Impact of the COVID-19 Pandemic on Epidemiological Trends in Pediatric Cervical Abscess-Forming Infections
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, F.; Allen, S.M.; Stocks, R.M.; Thompson, J.W. Deep neck infection. Otolaryngol. Clin. N. Am. 2008, 41, 459–483, vii. [Google Scholar] [CrossRef]
- Page, N.C.; Bauer, E.M.; Lieu, J.E. Clinical features and treatment of retropharyngeal abscess in children. Otolaryngol. Head Neck Surg. 2008, 138, 300–306. [Google Scholar] [CrossRef]
- McClay, J.E.; Murray, A.D.; Booth, T. Intravenous antibiotic therapy for deep neck abscesses defined by computed tomography. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.; Brown, C.; Mills, N.; Spielmann, P.; Neeff, M. To drain or not to drain-management of pediatric deep neck abscesses: A case-control study. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Sichel, J.Y.; Dano, I.; Hocwald, E.; Biron, A.; Eliashar, R. Nonsurgical management of parapharyngeal space infections: A prospective study. Laryngoscope 2002, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Kharel, B.; Shahi, K.; Gurung, U. Antibiotic Resistance Pattern in Pediatric Deep Neck Space Infection. Int. Arch. Otorhinolaryngol. 2022, 26, e585–e591. [Google Scholar] [CrossRef]
- Nagasawa, M.; Udagawa, T.; Okada, M.; Nakagawa, R.; Yokoyama, H.; Kato, T.; Furuya, M.; Sakaguchi, H. COVID-19 pandemic-altered epidemiology of respiratory syncytial virus and human metapneumovirus infections in young children. GHM Open 2024, 4, 47–49. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Mustard, C.; Smith, P.; Siddiqi, A.; Cheng, Y.; Burdorf, A.; Rugulies, R. Work as a social determinant of health in high-income countries: Past, present, and future. Lancet 2023, 402, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liu, H. Impact of non-pharmaceutical interventions during the COVID-19 pandemic on common childhood respiratory viruses—An epidemiological study based on hospital data. Microbes Infect. 2022, 24, 104911. [Google Scholar] [CrossRef]
- Yang, M.C.; Su, Y.T.; Chen, P.H.; Tsai, C.C.; Lin, T.I.; Wu, J.R. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front. Cell Infect. Microbiol. 2023, 13, 1200617. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; De Guido, C.; Pappalardo, M.; Laudisio, S.; Meccariello, G.; Capoferri, G.; Rahman, S.; Vicini, C.; Principi, N. Retropharyngeal, Parapharyngeal and Peritonsillar Abscesses. Children 2022, 9, 618. [Google Scholar] [CrossRef]
- Carbone, P.N.; Capra, G.G.; Brigger, M.T. Antibiotic therapy for pediatric deep neck abscesses: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Tansey, J.B.; Hamblin, J.; Mamidala, M.; Thompson, J.; McLevy, J.; Wood, J.; Sheyn, A. Dexamethasone Use in the Treatment of Pediatric Deep Neck Space Infections. Ann. Otol. Rhinol. Laryngol. 2020, 129, 376–379. [Google Scholar] [CrossRef]
- Akhavan, M. Ear, Nose, Throat: Beyond Pharyngitis: Retropharyngeal Abscess, Peritonsillar Abscess, Epiglottitis, Bacterial Tracheitis, and Postoperative Tonsillectomy. Emerg. Med. Clin. N. Am. 2021, 39, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Center TMIDS. Available online: https://idsc.tmiph.metro.tokyo.lg.jp/weekly/ (accessed on 24 September 2024).
