The Response Regulator OmpR Negatively Controls the Expression of Genes Implicated in Tilimycin and Tilivalline Cytotoxin Production in Klebsiella oxytoca
Abstract
1. Introduction
2. Materials and Methods
3. Results
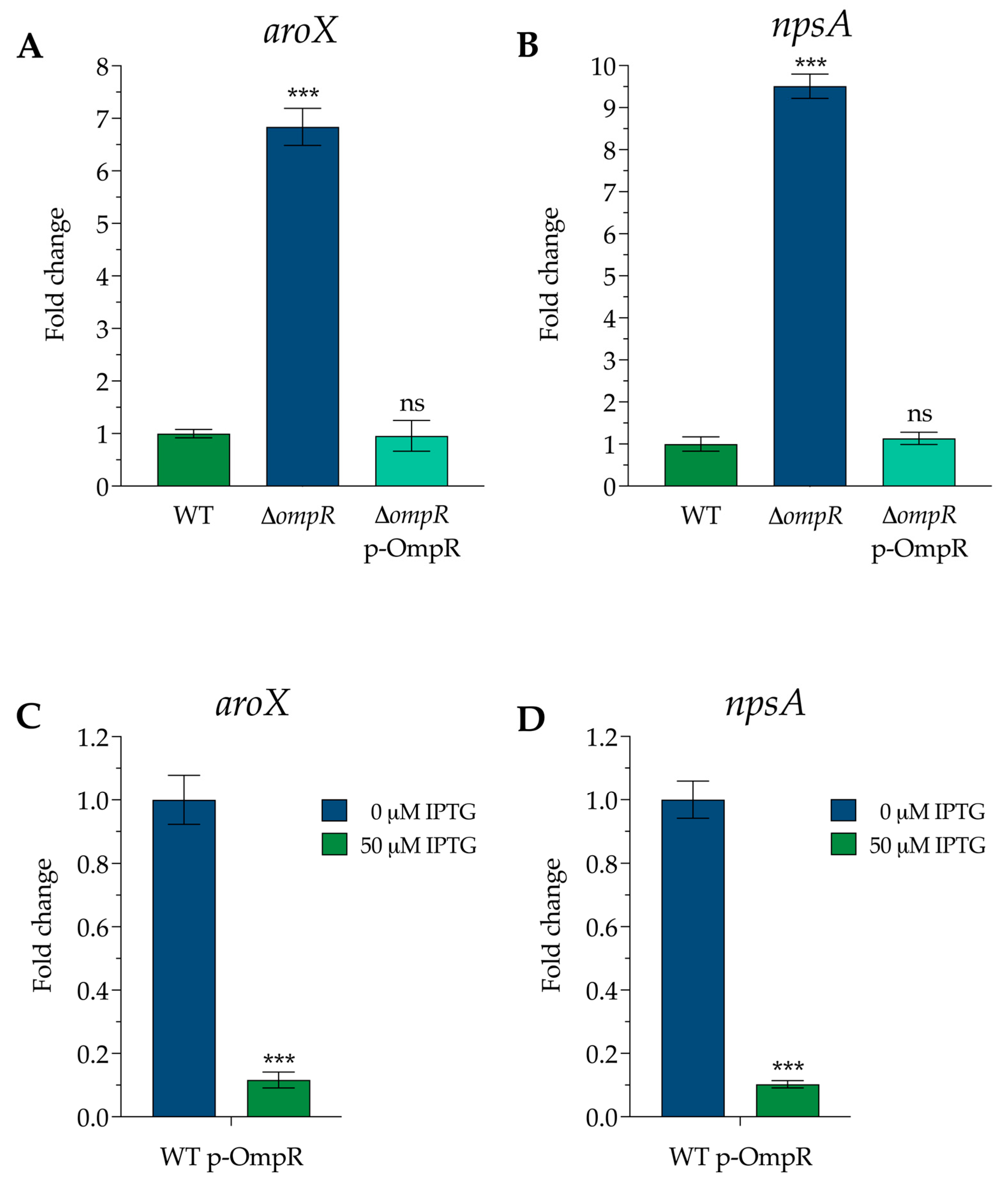
3.1. OmpR Is a Repressor of aroX and npsA Gene Expression
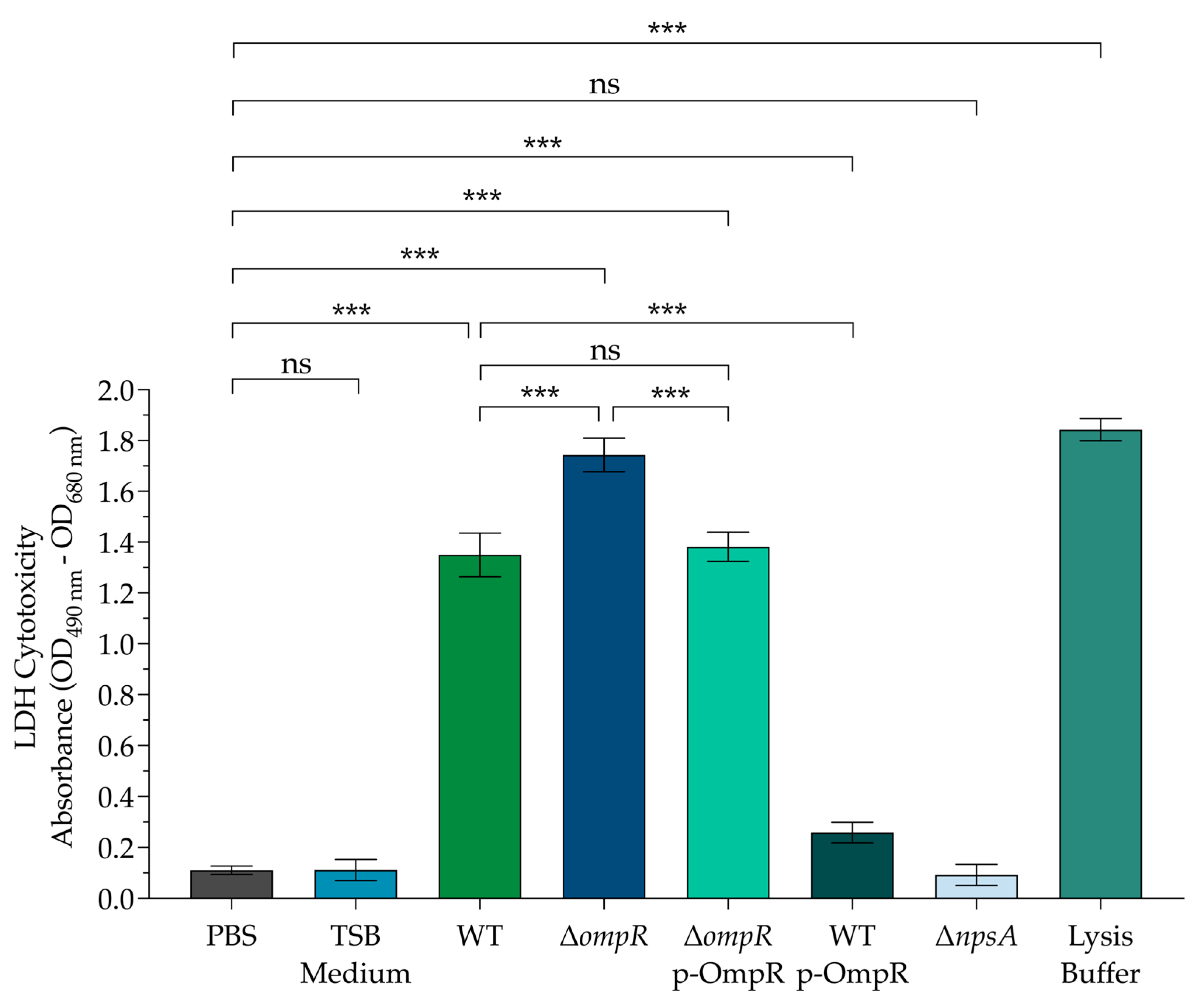
3.2. The Cytotoxic Effect on Colonic Epithelial Cells Is Affected by OmpR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikhimiukor, O.O.; Souza, S.S.R.; Akintayo, I.J.; Marcovici, M.M.; Workman, A.; Martin, I.W.; Andam, C.P. Phylogenetic Lineages and Antimicrobial Resistance Determinants of Clinical Klebsiella oxytoca Spanning Local to Global Scales. Microbiol. Spectr. 2023, 11, e0054923. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Baloch, Z.; Shah, Z.; Cui, X.; Xia, X. The Intestinal Microbiota: Impacts of Antibiotics Therapy, Colonization Resistance, and Diseases. Int. J. Mol. Sci. 2021, 22, 6597. [Google Scholar] [CrossRef]
- Greimel, T.M.; Stampfer, L.; Leitner, E.; Kienesberger, S.; Zechner, E.L.; Bozic, M.; Wagner, G.E.; Unterhauser, K.; Kitsera, M.; Hauer, A.C.; et al. Toxin-Producing Klebsiella oxytoca in Healthy Infants: Commensal or Pathobiont? J. Pediatr. Gastroenterol. Nutr. 2022, 74, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Lee, S.W.; Cho, H.H.; Park, S.; Chung, H.S.; Seo, J.W. Antibiotics-Associated Hemorrhagic Colitis Caused by Klebsiella oxytoca: Two Case Reports. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Leitner, E.; Bozic, M.; Kienesberger, S.; Cosic, A.; Landt, O.; Högenauer, C.; Kessler, H.H. Improved Diagnosis of Antibiotic-Associated Haemorrhagic Colitis (AAHC) in Faecal Specimens by a New Qualitative Real-Time PCR Assay Detecting Relevant Toxin Genes of Klebsiella oxytoca Sensu Lato. Clin. Microbiol. Infect. 2022, 28, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Högenauer, C.; Beubler, E.; Lippe, I.T.; Schicho, R.; Gorkiewicz, G.; Krause, R.; Gerstgrasser, N.; Krejs, G.J.; Hinterleitner, T.A. Klebsiella oxytoca as a Causative Organism of Antibiotic-Associated Hemorrhagic Colitis. N. Engl. J. Med. 2006, 355, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Sadotra, S.; Lou, Y.C.; Tang, H.C.; Chiu, Y.C.; Hsu, C.H.; Chen, C. Structural Basis for Promoter DNA Recognition by the Response Regulator OmpR. J. Struct. Biol. 2021, 213, 107638. [Google Scholar] [CrossRef]
