Effect of Relocation, Social Housing Changes, and Diarrhea Status on Microbiome Composition of Juvenile Cynomolgus Macaques (Macaca fascicularis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. Ethics Statement
2.3. Study Design
2.4. Specimen Collection
2.5. Microbiome Sequencing
2.6. Cortisol Measures
2.7. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. No Differences in Cortisol Levels
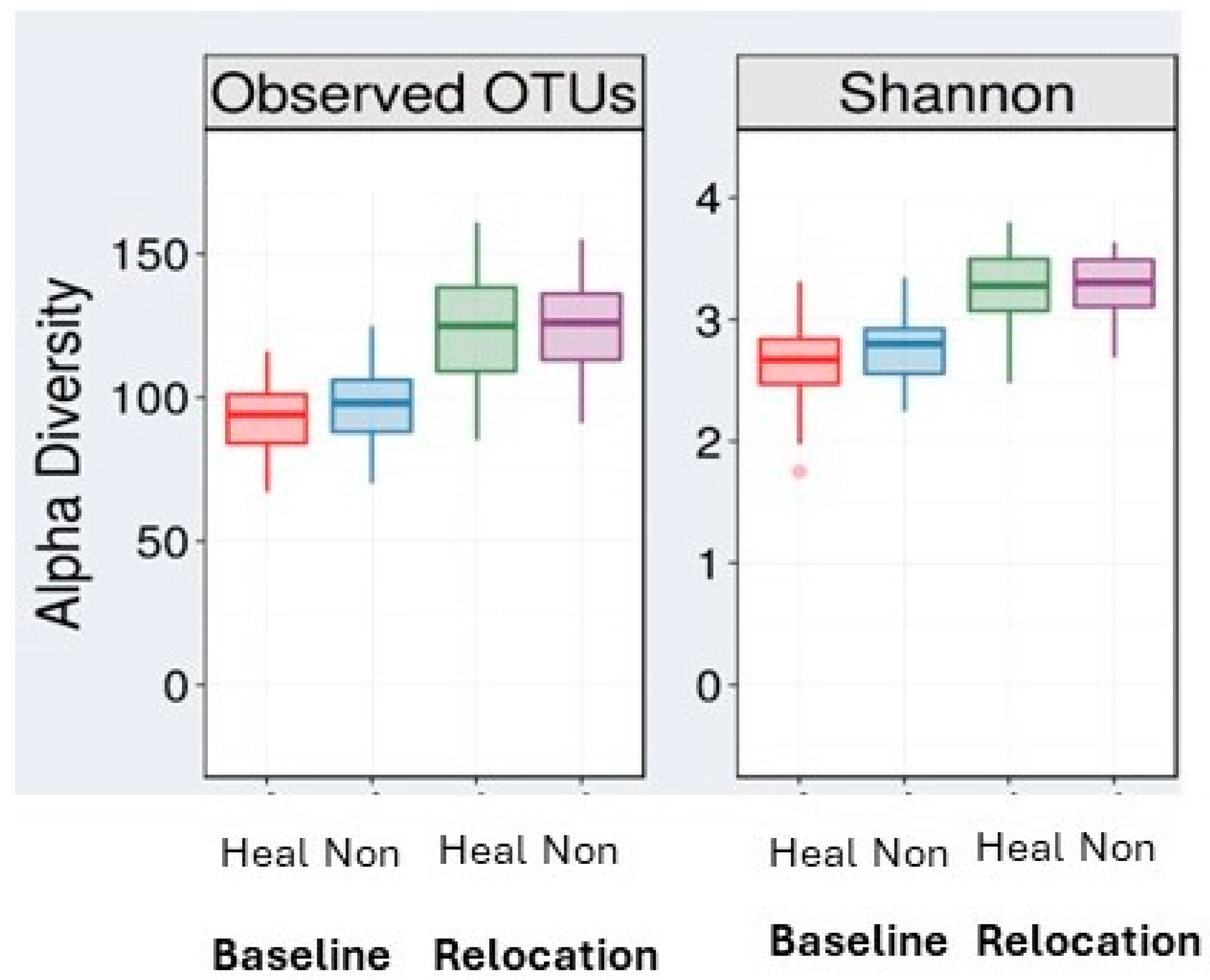
3.3. Animal Relocation Alters the Microbiome Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, A.; Morton, D. Gastrointestinal system: Approach to diarrhea diagnosis and treatment. In Nonhuman Primates in Biomedical Research: Diseases; Academic Press: San Diego, CA, USA, 1998; pp. 81–87. [Google Scholar]
- Maaskant, A.; Voermans, B.; Levin, E.; de Goffau, M.C.; Plomp, N.; Schuren, F.; Remarque, E.J.; Smits, A.; Langermans, J.A.M.; Bakker, J.; et al. Microbiome signature suggestive of lactose-intolerance in rhesus macaques (Macaca mulatta) with intermittent chronic diarrhea. Anim. Microbiome 2024, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Prongay, K.; Park, B.; Murphy, S.J. Risk factor analysis may provide clues to diarrhea prevention in outdoor-housed rhesus macaques (Macaca mulatta). Am. J. Primatol. 2013, 75, 872–882. [Google Scholar] [CrossRef]
- Ferrecchia, C.E.; Hobbs, T.R. Efficacy of oral fecal bacteriotherapy in rhesus macaques (Macaca mulatta) with chronic diarrhea. Comp. Med. 2013, 63, 71–75. [Google Scholar]
- Populin, L.C.; Rajala, A.Z.; Matkowskyj, K.A.; Saha, S.; Zeng, W.; Christian, B.; McVea, A.; Tay, E.X.; Mueller, E.M.; Malone, M.E.; et al. Characterization of idiopathic chronic diarrhea and associated intestinal inflammation and preliminary observations of effects of vagal nerve stimulation in a non-human primate. Neurogastroenterol. Motil. 2024, 36, e14876. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.; White, D.; Ingram, S.; Jackson, R.; Larin, J.; Morales, P.; Garcia, A.P.; Hicks, C.; Hopper, K.; Wagner, J. A bio-behavioral study of chronic idiopathic colitis in the rhesus macaque (Macaca mulatta). Appl. Anim. Behav. Sci. 2012, 137, 208–220. [Google Scholar] [CrossRef]
- Brady, A.G.; Carville, A.A. Digestive system diseases of nonhuman primates. In Nonhuman Primates in Biomedical Research; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 589–627. [Google Scholar]
- Bacon, R.L.; Hodo, C.L.; Wu, J.; Welch, S.; Nickodem, C.; Vinasco, J.; Threadgill, D.; Gray, S.B.; Norman, K.N.; Lawhon, S.D. Diversity of Campylobacter spp. circulating in a rhesus macaque (Macaca mulatta) breeding colony using culture and molecular methods. mSphere 2024, 9, e0056024. [Google Scholar] [CrossRef]
- Gottlieb, D.H.; Del Rosso, L.; Sheikhi, F.; Gottlieb, A.; McCowan, B.; Capitanio, J.P. Personality, environmental stressors, and diarrhea in Rhesus macaques: An interactionist perspective. Am. J. Primatol. 2018, 80, e22908. [Google Scholar] [CrossRef]