- Marom, T.; Alvarez-Fernandez, P.E.; Jennings, K.; Patel, J.A.; McCormick, D.P.; Chonmaitree, T. Acute bacterial sinusitis complicating viral upper respiratory tract infection in young children. Pediatr. Infect. Dis. J. 2014, 33, 803–808. [Google Scholar] [CrossRef]
- Brook, I. Acute sinusitis in children. Pediatr. Clin. N. Am. 2013, 60, 409–424. [Google Scholar] [CrossRef]
- Autio, T.J.; Tapiainen, T.; Koskenkorva, T.; Närkiö, M.; Lappalainen, M.; Nikkari, S.; Hemmilä, H.; Koskela, K.A.; Koskela, M.; Koivunen, P.; et al. The role of microbes in the pathogenesis of acute rhinosinusitis in young adults. Laryngoscope 2015, 125, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Alho, O.P. Nasal airflow, mucociliary clearance, and sinus functioning during viral colds: Effects of allergic rhinitis and susceptibility to recurrent sinusitis. Am. J. Rhinol. 2004, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Foster, D.; Nicoli, E.; Trotter, C.; Vipond, B.; Muir, P.; Gonçalves, G.; Januário, L.; Finn, A. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in children attending daycare. Pediatr. Infect. Dis. J. 2013, 32, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, K.; Harigopal, S.; Reddy, V.; Taylor, N.; van Saene, H.K. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 2006, 61, 611–615. [Google Scholar] [CrossRef]
- Lehtinen, P.; Jartti, T.; Virkki, R.; Vuorinen, T.; Leinonen, M.; Peltola, V.; Ruohola, A.; Ruuskanen, O. Bacterial coinfections in children with viral wheezing. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ren, A.; Wang, X.; Fan, X.; Zhao, Y.; Gao, G.F.; Cleary, P.; Wang, B. Influenza viral neuraminidase primes bacterial coinfection through TGF-β-mediated expression of host cell receptors. Proc. Natl. Acad. Sci. USA 2015, 112, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Van Ewijk, B.E.; Wolfs, T.F.; Aerts, P.C.; Van Kessel, K.P.; Fleer, A.; Kimpen, J.L.; Van der Ent, C.K. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr. Res. 2007, 61, 398–403. [Google Scholar] [CrossRef]