- Kenney, L.J.; Anand, G.S. EnvZ/OmpR Two-Component Signaling: An Archetype System That Can Function Noncanonically. EcoSal Plus 2020, 9, 10.1128. [Google Scholar] [CrossRef]
- Darby, A.; Lertpiriyapong, K.; Sarkar, U.; Seneviratne, U.; Park, D.S.; Gamazon, E.R.; Batchelder, C.; Cheung, C.; Buckley, E.M.; Taylor, N.S.; et al. Cytotoxic and Pathogenic Properties of Klebsiella oxytoca Isolated from Laboratory Animals. PLoS ONE 2014, 9, e100542. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-Step Inactivation of Chromosomal Genes in Escherichia coli K-12 Using PCR Products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Hirakawa, H.; Nishino, K.; Hirata, T.; Yamaguchi, A. Comprehensive Studies of Drug Resistance Mediated by Overexpression of Response Regulators of Two-Component Signal Transduction Systems in Escherichia coli. J. Bacteriol. 2003, 185, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Nishino, K.; Yamada, J.; Hirata, T.; Yamaguchi, A. Beta-Lactam Resistance Modulated by the Overexpression of Response Regulators of Two-Component Signal Transduction Systems in Escherichia coli. J. Antimicrob. Chemother. 2003, 52, 576–582. [Google Scholar] [CrossRef]
- Rodríguez-Valverde, D.; León-Montes, N.; Soria-Bustos, J.; Martínez-Cruz, J.; González-Ugalde, R.; Rivera-Gutiérrez, S.; González-Y-Merchand, J.A.; Rosales-Reyes, R.; García-Morales, L.; Hirakawa, H.; et al. CAMP Receptor Protein Positively Regulates the Expression of Genes Involved in the Biosynthesis of Klebsiella oxytoca Tilivalline Cytotoxin. Front. Microbiol. 2021, 12, 743594. [Google Scholar] [CrossRef]
- Aranda, P.S.; Lajoie, D.M.; Jorcyk, C.L. Bleach Gel: A Simple Agarose Gel for Analyzing RNA Quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Joainig, M.M.; Gorkiewicz, G.; Leitner, E.; Weberhofer, P.; Zollner-Schwetz, I.; Lippe, I.; Feierl, G.; Krause, R.; Hinterleitner, T.; Zechner, E.L.; et al. Cytotoxic Effects of Klebsiella oxytoca Strains Isolated from Patients with Antibiotic-Associated Hemorrhagic Colitis or Other Diseases Caused by Infections and from Healthy Subjects. J. Clin. Microbiol. 2010, 48, 817–824. [Google Scholar] [CrossRef]
