Scola, B.; Aherfi, S. SARS-CoV-2 Testing of Aircraft Wastewater Shows That Mandatory Tests and Vaccination Pass before Boarding Did Not Prevent Massive Importation of Omicron Variant into Europe. Viruses 2022, 14, 1511. [Google Scholar] [CrossRef]
- Manoha, C.; Dequiedt, A.-L.; Thery, L.; Marotel, M.; Pez, F.; Vouillon, B.; Gueneau, E.; de Rougemont, A. Multisite Community-Scale Monitoring of Respiratory and Enteric Viruses in the Effluent of a Nursing Home and in the Inlet of the Local Wastewater Treatment Plant. Appl. Environ. Microbiol. 2024, 90, e0115824. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Iverson, G.; Wright, L.; Fallon, J.T., 3rd; Briley, K.P.; Yin, C.; Huang, W.; Humphrey, C. Wastewater Based Epidemiology as a Surveillance Tool during the Current COVID-19 Pandemic on a College Campus (East Carolina University) and Its Accuracy in Predicting SARS-CoV-2 Outbreaks in Dormitories. PLoS ONE 2024, 19, e0289906. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, K.; Xue, B.; Deng, Y.; Xu, X.; Yan, W.; Rong, C.; Leung, K.; Wu, J.T.; Leung, G.M.; et al. Tracking Diarrhea Viruses and Mpox Virus Using the Wastewater Surveillance Network in Hong Kong. Water Res. 2024, 255, 121513. [Google Scholar] [CrossRef]
- Ares-Gómez, S.; Mallah, N.; Santiago-Pérez, M.-I.; Pardo-Seco, J.; Pérez-Martínez, O.; Otero-Barrós, M.-T.; Suárez-Gaiche, N.; Kramer, R.; Jin, J.; Platero-Alonso, L.; et al. Effectiveness and Impact of Universal Prophylaxis with Nirsevimab in Infants against Hospitalisation for Respiratory Syncytial Virus in Galicia, Spain: Initial Results of a Population-Based Longitudinal Study. Lancet Infect. Dis. 2024, 24, 817–828. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurtz, N.; Maggiore, L.; Boschi, C.; Annessi, A.; Berges, F.; Lacoste, A.; Chaudet, H.; Colson, P.; Scola, B.L.; Aherfi, S. Long-Term Sewage Survey of SARS-CoV-2, Influenza A and Respiratory Syncytial Virus (RSV), and Correlation to Human Cases in a City with One Million Inhabitants. Microorganisms 2025, 13, 2268. https://doi.org/10.3390/microorganisms13102268
Wurtz N, Maggiore L, Boschi C, Annessi A, Berges F, Lacoste A, Chaudet H, Colson P, Scola BL, Aherfi S. Long-Term Sewage Survey of SARS-CoV-2, Influenza A and Respiratory Syncytial Virus (RSV), and Correlation to Human Cases in a City with One Million Inhabitants. Microorganisms. 2025; 13(10):2268. https://doi.org/10.3390/microorganisms13102268
Chicago/Turabian StyleWurtz, Nathalie, Lea Maggiore, Céline Boschi, Alexandre Annessi, Franck Berges, Alexandre Lacoste, Herve Chaudet, Philippe Colson, Bernard La Scola, and Sarah Aherfi. 2025. "Long-Term Sewage Survey of SARS-CoV-2, Influenza A and Respiratory Syncytial Virus (RSV), and Correlation to Human Cases in a City with One Million Inhabitants" Microorganisms 13, no. 10: 2268. https://doi.org/10.3390/microorganisms13102268
APA StyleWurtz, N., Maggiore, L., Boschi, C., Annessi, A., Berges, F., Lacoste, A., Chaudet, H., Colson, P., Scola, B. L., & Aherfi, S. (2025). Long-Term Sewage Survey of SARS-CoV-2, Influenza A and Respiratory Syncytial Virus (RSV), and Correlation to Human Cases in a City with One Million Inhabitants. Microorganisms, 13(10), 2268. https://doi.org/10.3390/microorganisms13102268





