Effects of Combined Application of Biogas Slurry and Chemical Fertilizers on Silage Corn, Soil Nutrients, and Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Soil Sample Collection and Processing
2.4. Test Methods for Physicochemical Properties
2.5. Extraction, Amplification, and Quantification of Soil Microbial Genomic DNA
2.6. Data Processing and Analysis
3. Results and Analysis
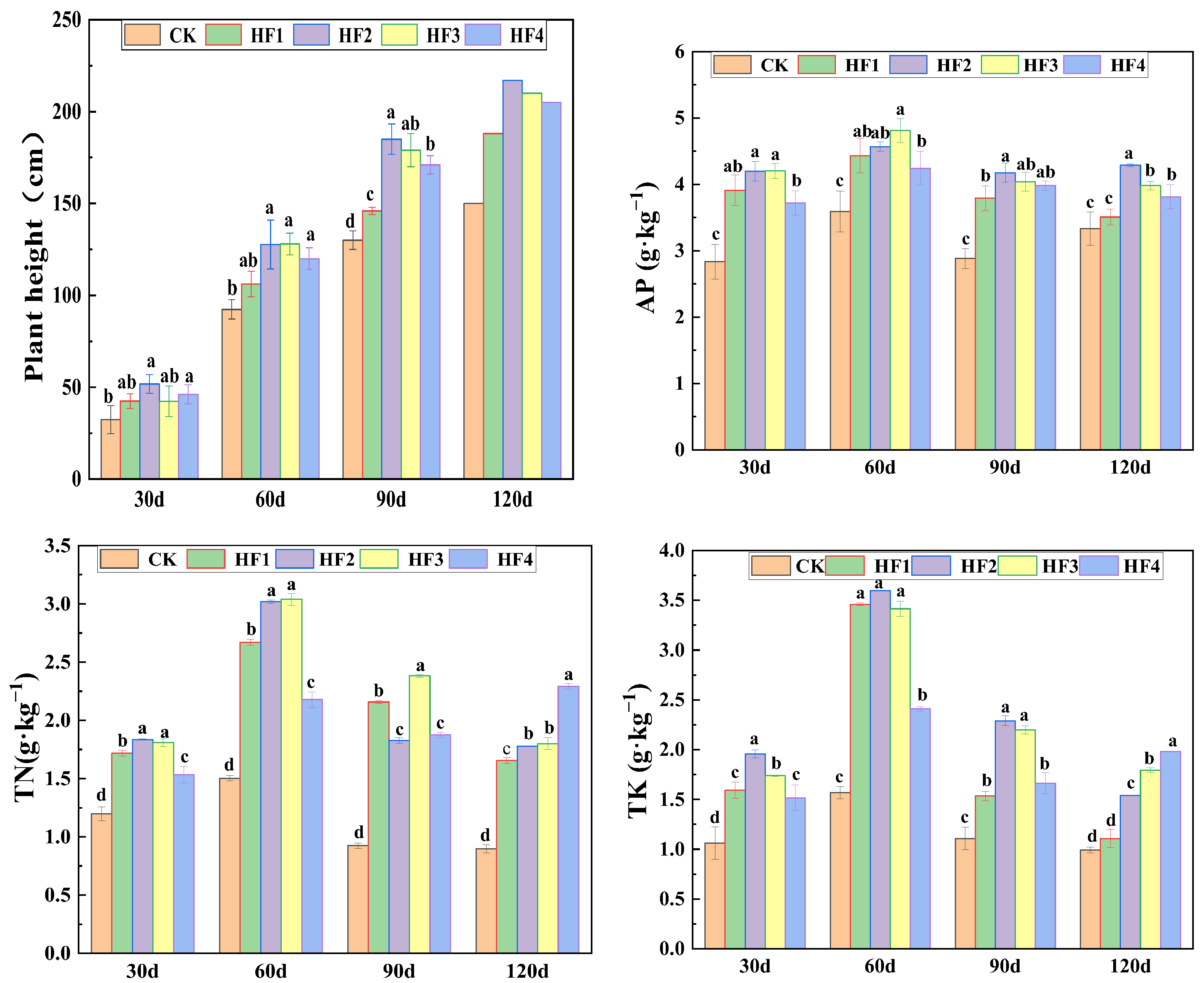
3.1. Growth and Nutrient Response Patterns of Silage Corn
3.2. Characteristics of Changes in Soil Microbial Community Structure in the Harvest Period of Silage Corn
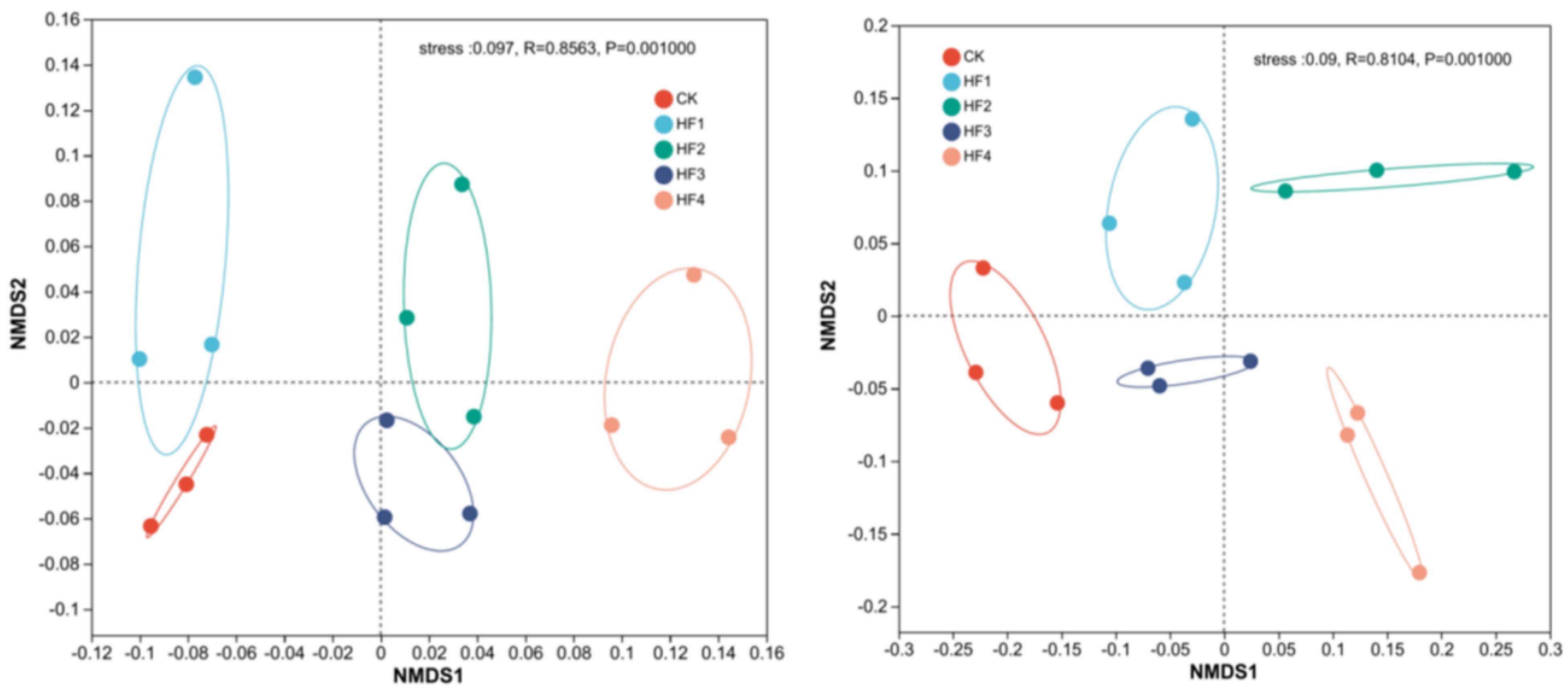
3.2.1. Analysis of Soil Microbial Diversity
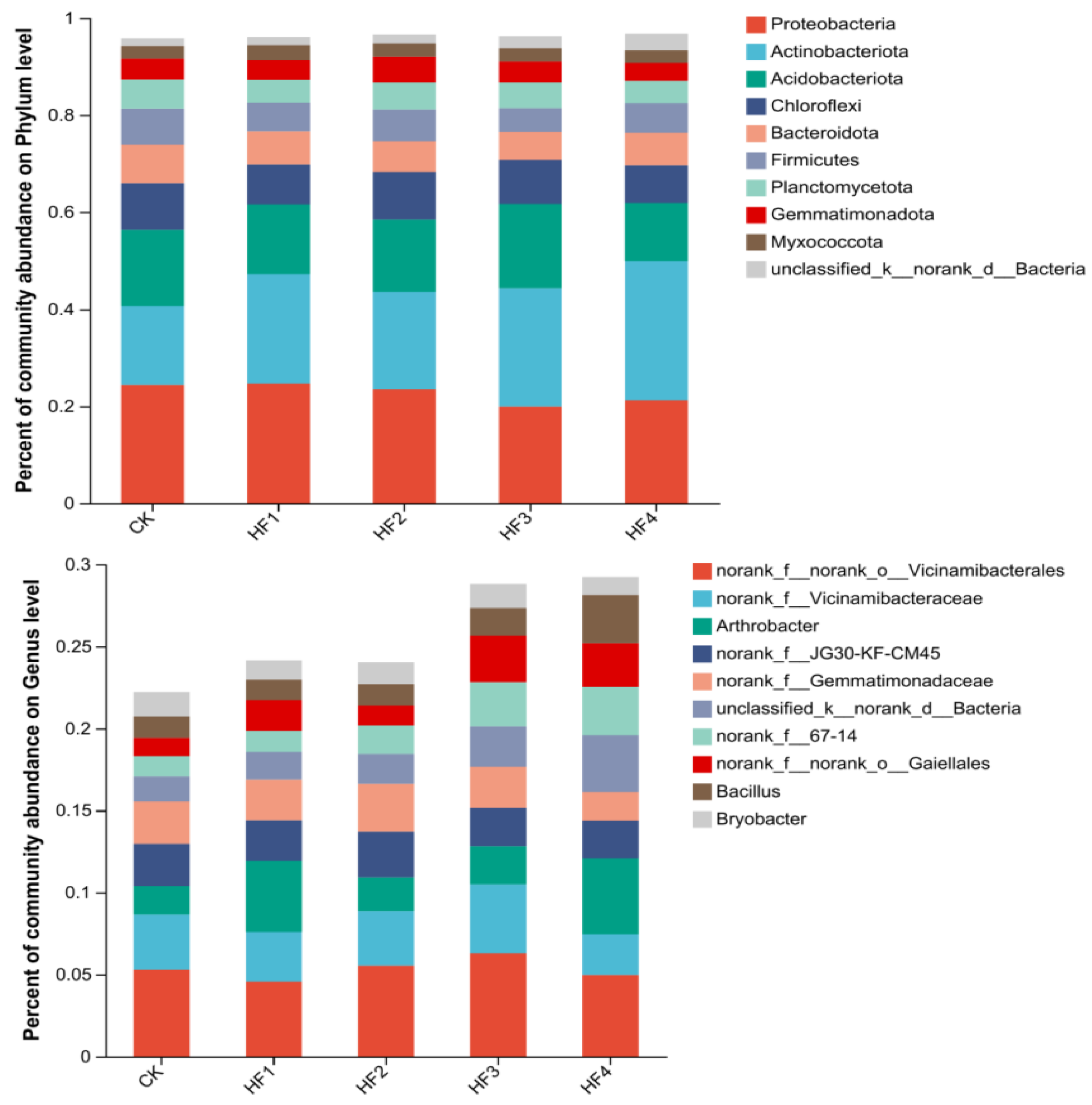
3.2.2. Characteristics of Changes in Soil Microbial Community Structure
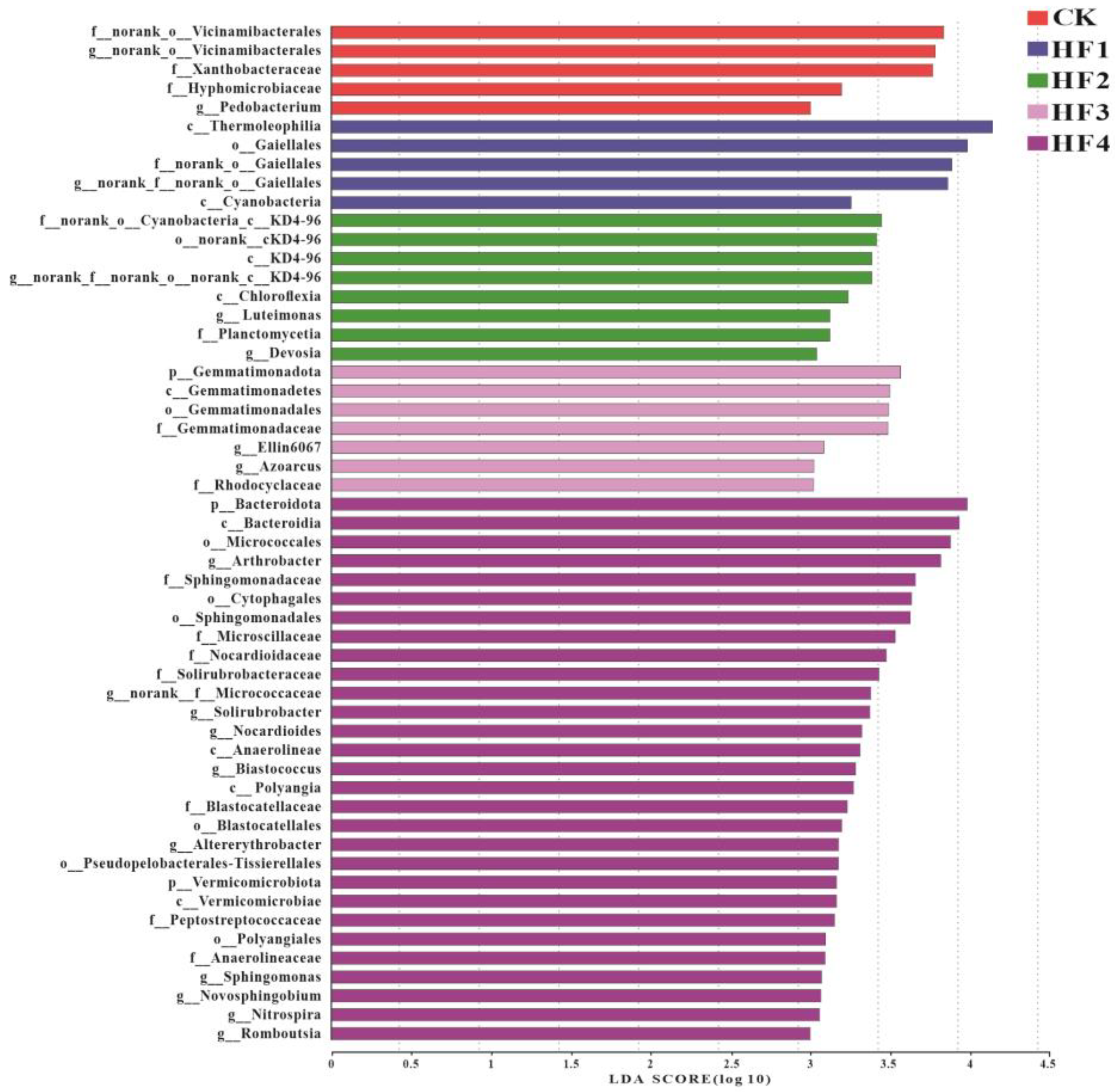
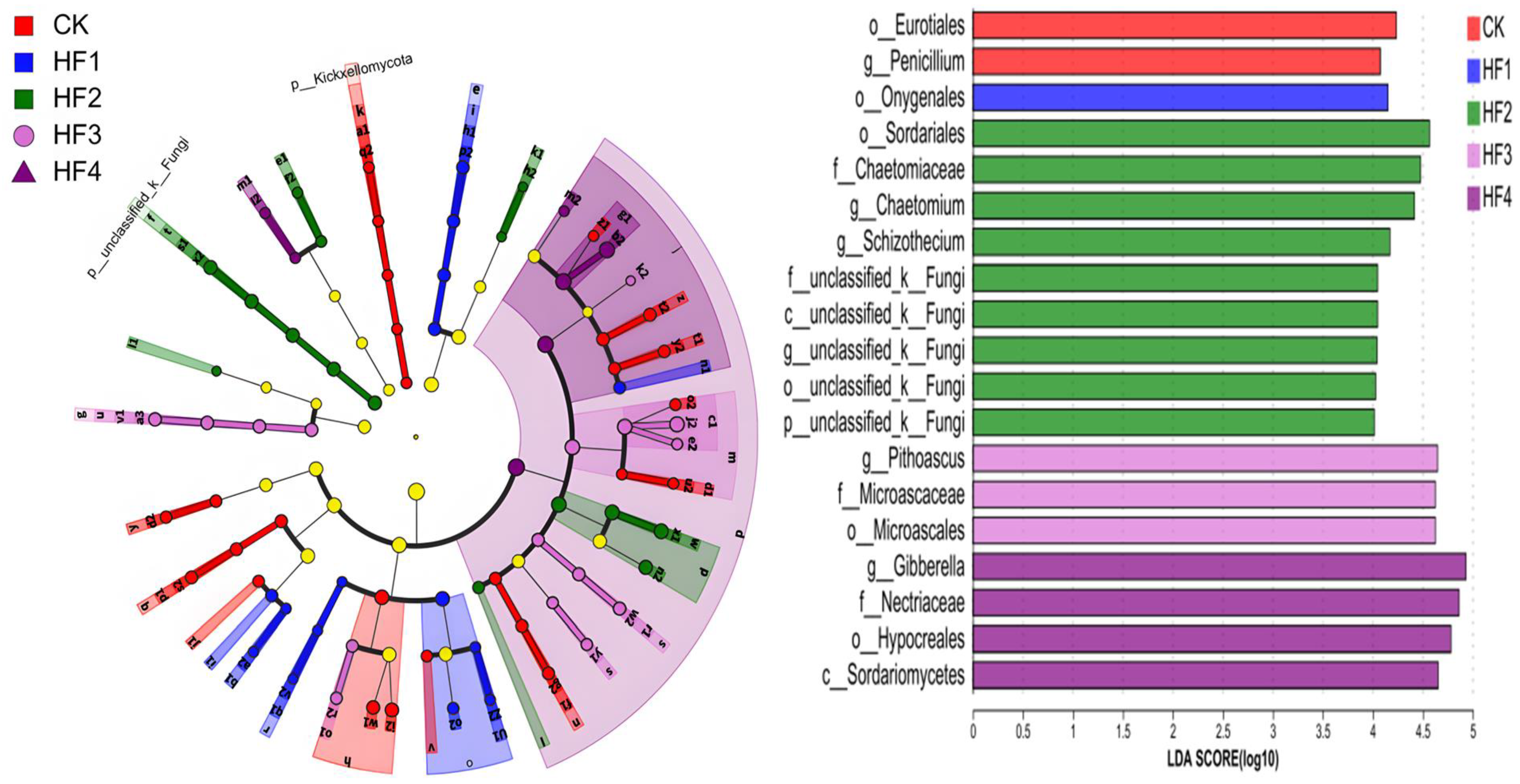
3.2.3. Key Populations with Differences in Microbial Communities
3.3. Correlation Between Microbial Community Diversity and Soil Physicochemical Properties
4. Discussion
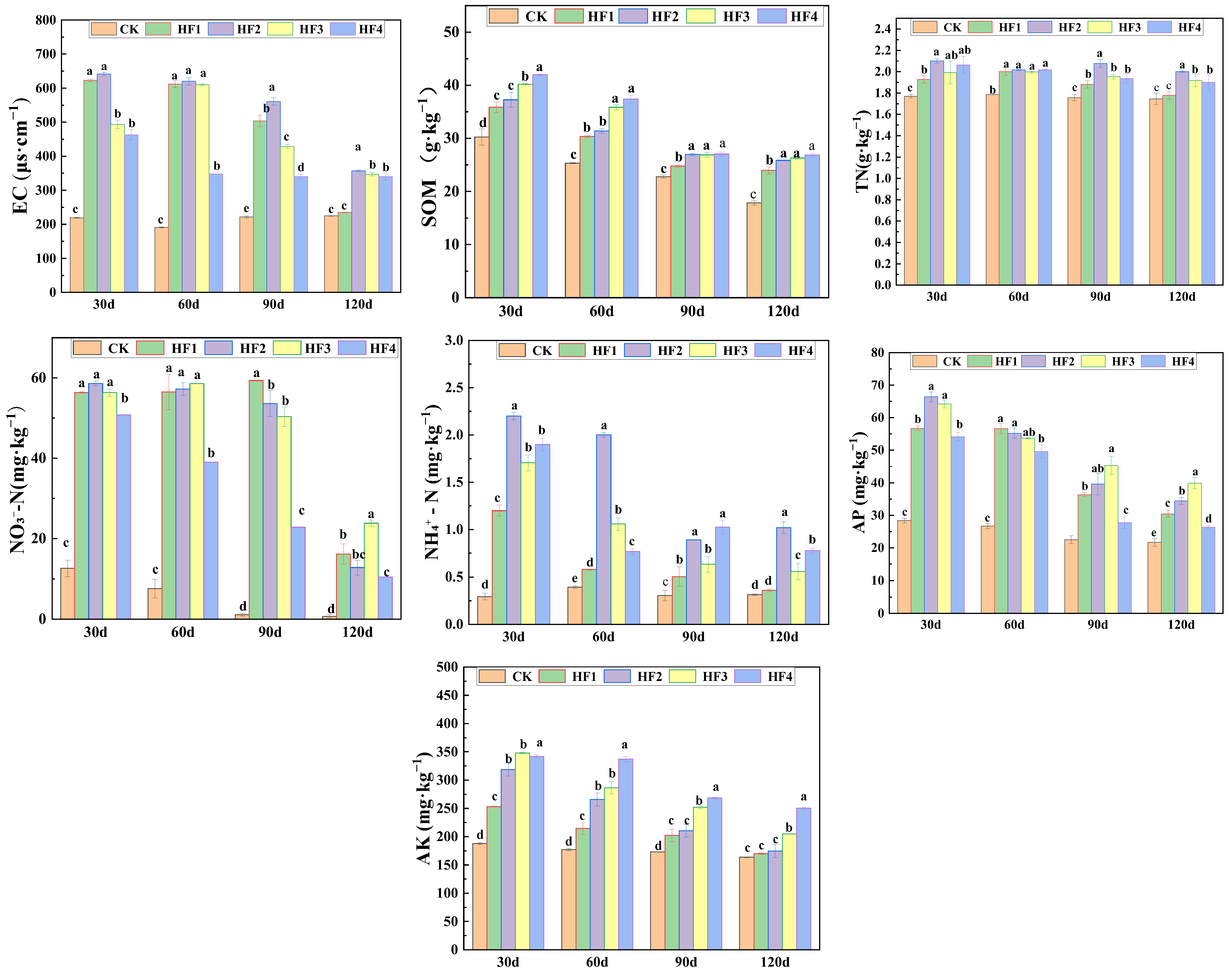
4.1. Impact of Combined Application of Biogas Slurry on Silage Corn and Soil Physicochemical Properties
4.2. Succession Characteristics of Soil Microbial Community Structure of Silage Corn Under the Combined Application of Biogas Slurry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.P.; Sun, G.F.; Wang, Z.C.; Zong, J.; Zhou, W.; Sheng, J. Analysis of rice seedling growth restriction factors under biogas slurry application. J. Agro-Environ. Sci. 2021, 40, 2537–2543. [Google Scholar]