- Rynda-Apple, A.; Robinson, K.M.; Alcorn, J.F. Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease. Infect. Immun. 2015, 83, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Kolls, J.K.; Alcorn, J.F. The immunology of influenza virus-associated bacterial pneumonia. Curr. Opin. Immunol. 2015, 34, 59–67. [Google Scholar] [CrossRef]
- Willen, L.; Ekinci, E.; Cuypers, L.; Theeten, H.; Desmet, S. Infant Pneumococcal Carriage in Belgium Not Affected by COVID-19 Containment Measures. Front. Cell Infect. Microbiol. 2021, 11, 825427. [Google Scholar] [CrossRef]
- McCullers, J.A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; van der Beek, B.A.; Ben-Shimol, S.; Greenberg, D.; Shemer-Avni, Y.; Weinberger, D.M.; Danino, D. The COVID-19 pandemic as an opportunity for unravelling the causative association between respiratory viruses and pneumococcus-associated disease in young children: A prospective study. EBioMedicine 2023, 90, 104493. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.R.; Kasper, D.L.; Panzo, R.J.; Chitnis, T.; Grusby, M.J.; Sayegh, M.H.; Tzianabos, A.O. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J. Immunol. 2003, 170, 1958–1963. [Google Scholar] [CrossRef]
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Wu, Q.; Pan, X.; Han, D.; Ma, Z.; Zhang, H. New Insights into the Epidemiological Characteristics of Mycoplasma pneumoniae Infection before and after the COVID-19 Pandemic. Microorganisms 2024, 12, 2019. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Nagaoka, K.; Kawasuji, H.; Kawagishi, T.; Fuchigami, T.; Ikeda, K.; Kanatani, J.I.; Doi, T.; Oishi, K.; Yamamoto, Y. COVID-19 complicated with severe M1(UK)-lineage Streptococcus pyogenes infection in elderly patients: A report of two cases. Int. J. Infect. Dis. 2024, 148, 107246. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.; Bajanca-Lavado, M.P.; Mamede, R.; Araújo Carvalho, A.; Rodrigues, F.; Melo-Cristino, J.; Ramirez, M.; Friães, A. Sustained increase of paediatric invasive Streptococcus pyogenes infections dominated by M1(UK) and diverse emm12 isolates, Portugal, September 2022 to May 2023. Euro Surveill 2023, 28, 2300427. [Google Scholar] [CrossRef] [PubMed]