- De la Cruz, M.A.; Valdez-Salazar, H.A.; Robles-Leyva, N.; Siqueiros-Cendón, T.; Rascón-Cruz, Q.; Rodríguez-Valverde, D.; León-Montes, N.; Soria-Bustos, J.; Rosales-Reyes, R.; Cedillo, M.L.; et al. The Transcriptional Regulator Lrp Activates the Expression of Genes Involved in Tilivalline Enterotoxin Biosynthesis in Klebsiella oxytoca. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Dornisch, E.; Pletz, J.; Glabonjat, R.A.; Martin, F.; Lembacher-Fadum, C.; Neger, M.; Högenauer, C.; Francesconi, K.; Kroutil, W.; Zangger, K.; et al. Biosynthesis of the Enterotoxic Pyrrolobenzodiazepine Natural Product Tilivalline. Angew. Chem. Int. Ed. Engl. 2017, 56, 14753–14757. [Google Scholar] [CrossRef]
- Schneditz, G.; Rentner, J.; Roier, S.; Pletz, J.; Herzog, K.A.T.; Bücker, R.; Troeger, H.; Schild, S.; Weber, H.; Breinbauer, R.; et al. Enterotoxicity of a Nonribosomal Peptide Causes Antibiotic-Associated Colitis. Proc. Natl. Acad. Sci. USA 2014, 111, 13181–13186. [Google Scholar] [CrossRef] [PubMed]
- Osbelt, L.; Wende, M.; Almási, É.; Derksen, E.; Muthukumarasamy, U.; Lesker, T.R.; Galvez, E.J.C.; Pils, M.C.; Schalk, E.; Chhatwal, P.; et al. Klebsiella oxytoca Causes Colonization Resistance against Multidrug-Resistant K. Pneumoniae in the Gut via Cooperative Carbohydrate Competition. Cell Host. Microbe 2021, 29, 1663–1679.e7. [Google Scholar] [CrossRef]
- Hering, N.A.; Fromm, A.; Bücker, R.; Gorkiewicz, G.; Zechner, E.; Högenauer, C.; Fromm, M.; Schulzke, J.D.; Troeger, H. Tilivalline- and Tilimycin-Independent Effects of Klebsiella oxytoca on Tight Junction-Mediated Intestinal Barrier Impairment. Int. J. Mol. Sci. 2019, 20, 5595. [Google Scholar] [CrossRef]
- Unterhauser, K.; Pöltl, L.; Schneditz, G.; Kienesberger, S.; Glabonjat, R.A.; Kitsera, M.; Pletz, J.; Josa-Prado, F.; Dornisch, E.; Lembacher-Fadum, C.; et al. Klebsiella oxytoca Enterotoxins Tilimycin and Tilivalline Have Distinct Host DNA-Damaging and Microtubule-Stabilizing Activities. Proc. Natl. Acad. Sci. USA 2019, 116, 3774–3783. [Google Scholar] [CrossRef]
- Osbelt, L.; Almási, É.d.H.; Wende, M.; Kienesberger, S.; Voltz, A.; Lesker, T.R.; Muthukumarasamy, U.; Knischewski, N.; Nordmann, E.; Bielecka, A.A.; et al. Klebsiella oxytoca Inhibits Salmonella Infection through Multiple Microbiota-Context-Dependent Mechanisms. Nat. Microbiol. 2024, 9, 1792–1811. [Google Scholar] [CrossRef]
- Mattison, K.; Oropeza, R.; Byers, N.; Kenney, L.J. A Phosphorylation Site Mutant of OmpR Reveals Different Binding Conformations at OmpF and OmpC. J. Mol. Biol. 2002, 315, 497–511. [Google Scholar] [CrossRef]