- Gao, X. Effects of Integrated Application of Biogas Slurry Water and Fertilizer Instead of Chemical Fertilizer on Greenhouse Crops and Soil Properties. Master’s Thesis, Northwest A & F University, Xianyang, China, 2019. [Google Scholar]
- Wentzel, S.; Schmidt, R.; Piepho, H.-P.; Semmler-Busch, K.; Joergensen, R.G. Response of soil fertilityindices to long-term application of biogas and raw slurry under organic farming. Appl. Soil Ecol. 2015, 96, 99–107. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Ren, T.; Yu, X.; Liao, J.; Du, Y.; Zhu, Y.; Jin, L.; Wang, B.; Xu, H.; Xiao, W.; Chen, H.Y.H.; et al. Application of biogas slurry rather than biochar increases soil microbial functional gene signal intensity and diversity in a poplar plantation. Soil Biol. Biochem. 2020, 146, 107825. [Google Scholar] [CrossRef]
- Shi, Y.; Rahaman, M.A.; Zhang, Q.; Zhan, X.; Zheng, L. Effects of partial substitution of chemical fertilizer with biogas slurry on nitrous oxide emissions and the related nitrifier and denitrifier in a saline-alkali soil. Environ. Technol. Innov. 2022, 28, 102900. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol. Biochem. 2010, 43, 795–803. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Yu, X.Y.; Zhu, Y.J.; Jin, L.; Wang, B.T.; Xu, X.; Zou, X.M.; Ruan, H.H.; Jin, F.J. Contrasting responses of fungal and bacterial communities to biogas slurry addition in rhizospheric soil of poplar plantations. Appl. Soil Ecol. 2022, 175, 104427. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.X.; Sun, X.M.; Chen, N.L. Effects of Biogas Slurry on Soil Microbial Functional Diversity of Vineyard in Hexi Oasis. Soils 2020, 52, 1203–1211. [Google Scholar]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Mingorance.;F. Fornasier.;C. Mondini. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Song, Y.G.; Xia, Y.X.; Zhang, Y.P.; Wang, L. Effects of biogas slurry nitrogen replacing fertilizer nitrogen on rice yield and soil physicochemical properties. Mod. Agric. Sci. Technol. 2023, 15–18. [Google Scholar] [CrossRef]
- Wang, J. Effects of Biogas Slurry Instead of Chemical Fertilizer Topdressing on Pepper Growth and Soil Quality in Facilities. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2022. [Google Scholar]
- Wang, W.; Zhu, S.D.; Yuan, L.Y.; Hao, X.J.; Li, R.; Long, J. Application of Biogas Liquid and Biogas Residue in Soil less Culture of Peppers. J. Anhui Agri. Sci. 2009, 37, 11499–11500. [Google Scholar]
- Liu, Y.D.; Chi, F.Q.; Gu, S.Y.; Zhang, J.M.; Zhang, Y.W.; Kuang, E.J.; Su, Q.R. Effects of Organic Fertilizer Substituted by Mineral Nitrogen Fertilizer on Yield of Spring Wheat and Nitrogen Utilization Efficiency. Chin. J. Soil Sci. 2020, 51, 442–448. [Google Scholar]
- Xin, X.; Qin, S.; Zhang, J.; Zhu, A.; Yang, W.; Zhang, X. Yield, phosphorus use efficiency and balance response to substituting long-term chemical fertilizer use with organic manure in a wheat-maize system. Field Crops Res. 2017, 208, 27–33. [Google Scholar] [CrossRef]
- Wu, C.L.; Shen, Q.R.; Zhang, S.L.; Zhang, Y.J.; Xu, Y.C. Mechanisms for the Increased Utilization of Fertilizer N under the Integrated Use of Inorganic and Organic Fertilizers in a Winter Wheat-Rice Rotation System I: Fate of Fertilizer 15N during Winter Wheat Growth Stages. Chin. J. Soil Sci. A 2010, 47, 1170–1179. [Google Scholar]