- Peetermans, M.; Matheeussen, V.; Moerman, C.; De Rydt, F.; Thieren, S.; Pollet, E.; Casaer, M.; De Backer, B.; De Paep, R.; Debaveye, Y.; et al. Clinical and molecular epidemiological features of critically ill patients with invasive group A Streptococcus infections: A Belgian multicenter case-series. Ann. Intensive Care 2024, 14, 19. [Google Scholar] [CrossRef]
- Vieira, A.; Wan, Y.; Ryan, Y.; Li, H.K.; Guy, R.L.; Papangeli, M.; Huse, K.K.; Reeves, L.C.; Soo, V.W.C.; Daniel, R.; et al. Rapid expansion and international spread of M1(UK) in the post-pandemic UK upsurge of Streptococcus pyogenes. Nat. Commun. 2024, 15, 3916. [Google Scholar] [CrossRef]
- Tromp, A.T.; van Strijp, J.A.G. Studying Staphylococcal Leukocidins: A Challenging Endeavor. Front. Microbiol. 2020, 11, 611. [Google Scholar] [CrossRef]
- Diep, B.A.; Otto, M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008, 16, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Irenji, N.; Pillai, S.K.G.; West-Jones, J.S. Serious life-threatening multifocal infection in a child, caused by Panton-Valentine leucocidin-producing Staphylococcus aureus (PVL-MSSA). BMJ Case Rep. 2018, 2018, bcr2017222138. [Google Scholar] [CrossRef] [PubMed]
- Gopal Rao, G.; Batura, R.; Nicholl, R.; Coogan, F.; Patel, B.; Bassett, P.; Kearns, A.M. Outbreak report of investigation and control of an outbreak of Panton-Valentine Leukocidin-positive methicillin-sensitive Staphylococcus aureus (PVL-MSSA) infection in neonates and mothers. BMC Infect. Dis. 2019, 19, 178. [Google Scholar] [CrossRef]
- Sunagar, R.; Hegde, N.R.; Archana, G.J.; Sinha, A.Y.; Nagamani, K.; Isloor, S. Prevalence and genotype distribution of methicillin-resistant Staphylococcus aureus (MRSA) in India. J. Glob. Antimicrob. Resist. 2016, 7, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Singh, W.; Raj, V.S.; Pokhrel, B.M.; Mohapatra, T.M. High prevalence of Panton-Valentine leukocidin (PVL) genes in nosocomial-acquired Staphylococcus aureus isolated from tertiary care hospitals in Nepal. Biomed. Res. Int. 2014, 2014, 790350. [Google Scholar] [CrossRef]