- Chakraborty, S.; Winardhi, R.S.; Morgan, L.K.; Yan, J.; Kenney, L.J. Non-Canonical Activation of OmpR Drives Acid and Osmotic Stress Responses in Single Bacterial Cells. Nat. Commun. 2017, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Ludwiczak, M.; Murawska, E.; Raczkowska, A.; Brzostek, K. The Regulator OmpR in Yersinia enterocolitica Participates in Iron Homeostasis by Modulating Fur Level and Affecting the Expression of Genes Involved in Iron Uptake. Int. J. Mol. Sci. 2021, 22, 1475. [Google Scholar] [CrossRef]
- Nieckarz, M.; Jaworska, K.; Raczkowska, A.; Brzostek, K. The Regulatory Circuit Underlying Downregulation of a Type III Secretion System in Yersinia enterocolitica by Transcription Factor OmpR. Int. J. Mol. Sci. 2022, 23, 4758. [Google Scholar] [CrossRef]
- Nieckarz, M.; Raczkowska, A.; Debski, J.; Kistowski, M.; Dadlez, M.; Heesemann, J.; Rossier, O.; Brzostek, K. Impact of OmpR on the Membrane Proteome of Yersinia enterocolitica in Different Environments: Repression of Major Adhesin YadA and Heme Receptor HemR. Env. Microbiol. 2016, 18, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, D.E.; Bina, X.R.; Bina, J.E. Vibrio cholerae OmpR Contributes to Virulence Repression and Fitness at Alkaline PH. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, D.E.; Bina, T.F.; Bina, X.R.; Binaa, J.E. Vibrio cholerae OmpR Represses the ToxR Regulon in Response to Membrane Intercalating Agents That Are Prevalent in the Human Gastrointestinal Tract. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhi, T.; Hu, R.; Fan, R.; Long, Y.; Tian, F.; Zhao, Z. The OmpR-like Transcription Factor as a Negative Regulator of HrpR/S in Pseudomonas syringae Pv. Actinidiae. Int. J. Mol. Sci. 2022, 23, 12306. [Google Scholar] [CrossRef]
- Wang, S.T.; Kuo, C.J.; Huang, C.W.; Lee, T.M.; Chen, J.W.; Chen, C.S. OmpR Coordinates the Expression of Virulence Factors of Enterohemorrhagic Escherichia coli in the Alimentary Tract of Caenorhabditis Elegans. Mol. Microbiol. 2021, 116, 168–183. [Google Scholar] [CrossRef]
| Strain or Plasmid | Description * | Reference |
|---|---|---|
| Strains | ||
| K. oxytoca WT | Wild-type K. oxytoca strain MIT 09-7231 | [9] |
| K. oxytoca ΔompR | K. oxytoca ΔompR::kan | This study |
| K. oxytoca ΔompR p-OmpR | K. oxytoca ΔompR::kan + p-OmpR | This study |