- Wilk, J.L.; Maginnis, G.M.; Coleman, K.; Lewis, A.; Ogden, B. Evaluation of the use of coconut to treat chronic diarrhea in rhesus macaques (Macaca mulatta). J. Med. Primatol. 2008, 37, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Bacon, R.L.; Taylor, L.; Gray, S.B.; Hodo, C.L. Analysis of cell populations in the normal rhesus macaque (Macaca mulatta) lower intestinal tract and diagnostic thresholds for chronic enterocolitis. Vet. Pathol. 2024, 61, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, X.; Liu, Z.; Liu, N. Social status predicts physiological and behavioral responses to chronic stress in rhesus monkeys. iScience 2024, 27, 110073. [Google Scholar] [CrossRef]
- Capitanio, J.P.; Mendoza, S.P.; Cole, S.W. Nervous temperament in infant monkeys is associated with reduced sensitivity of leukocytes to cortisol’s influence on trafficking. Brain Behav. Immun. 2011, 25, 151–159. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- Bailey, M.T.; Coe, C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999, 35, 146–155. [Google Scholar] [CrossRef]
- Sarkar, A.; McInroy, C.J.A.; Harty, S.; Raulo, A.; Ibata, N.G.O.; Valles-Colomer, M.; Johnson, K.V.; Brito, I.L.; Henrich, J.; Archie, E.A.; et al. Microbial transmission in the social microbiome and host health and disease. Cell 2024, 187, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Makris, A.P.; Karianaki, M.; Tsamis, K.I.; Paschou, S.A. The role of the gut-brain axis in depression: Endocrine, neural, and immune pathways. Hormones 2021, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.T.; Merriam, D.; Shock, B.C.; Mills, S.; Spinner, A.; Reader, R.; Hartigan-O’Connor, D.J. Idiopathic Colitis in Rhesus Macaques Is Associated With Dysbiosis, Abundant Enterochromaffin Cells and Altered T-Cell Cytokine Expression. Vet. Pathol. 2018, 55, 741–752. [Google Scholar] [CrossRef]
- Seekatz, A.M.; Panda, A.; Rasko, D.A.; Toapanta, F.R.; Eloe-Fadrosh, E.A.; Khan, A.Q.; Liu, Z.; Shipley, S.T.; Detolla, L.J.; Sztein, M.B.; et al. Differential response of the cynomolgus macaque gut microbiota to Shigella infection. PLoS ONE 2013, 8, e64212. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Guard, B.C.; Barr, J.W.; Reddivari, L.; Klemashevich, C.; Jayaraman, A.; Steiner, J.M.; Vanamala, J.; Suchodolski, J.S. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 2015, 10, e0127259. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Foster, M.L.; Sohail, M.U.; Leutenegger, C.; Queen, E.V.; Steiner, J.M.; Marks, S.L. The fecal microbiome in cats with diarrhea. PLoS ONE 2015, 10, e0127378. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Zijlstra, R.T.; Willing, B.P. The role of gut microbiota in the health and disease of pigs. Anim. Front. 2016, 6, 30–36. [Google Scholar] [CrossRef]