- Tabassum, H.; Gull, M.; Rasheed, A.; Bano, A.; Ejaz, H.; Javed, N. Molecular analysis of Panton-Valentine Leucocidin (pvl) gene among MRSA and MSSA isolates. Braz. J. Biol. 2023, 83, e250351. [Google Scholar] [CrossRef]
- Lee, Y.Q.; Kanagalingam, J. Bacteriology of deep neck abscesses: A retrospective review of 96 consecutive cases. Singapore Med. J. 2011, 52, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Tanriverdi, M.H.; Gün, R.; Yorgancilar, A.E.; Yildirim, M.; Tekbaş, G.; Palanci, Y.; Meriç, K.; Topçu, I. Deep neck space infections: A retrospective review of 173 cases. Am. J. Otolaryngol. 2012, 33, 56–63. [Google Scholar] [CrossRef]
- Cengiz, A.B.; Kara, A.; Kanra, G.; Seçmeer, G.; Ceyhan, M.; Ozen, M. Acute neck infections in children. Turk. J. Pediatr. 2004, 46, 153–158. [Google Scholar] [PubMed]

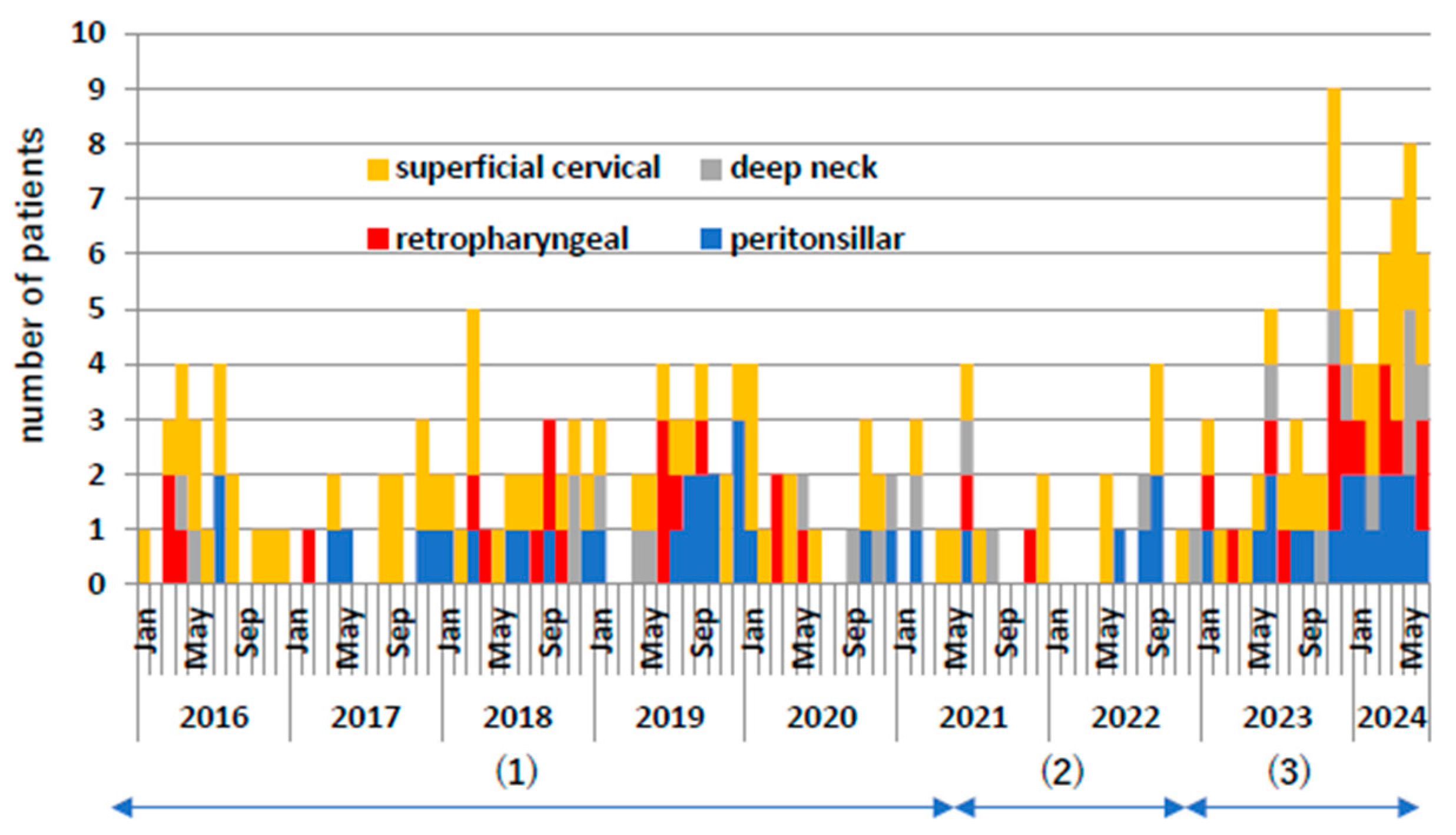
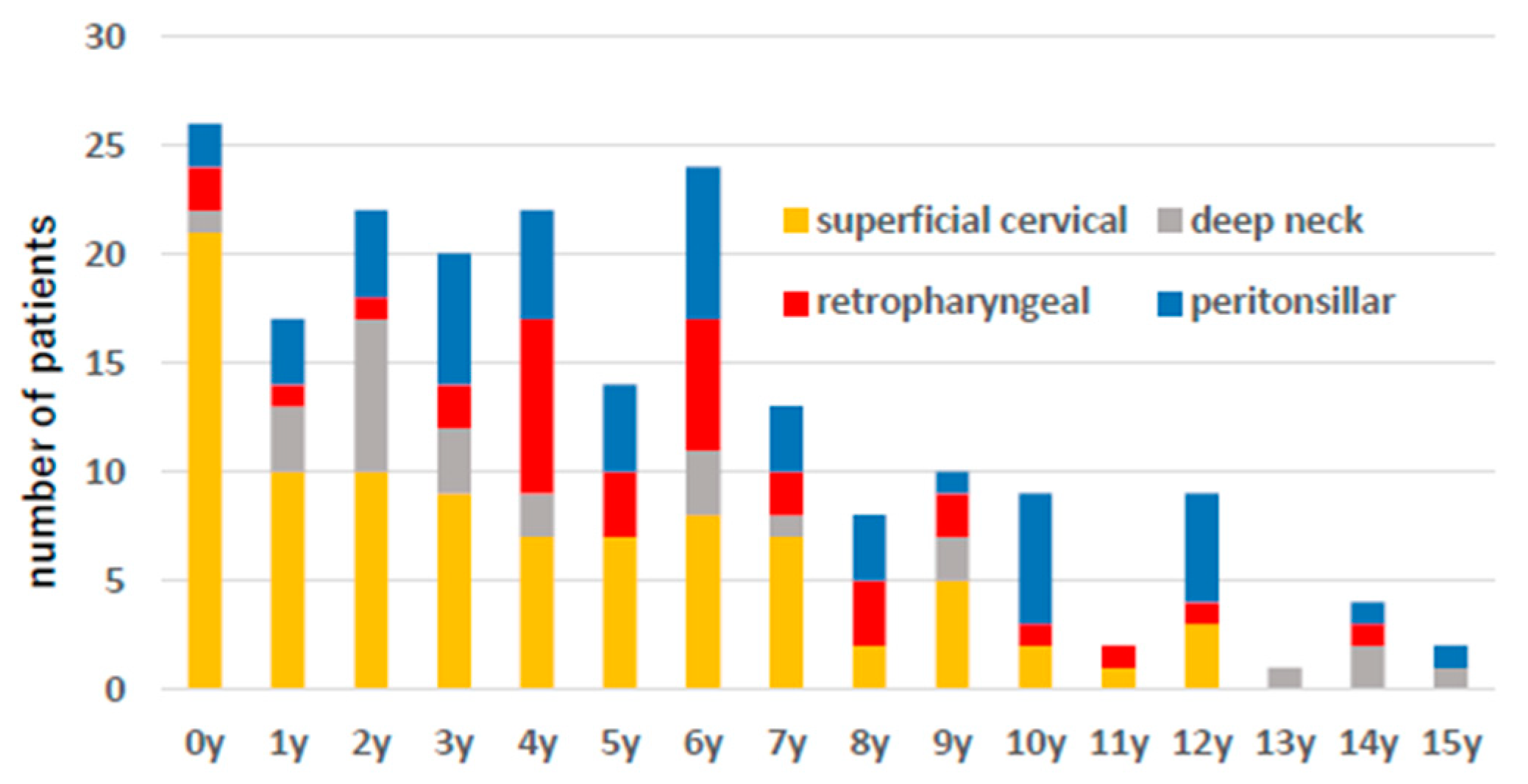
| n | n/Month | Age (y) | Antibiotics Use Prior to Admission | GAS Ag Positive (Test) | Puncture | Cultured Bacteria | Steroid Use | Antibiotics Therapy | Hospital Stay (Days) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iv (Days) | Po (Days) | iv + po (Days) | ||||||||||
| superficial cervical | 96 | 0.94 ± 0.92 | 3.71 ± 3.37 | 43 | 11(57) | 57 | 48 | 1 | 9.1 ± 4.6 | 7.2 ± 5.1 | 15.8 ± 7.7 | 9.9 ± 4.3 |
| ① 2016.1–2020.6 | 51 | 0.96 ± 0.79 | 3.35 ± 3.20 | 24 | 2(28) | 38 | 31 | 0 | 9.0 ± 3.6 | 7.6 ± 6.4 | 15.9 ± 8.9 | 9.7 ± 3.8 |
| ② 2020.7–2022.12 | 15 | 0.50 ± 0.72 | 2.93 ± 3.32 | 8 | 0(7) | 11 | 11 | 0 | 9.9 ± 5.2 | 6.7 ± 2.9 | 16.6 ± 6.5 | 12.1 ± 5.9 |
| ③ 2023.1–2024.6 | 30 | 1.67 ± 1.11 | 4.72 ± 3.47 | 11 | 9(22) | 8 | 6 | 1 | 9.2 ± 5.6 | 6.5 ± 2.5 | 15.0 ± 4.9 | 9.4 ± 3.2 |
| deep cervical | 111 | 1.09 ± 1.26 | 5.88 ± 3.77 | 35 | 30(58) | 40 | 31 | 17 | 10.2 ± 5.3 | 7.8 ± 6.8 | 17.2 ± 9.2 | 10.7 ± 5.6 |
| ① 2016.1–2020.6 | 50 | 0.93 ± 0.96 | 5.12 ± 3.61 | 18 | 19(33) | 19 | 16 | 9 | 11.0 ± 6.2 | 7.4 ± 5.5 | 17.3 ± 8.5 | 11.4 ± 6.1 |
| ② 2020.7–2022.12 | 18 | 0.60 ± 0.84 | 5.90 ± 4.83 | 4 | 1(7) | 8 | 7 | 3 | 10.5 ± 5.5 | 8.9 ± 9.0 | 18.8 ± 11.0 | 11.0 ± 6.7 |