- Ren, K.Y.; Lu, D.M.; Zou, H.Q.; Wang, H.Y.; Xu, F.H.; Lu, C.A.; Duan, Y.H. Effects of substituting manure for fertilizer on yield and nitrogen content of rice grain in the Yangtze River basin. J. Agric. Resour. Environ. 2022, 39, 716–725. [Google Scholar]
- Breda, C.C.; Soares, M.B.; Tavanti, R.F.R.; Viana, D.G.; da Silva Freddi, O.; Piedade, A.R.; Mahl, D.; Traballi, R.C.; Guerrini, I.A. Amaral Guerrini. Successive sewage sludge fertilization: Recycling for sustainable agriculture. Waste Manag. 2020, 109, 38–50. [Google Scholar] [CrossRef]
- Liu, B.X.; Wang, Z.G.; Liang, H.Y.; Yang, M.S. Effects of salt stress on physiological characters and salt-tolerance of Ulmus pumila in differ ent habitats. Chin. J. Appl. Ecol. 2012, 23, 1481–1489. [Google Scholar]
- Zhu, W.; Zhang, X.B.; Geng, X.Y.; Chen, Y.L.; Dai, Q.G.; Zhou, G.S.; Wei, H.H.; Meng, T.Y. Research Progress on the Effects of Salt Stress and Drought on the Root Morphophysiology and Yield Formation of Rice and Their Mechanisms. China Rice 2023, 29, 34–40. [Google Scholar]
- Yu, F.B.; Luo, X.P.; Song, C.F.; Zhang, M.X.; Shan, S.D. Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2010, 60, 262–268. [Google Scholar] [CrossRef]
- Qian, J.H.; Lin, C.; Wang, J.H.; Wang, X.L. Effect of biogas residues on apples’ quality and soil fertility. Renew. Energy 2005, 4, 34–36. [Google Scholar]
- Yao, Y.S.; Sun, X.L.; Liu, W.Y.; You, Y.J.; Li, F.Y. Effects of straw returning and biogas slurry combined application on salinized soil nutrients and enzyme activities. Agric. Res. Arid. Areas 2024, 42, 117–126. [Google Scholar]