| K. oxytoca WT p-OmpR | K. oxytoca WT + p-OmpR | This study |
| Plasmids | ||
| pTrc99A-ompR | ompR expression plasmid, IPTG-inducible trc promoter, AmpR | [11] |
| pTrc99K | Expression plasmid, IPTG-inducible trc promoter, KanR | [12] |
| p-OmpR | ompR expression plasmid, IPTG-inducible trc promoter, KanR | This study |
| pKD119 | pINT-ts derivative containing the lambda Red recombinase system under an arabinose-inducible promoter, TetR | [10] |
| pKD4 | pANTsy derivative template plasmid containing the kanamycin cassette for lambda Red recombination, AmpR | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-Nájera, R.G.; De la Cruz, M.A.; Soria-Bustos, J.; González-Horta, C.; Delgado-Gardea, M.C.E.; Yáñez-Santos, J.A.; Cedillo, M.L.; Hirakawa, H.; Fox, J.G.; Sánchez-Ramírez, B.; et al. The Response Regulator OmpR Negatively Controls the Expression of Genes Implicated in Tilimycin and Tilivalline Cytotoxin Production in Klebsiella oxytoca. Microorganisms 2025, 13, 158. https://doi.org/10.3390/microorganisms13010158
Varela-Nájera RG, De la Cruz MA, Soria-Bustos J, González-Horta C, Delgado-Gardea MCE, Yáñez-Santos JA, Cedillo ML, Hirakawa H, Fox JG, Sánchez-Ramírez B, et al. The Response Regulator OmpR Negatively Controls the Expression of Genes Implicated in Tilimycin and Tilivalline Cytotoxin Production in Klebsiella oxytoca. Microorganisms. 2025; 13(1):158. https://doi.org/10.3390/microorganisms13010158
Chicago/Turabian StyleVarela-Nájera, Ramón G., Miguel A. De la Cruz, Jorge Soria-Bustos, Carmen González-Horta, Ma Carmen E. Delgado-Gardea, Jorge A. Yáñez-Santos, María L. Cedillo, Hidetada Hirakawa, James G. Fox, Blanca Sánchez-Ramírez, and et al. 2025. "The Response Regulator OmpR Negatively Controls the Expression of Genes Implicated in Tilimycin and Tilivalline Cytotoxin Production in Klebsiella oxytoca" Microorganisms 13, no. 1: 158. https://doi.org/10.3390/microorganisms13010158
APA StyleVarela-Nájera, R. G., De la Cruz, M. A., Soria-Bustos, J., González-Horta, C., Delgado-Gardea, M. C. E., Yáñez-Santos, J. A., Cedillo, M. L., Hirakawa, H., Fox, J. G., Sánchez-Ramírez, B., & Ares, M. A. (2025). The Response Regulator OmpR Negatively Controls the Expression of Genes Implicated in Tilimycin and Tilivalline Cytotoxin Production in Klebsiella oxytoca. Microorganisms, 13(1), 158. https://doi.org/10.3390/microorganisms13010158