| ③ 2023.1–2024.6 | 43 | 2.39 ± 1.70 | 6.56 ± 3.24 | 13 | 10(18) | 13 | 8 | 5 | 8.9 ± 3.8 | 7.8 ± 7.4 | 16.1 ± 9.4 | 9.7 ± 4.4 |
| retropharyngeal | 34 | 0.33 ± 0.66 | 5.74 ± 3.15 | 13 | 9(15) | 13 | 8 | 8 | 11.8 ± 5.4 | 7.1 ± 4.1 | 18.4 ± 6.1 | 11.9 ± 5.6 |
| ① 2016.1–2020.6 | 18 | 0.33 ± 0.67 | 5.11 ± 3.03 | 7 | 7(11) | 8 | 6 | 4 | 13.4 ± 6.2 | 6.4 ± 4.4 | 19.8 ± 6.4 | 13.2 ± 6.6 |
| ② 2020.7–2022.12 | 2 | 1 | 0(1) | 0 | 0 | 0 | ||||||
| ③ 2023.1–2024.6 | 14 | 0.78 ± 0.85 | 6.79 ± 2.81 | 5 | 2(3) | 5 | 2 | 4 | 9.3 ± 3.3 | 6.2 ± 4.0 | 15.5 ± 4.3 | 9.8 ± 3.4 |
| peritonsillar | 51 | 0.50 ± 0.72 | 6.16 ± 3.79 | 15 | 14(30) | 20 | 17 | 6 | 7.8 ± 3.8 | 7.8 ± 7.4 | 14.6 ± 9.9 | 8.4 ± 4.4 |
| ① 2016.1–2020.6 | 24 | 0.44 ± 0.71 | 5.38 ± 3.57 | 8 | 8(18) | 10 | 9 | 4 | 8.2 ± 3.8 | 7.1 ± 5.4 | 14.1 ± 7.3 | 8.9 ± 4.0 |
| ② 2020.7–2022.12 | 8 | 0.27 ± 0.51 | 6.86 ± 5.14 | 1 | 1(3) | 2 | 2 | 1 | 6.3 ± 3.5 | 7.0 ± 5.8 | 13.3 ± 9.2 | 6.3 ± 4.4 |
| ③ 2023.1–2024.6 | 19 | 1.06 ± 0.78 | 6.85 ± 3.28 | 6 | 5(9) | 8 | 6 | 1 | 7.7 ± 3.9 | 8.9 ± 9.6 | 15.6 ± 12.5 | 8.4 ± 4.6 |
| deep neck | 26 | 0.25 ± 0.54 | 5.15 ± 4.43 | 7 | 7(13) | 7 | 6 | 3 | 13.1 ± 5.5 | 9.9 ± 8.1 | 21.3 ± 9.4 | 14.2 ± 5.5 |
| ① 2016.1–2020.6 | 8 | 0.15 ± 0.40 | 4.38 ± 4.69 | 3 | 4(4) | 1 | 1 | 1 | 15.8 ± 8.7 | 14.3 ± 6.1 | 24.4 ± 11.9 | 17.4 ± 6.3 |
| ② 2020.7–2022.12 | 8 | 0.27 ± 0.44 | 5.64 ± 4.62 | 2 | 0(3) | 6 | 5 | 2 | 13.6 ± 4.8 | 11.2 ± 11.5 | 23.1 ± 11.1 | 14.3 ± 6.2 |
| ③ 2023.1–2024.6 | 10 | 0.56 ± 0.83 | 5.29 ± 3.61 | 2 | 3(6) | 0 | 0 | 0 | 11.6 ± 2.8 | 8.0 ± 5.0 | 18.4 ± 5.3 | 13.0 ± 3.4 |
| Pathogen Detected | Total Number | Superficial Cervical | Retropharyngeal | Deep Neck | Peritonsillar |
|---|---|---|---|---|---|
| Fusobacterium spp. | 3 | 2 | 1 | ||
| Prevottella spp. | 1 | 1 | |||
| Staphylococcus aureus (MRSA) | 2 | 2 | |||
| Staphylococcus aureus (MSSA) | 29 | 27 | 1 | 1 | |
| Streptocuccus pyogenes | 15 | 4 | 2 | 9 | |
| Streptocuccus spp. | 21 | 12 | 3 | 3 | 3 |
| Peptostreptococcus spp. | 1 | 1 | |||
| Others * | 7 | 3 | 1 | 3 |
| Puncture | n | Total Antibiotics Therapy (Days) | Hospital Stay (Days) | |||||