- Li, S.S.; Yang, J.C.; Jiang, H.M.; Zhang, J.F.; Li, L.L.; Zhang, W.Q.; Pan, P.; Guo, J.; Liu, L. Effects of Organic and Inorganic Fertilizer on Nitrogen Pool and Distribution of Residual N Fractions in Flu vo-aquic Soil Under the Winter Wheat System. J. Agro-Environ. Sci. 2013, 32, 1185–1193. [Google Scholar]
- Zhu, W.; Zhang, X.B.; Geng, X.Y.; Chen, Y.L.; Dai, Q.G.; Zhou, G.S.; Wei, H.H.; Meng, T.Y. Nitrogen leaching losses following biogas slurry irrigation to purple soil of the Three Gorges Reservoir Area. Environ. Sci. Pollut. Res. 2018, 25, 29096–29103. [Google Scholar]
- Luo, W.; Zhang, Z.H.; Wu, J.; Lai, X.; Meng, X.X.; Huang, B.H. Effects of Biogas Slurry on Soil Nitrogen, Phosphorus and Potassium Contents and Balance in Chengdu Plain. J. Soil Water Conserv. 2019, 33, 185–191. [Google Scholar]
- Yang, J.; Xu, X.Y. Influence of Applying Biogas Slurry on Yield and Quality of Lettuce and Soil Environmen. China Biogas 2013, 31, 51–54. [Google Scholar]
- Wang, W.P.; Zhu, F.X.; Chen, X.Y.; Xue, Z.Y.; Hong, C.L.; Yao, Y.L. Effects of Biogas Slurry Irrigation on the Yield of Open-field Watermelon and Soil Environment. J. Zhejiang Agric. Sci. 2015, 56, 1024–1026. [Google Scholar]
- Xiao, Y.; Tian, L.; Lu, Y.C.; Xu, L.J.; Huang, Y.X.; Meng, K.; Liu, Z.Q. Effects of Combined Application of Biogas Slurry, Biogas Residue and Chemical Fertilizeron Soil Fertility. Chin. Agric. Sci. Bull. 2016, 32, 78–81. [Google Scholar]
- Zhang, W.D.; Yin, F.; Xu, R.; Li, J.C.; Xu, L.; He, F.Q.; Xue, W.J.; Chen, Y.B. Effect of Biogas Liquid on Biological Properties of Soil. Hubei Agric. Sci. 2009, 48, 2403–2407. [Google Scholar]