- Zeineldin, M.; Aldridge, B.; Lowe, J. Dysbiosis of the fecal microbiota in feedlot cattle with hemorrhagic diarrhea. Microb. Pathog. 2018, 115, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- Amato, K.R.; Metcalf, J.L.; Song, S.J.; Hale, V.L.; Clayton, J.; Ackermann, G.; Humphrey, G.; Niu, K.; Cui, D.; Zhao, H. Using the gut microbiota as a novel tool for examining colobine primate GI health. Glob. Ecol. Conserv. 2016, 7, 225–237. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals: Eighth Edition; The National Academies Press: Washington, DC, USA, 2011. [CrossRef]
- Niven, D.J.; Berthiaume, L.R.; Fick, G.H.; Laupland, K.B. Matched case-control studies: A review of reported statistical methodology. Clin. Epidemiol. 2012, 4, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Nehete, P.N.; Shelton, K.A.; Nehete, B.P.; Chitta, S.; Williams, L.E.; Schapiro, S.J.; Abee, C.R. Effects of transportation, relocation, and acclimation on phenotypes and functional characteristics of peripheral blood lymphocytes in rhesus monkeys (Macaca mulatta). PLoS ONE 2017, 12, e0188694. [Google Scholar] [CrossRef]
- Field, A.E.; Jones, C.L.; Kelly, R.; Marko, S.T.; Kern, S.J.; Rico, P.J. Measurement of fecal corticosterone metabolites as a predictor of the habituation of rhesus macaques (Macaca mulatta) to jacketing. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 59–65. [Google Scholar] [PubMed]
- Whitten, P.L.; Stavisky, R.; Aureli, F.; Russell, E. Response of fecal cortisol to stress in captive chimpanzees (Pan troglodytes). Am. J. Primatol. 1998, 44, 57–69. [Google Scholar] [CrossRef]
- Minot, S.S.; Krumm, N.; Greenfield, N.B. One Codex: A Sensitive and Accurate Data Platform for Genomic Microbial identification. bioRxiv 2015. [Google Scholar] [CrossRef]
- Hagerty, S.L.; Hutchison, K.E.; Lowry, C.A.; Bryan, A.D. An empirically derived method for measuring human gut microbiome alpha diversity: Demonstrated utility in predicting health-related outcomes among a human clinical sample. PLoS ONE 2020, 15, e0229204. [Google Scholar] [CrossRef] [PubMed]
- Finotello, F.; Mastrorilli, E.; Di Camillo, B. Measuring the diversity of the human microbiota with targeted next-generation sequencing. Brief. Bioinform. 2018, 19, 679–692. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Atwood, R.E.; Golden, D.M.; Kaba, S.A.; Bradley, M.J. Characterization of the cortisol response to traumatic hemorrhage and intra-abdominal contamination models in Cynomologus Macaques. Mol. Cell. Endocrinol. 2020, 518, 111036. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Community Ecology Package; R Package Version 2; R: Vienna, Austria, 2013; pp. 321–326. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dwass, M. Modified randomization tests for nonparametric hypotheses. Ann. Math. Stat. 1957, 28, 181–187. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Strait, K.; Else, J.G.; Eberhard, M.L. Parasitic diseases of nonhuman primates. In Nonhuman Primates in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2012; pp. 197–297. [Google Scholar]