|---|---|---|---|---|---|---|---|---|
| superficial cervical | ||||||||
| yes | 48 | 16.9 ± 9.6 | p = 0.35 | 10.7 ± 4.9 | p < 0.05 | |||
| no | 35 | 14.3 ± 2.8 | 8.9 ± 3.0 | |||||
| deep cervical | ||||||||
| yes | 39 | 15.0 ± 4.9 | p = 0.16 | 8.6 ± 4.0 | p < 0.01 | |||
| no | 60 | 18.6 ± 10.9 | 12.1 ± 6.0 | |||||
| retropharyngeal | ||||||||
| yes | 12 | 17.2 ± 4.2 | p = 0.62 | 10.4 ± 4.0 | p = 0.25 | |||
| no | 18 | 19.2 ± 6.9 | 12.9 ± 6.3 | |||||
| peritonsillar | ||||||||
| yes | 20 | 11.8 ± 2.2 | p = 0.42 | 6.2 ± 2.0 | p < 0.01 | |||
| no | 27 | 16.6 ± 12.5 | 10.0 ± 4.9 | |||||
| deep neck | ||||||||
| yes | 7 | 20.6 ± 4.3 | p = 0.43 | 12.4 ± 3.8 | p = 0.27 | |||
| no | 15 | 21.6 ± 11.0 | 15.1 ± 5.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, S.; Kishino, A.; Miyai, K.; Takishima, S.; Omori, T.; Furuno, H.; Iemura, R.; Ono, M.; Ogasawara, K.; Sutani, A.; et al. Impact of the COVID-19 Pandemic on Epidemiological Trends in Pediatric Cervical Abscess-Forming Infections. Microorganisms 2025, 13, 190. https://doi.org/10.3390/microorganisms13010190
Takahashi S, Kishino A, Miyai K, Takishima S, Omori T, Furuno H, Iemura R, Ono M, Ogasawara K, Sutani A, et al. Impact of the COVID-19 Pandemic on Epidemiological Trends in Pediatric Cervical Abscess-Forming Infections. Microorganisms. 2025; 13(1):190. https://doi.org/10.3390/microorganisms13010190
Chicago/Turabian StyleTakahashi, Shuhei, Ai Kishino, Kentaro Miyai, Shigeru Takishima, Tae Omori, Hidehiro Furuno, Ryosei Iemura, Makoto Ono, Keisuke Ogasawara, Akito Sutani, and et al. 2025. "Impact of the COVID-19 Pandemic on Epidemiological Trends in Pediatric Cervical Abscess-Forming Infections" Microorganisms 13, no. 1: 190. https://doi.org/10.3390/microorganisms13010190
APA StyleTakahashi, S., Kishino, A., Miyai, K., Takishima, S., Omori, T., Furuno, H., Iemura, R., Ono, M., Ogasawara, K., Sutani, A., & Nagasawa, M. (2025). Impact of the COVID-19 Pandemic on Epidemiological Trends in Pediatric Cervical Abscess-Forming Infections. Microorganisms, 13(1), 190. https://doi.org/10.3390/microorganisms13010190