- Zheng, X.B.; Fan, J.B.; Cui, J.; Xu, L.; Zhu, Z.Q.; Zhou, J.; He, Y.Q. Analysis on metabolic characteristics and functional diversity of soil edaphon communities in upland red soil under biogas slurry application. Acta Ecol. Sin. 2016, 36, 5865–5875. [Google Scholar]
- Zhang, H.; Li, S.; Zheng, X.; Zhang, J.Q.; Bai, N.L.; Zhang, H.Y.; Lv, W.G. Effects of Biogas Slurry Combined With Chemical Fertilizer on Soil Bacterial and Fungal Community Composition in a Paddy Field. Front. Microbiol. 2021, 12, 655515. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.J.; Zhang, J.T.; Li, L.; Wang, Q.; Diao, F.W.; Gao, M.; Wang, X.H.; Shi, X.Y. Effects of biogas slurry combined with chem ical fertilizer on Allium fistulosum yields, soil nutrients, microorganisms, and enzymes activities. Chin. J. Eco-Agric. 2024, 32, 95–105. [Google Scholar]
- Xing, P.F.; Wu, X.S.; Gao, S.C.; Li, H.J.; Zhao, T.K.; Zhou, X.L.; Shen, D.L.; Sun, J.D. Effects of Different Fertilization on Soil Microbial Community and Functional Diversity in Corn-Wheat Crop Rotation. J. Microbiol. 2016, 36, 22–29. [Google Scholar]
- Li, Y.; Shen, C.; Wang, Y.; Shen, C.; Wang, Y.M.; Xu, L.; Zhao, Y.L.; Yi, S.Q.; Zuo, W.G.; Yao, R.G.; et al. Alleviated environmental constraints and restructured fungal microbiome facilitate aggregate formation and stabilization in coastal mudflat saline soil amended by sewage sludge. Land Degrad. Dev. 2023, 34, 3064–3075. [Google Scholar] [CrossRef]