- Cinque, C.; De Marco, A.; Mairesse, J.; Giuli, C.; Sanna, A.; De Marco, L.; Zuena, A.R.; Casolini, P.; Catalani, A.; Thierry, B.; et al. Relocation stress induces short-term fecal cortisol increase in Tonkean macaques (Macaca tonkeana). Primates 2017, 58, 315–321. [Google Scholar] [CrossRef] [PubMed]
- McKenna, P.; Hoffmann, C.; Minkah, N.; Aye, P.P.; Lackner, A.; Liu, Z.; Lozupone, C.A.; Hamady, M.; Knight, R.; Bushman, F.D. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog. 2008, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fan, Z.; Li, J.; Wang, X.; Lan, Y.; Yue, B.; He, M.; Zhang, A.; Li, J. Assembly of novel microbial genomes from gut metagenomes of rhesus macaque (Macaca mulatta). Gut Microbes 2023, 15, 2188848. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef]
- Janiak, M.C.; Montague, M.J.; Villamil, C.I.; Stock, M.K.; Trujillo, A.E.; DePasquale, A.N.; Orkin, J.D.; Bauman Surratt, S.E.; Gonzalez, O.; Platt, M.L.; et al. Age and sex-associated variation in the multi-site microbiome of an entire social group of free-ranging rhesus macaques. Microbiome 2021, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Amaral, W.Z.; Lubach, G.R.; Proctor, A.; Lyte, M.; Phillips, G.J.; Coe, C.L. Social Influences on Prevotella and the Gut Microbiome of Young Monkeys. Psychosom. Med. 2017, 79, 888–897. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Clayton, J.B.; Vangay, P.; Huang, H.; Ward, T.; Hillmann, B.M.; Al-Ghalith, G.A.; Travis, D.A.; Long, H.T.; Tuan, B.V.; Minh, V.V.; et al. Captivity humanizes the primate microbiome. Proc. Natl. Acad. Sci. USA 2016, 113, 10376–10381. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M. The Prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe 2019, 26, 666–679.e667. [Google Scholar] [CrossRef]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.H.; Liu, Z.J.; Zhang, L.; Wei, H.; Song, L.J.; Zhu, S.W.; He, M.B.; Duan, L.P. Sex-based differences in fecal short-chain fatty acid and gut microbiota in irritable bowel syndrome patients. J. Dig. Dis. 2021, 22, 246–255. [Google Scholar] [CrossRef]
- Pafčo, B.; Sharma, A.K.; Petrželková, K.J.; Vlčková, K.; Todd, A.; Yeoman, C.J.; Wilson, B.A.; Stumpf, R.; White, B.A.; Nelson, K.E. Gut microbiome composition of wild western lowland gorillas is associated with individual age and sex factors. Am. J. Phys. Anthropol. 2019, 169, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hyun, J.E.; Thiesen, A.; Park, H.; Hotte, N.; Watanabe, H.; Higashiyama, T.; Madsen, K.L. Sex-specific differences in the gut microbiome in response to dietary fiber supplementation in IL-10-deficient mice. Nutrients 2020, 12, 2088. [Google Scholar] [CrossRef]