- Kang, L.Y.; Li, Z.L.; Feng, X.H.; Liang, C.F.; Wu, Z.; Zhou, W.; Liu, X.N.; Yang, Y.H.; Chen, L.Y. Photo-produced aromatic compounds stimulate microbial degradation of dissolved organic carbon in thermokarst lakes. Nat. Commun. 2023, 14, 3681. [Google Scholar]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota Members Dominate Fungal Communities during Straw Residue Decomposition in Arable Soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Kang, W.Q.; Xu, Y.L.; Xu, X.L.; Zhu, Y.Q.; Xu, W.Z.; Lin, C.W.; Li, X.Y.; Nie, G. Effects of Different Biogas Slurry Application Rates on Soil Nutrients and Soil Bacterial Community Structure. Chin. J. Grassl. 2022, 44, 75–83. [Google Scholar]


| TN | TP | TK | pH | Ni | As | Cu | |
| (g·kg−1) | (mg·kg−1) | (g·kg−1) | (μg·L−1) | (μg·L−1) | (mg·L−1) | ||
| Biogas Slurry | 3.38 | 254.00 | 2.54 | 8.20 | 598.09 | 84.78 | 4.98 |
| Zn | Cr | Cd | Pb | Fe | Ca | Mg | |
| (mg·L−1) | (μg·L−1) | (μg·L−1) | (μg·L−1) | (mg·L−1) | (mg·L−1) | (mg·L−1) | |
| Biogas Slurry | 21.47 | 634.50 | 9.28 | 120.96 | 91.48 | 783.98 | 634.76 |
| Treatment | Chao1 | Shannon | Coverage |
|---|---|---|---|
| CK | 4029.55 ± 46.01 c | 6.52 ± 0.02 c | 0.96 ± 0.00 a |
| HF1 | 4205.95 ± 13.98 b | 6.59 ± 0.03 b | 0.96 ± 0.00 a |
| HF2 | 4288.87 ± 20.64 a | 6.63 ± 0.03 ab | 0.96 ± 0.00 a |
| HF3 | 4266.93 ± 33.58 a | 6.64 ± 0.02 a | 0.96 ± 0.00 a |
| HF4 | 4151.81 ± 76.79 b | 6.64 ± 0.03 a | 0.96 ± 0.00 a |
| Treatment | Chao1 | Shannon | Coverage |
|---|---|---|---|
| CK | 556.89 ± 22.65 b | 4.11 ± 0.12 b | 0.99 ± 0.00 a |
| HF1 | 584.52 ± 16.40 ab | 4.22 ± 0.21 ab | 0.99 ± 0.00 a |
| HF2 | 606.93 ± 12.03 a | 4.48 ± 0.11 a | 0.99 ± 0.00 a |
| HF3 | 584.60 ± 17.07 ab | 4.21 ± 0.26 ab | 0.99 ± 0.00 a |
| HF4 | 557.69 ± 13.07 b | 3.75 ± 0.10 c | 0.99 ± 0.00 a |
| pH | SOM | EC | AP | AK | TN | NO3−-N | NH4+-N | ||
|---|---|---|---|---|---|---|---|---|---|
| bacterial | Chao1 | −0.525 ** | −0.100 | 0.375 | 0.685 *** | 0.598 ** | 0.059 | 0.648 *** | 0.191 |
| Shannon | −0.683 *** | 0.382 | 0.737 *** | 0.673 *** | 0.752 *** | 0.573 ** | 0.672 *** | 0.32 | |
| fungal | Chao1 | −0.464 * | −0.395 | 0.178 | 0.535 ** | 0.507 * | −0.328 | 0.457 * | 0.246 |
| Shannon | −0.237 | −0.615 ** | −0.153 | 0.371 | 0.256 | −0.669 *** | 0.236 | 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Cheng, Y.; Wu, X.; Zhou, J.; Liu, X. Effects of Combined Application of Biogas Slurry and Chemical Fertilizers on Silage Corn, Soil Nutrients, and Microorganisms. Microorganisms 2025, 13, 2. https://doi.org/10.3390/microorganisms13010002
Yang W, Cheng Y, Wu X, Zhou J, Liu X. Effects of Combined Application of Biogas Slurry and Chemical Fertilizers on Silage Corn, Soil Nutrients, and Microorganisms. Microorganisms. 2025; 13(1):2. https://doi.org/10.3390/microorganisms13010002
Chicago/Turabian StyleYang, Wencong, Yijing Cheng, Xia Wu, Jia Zhou, and Xiuping Liu. 2025. "Effects of Combined Application of Biogas Slurry and Chemical Fertilizers on Silage Corn, Soil Nutrients, and Microorganisms" Microorganisms 13, no. 1: 2. https://doi.org/10.3390/microorganisms13010002
APA StyleYang, W., Cheng, Y., Wu, X., Zhou, J., & Liu, X. (2025). Effects of Combined Application of Biogas Slurry and Chemical Fertilizers on Silage Corn, Soil Nutrients, and Microorganisms. Microorganisms, 13(1), 2. https://doi.org/10.3390/microorganisms13010002