- Forouzan, S.; Hoffman, K.L.; Kosten, T.A. Methamphetamine exposure and its cessation alter gut microbiota and induce depressive-like behavioral effects on rats. Psychopharmacology 2021, 238, 281–292. [Google Scholar] [CrossRef]
- Forouzan, S.; Hoffman, K.L.; Kosten, T.A. Probiotics alter the microbial and behavioral consequences of methamphetamine exposure in a sex-selective manner. Psychoactives 2024, 3, 318–336. [Google Scholar] [CrossRef]


| Measure | Male | Female | ||
|---|---|---|---|---|
| Healthy | Unhealthy | Healthy | Unhealthy | |
| Number | 12 | 13 | 16 | 20 |
| Age (years) | 2.97 ± 0.21 | 3.00 ± 0.19 | 3.37 ± 0.17 | 3.21 ± 0.16 |
| Body weight (kg) | 3.34 ± 0.21 | 3.43 ± 0.15 | 3.29 ± 0.15 | 3.08 ± 0.17 |
| OTUs | p-Value | Shannon | p-Value | ||
|---|---|---|---|---|---|
| Sex | Female | −23.44 | 0.10 | −0.4778 | 0.01 |
| Male | −34 | −0.8084 | |||
| Group | Healthy | −30.41 | 0.27 | −0.6781 | 0.23 |
| Unhealthy | −24.67 | −0.5287 |
| Taxa | Group | Baseline | Relocation | p-Value | FDR-Adj. p |
|---|---|---|---|---|---|
| Bacteroidota | Healthy | 0.56 | 0.50 | 0.07 | 0.11 |
| Firmicutes | Healthy | 0.38 | 0.39 | 0.54 | 0.54 |
| Actinobacteria | Healthy | 0.05 | 0.07 | 0.01 | 0.03 |
| Spirochaetota | Healthy | 0.01 | 0.03 | 0.02 | 0.04 |
| Bacteroidota | Unhealthy | 0.48 | 0.42 | 0.26 | 0.26 |
| Firmicutes | Unhealthy | 0.42 | 0.39 | 0.26 | 0.26 |
| Actinobacteria | Unhealthy | 0.04 | 0.11 | 0.00 | 0.001 |
| Spirochaetota | Unhealthy | 0.05 | 0.07 | 0.13 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGrew, K.; de Oca, N.M.; Kosten, T.A. Effect of Relocation, Social Housing Changes, and Diarrhea Status on Microbiome Composition of Juvenile Cynomolgus Macaques (Macaca fascicularis). Microorganisms 2025, 13, 98. https://doi.org/10.3390/microorganisms13010098
McGrew K, de Oca NM, Kosten TA. Effect of Relocation, Social Housing Changes, and Diarrhea Status on Microbiome Composition of Juvenile Cynomolgus Macaques (Macaca fascicularis). Microorganisms. 2025; 13(1):98. https://doi.org/10.3390/microorganisms13010098
Chicago/Turabian StyleMcGrew, Keely, Nicole Monts de Oca, and Therese A. Kosten. 2025. "Effect of Relocation, Social Housing Changes, and Diarrhea Status on Microbiome Composition of Juvenile Cynomolgus Macaques (Macaca fascicularis)" Microorganisms 13, no. 1: 98. https://doi.org/10.3390/microorganisms13010098
APA StyleMcGrew, K., de Oca, N. M., & Kosten, T. A. (2025). Effect of Relocation, Social Housing Changes, and Diarrhea Status on Microbiome Composition of Juvenile Cynomolgus Macaques (Macaca fascicularis). Microorganisms, 13(1), 98. https://doi.org/10.3390/microorganisms13010098





