Metabolomic Profiling Reveals Potential Biomarkers and Prominent Features in HIV/AIDS Patients Co-Infected with SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Study Cohorts
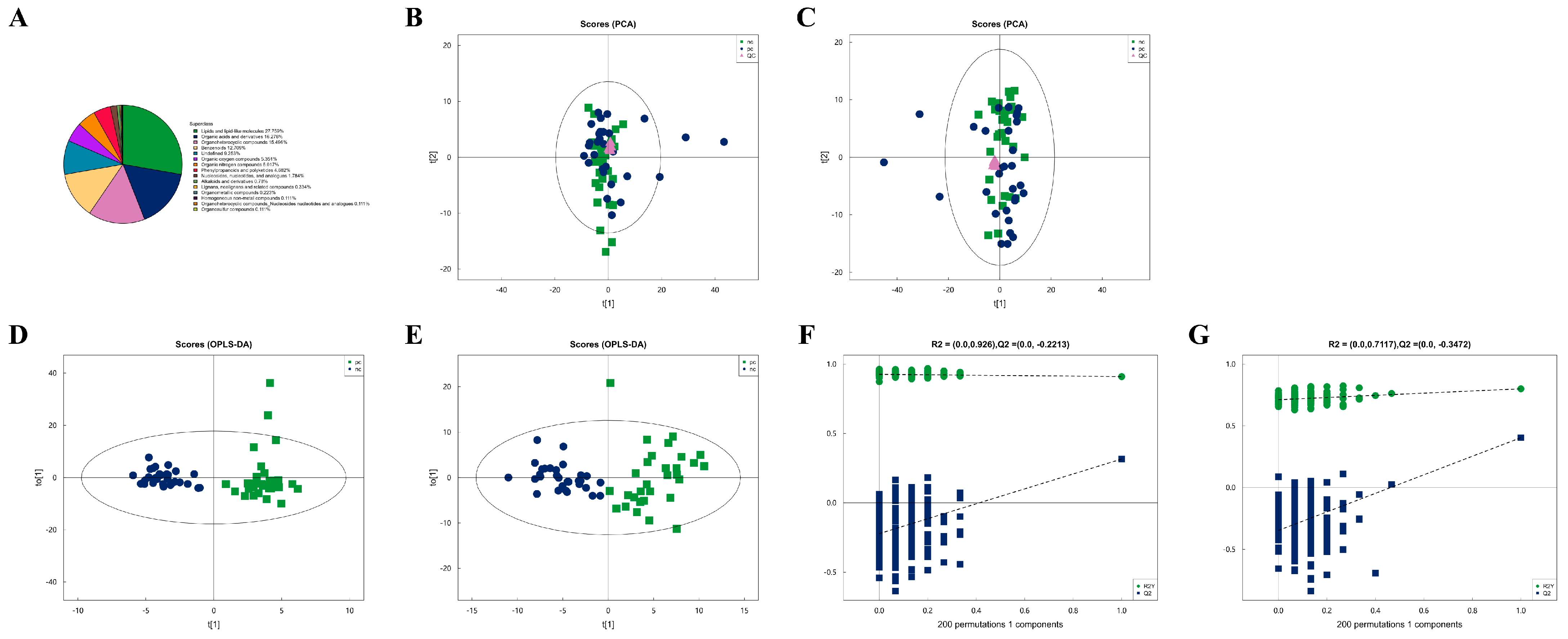
3.2. Metabolic Characteristics of Plasma Samples from Two Groups
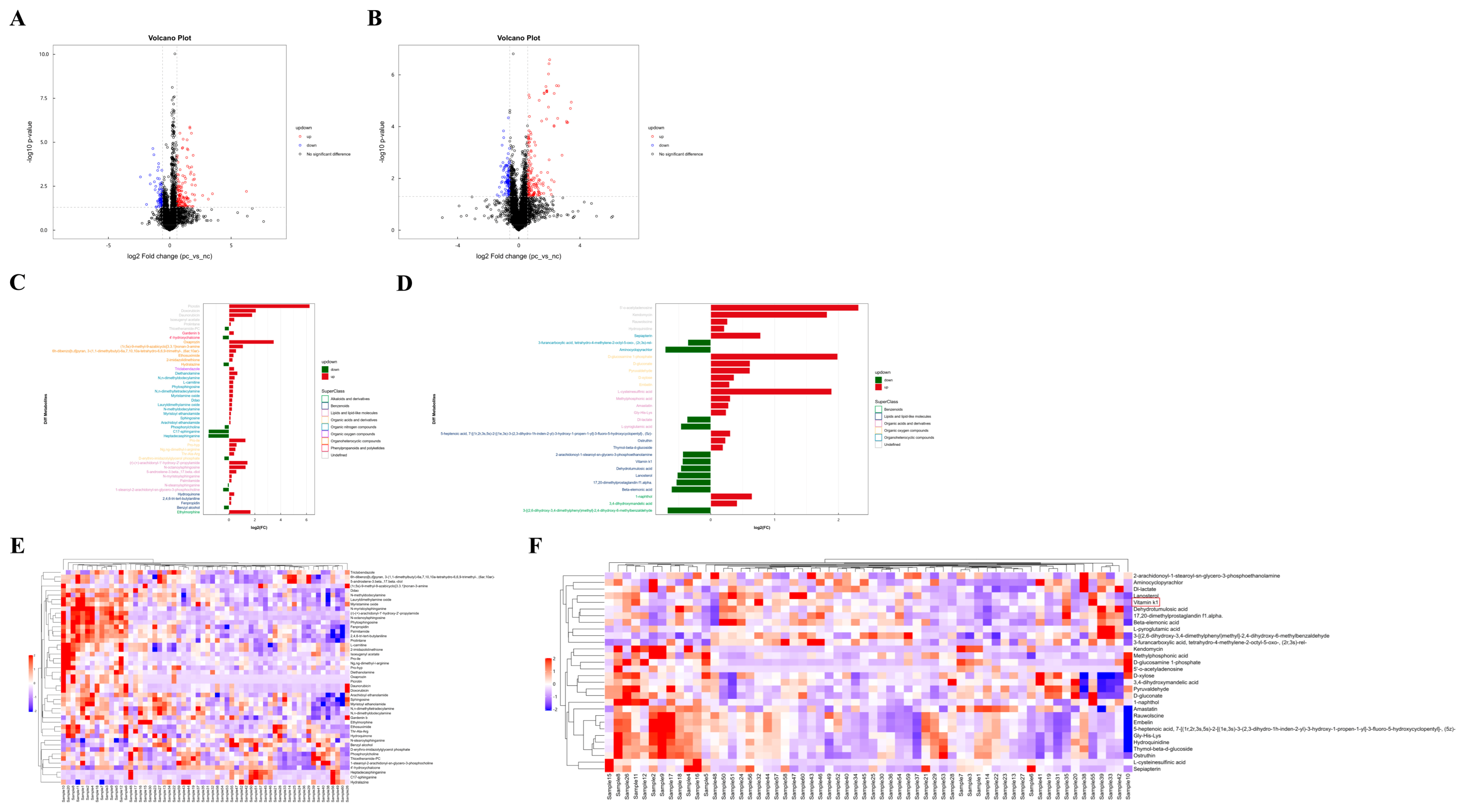
3.3. Difference Analysis of Metabolites Between Two Groups and Hierarchical Clustering Analysis (HCA) of Differential Metabolites
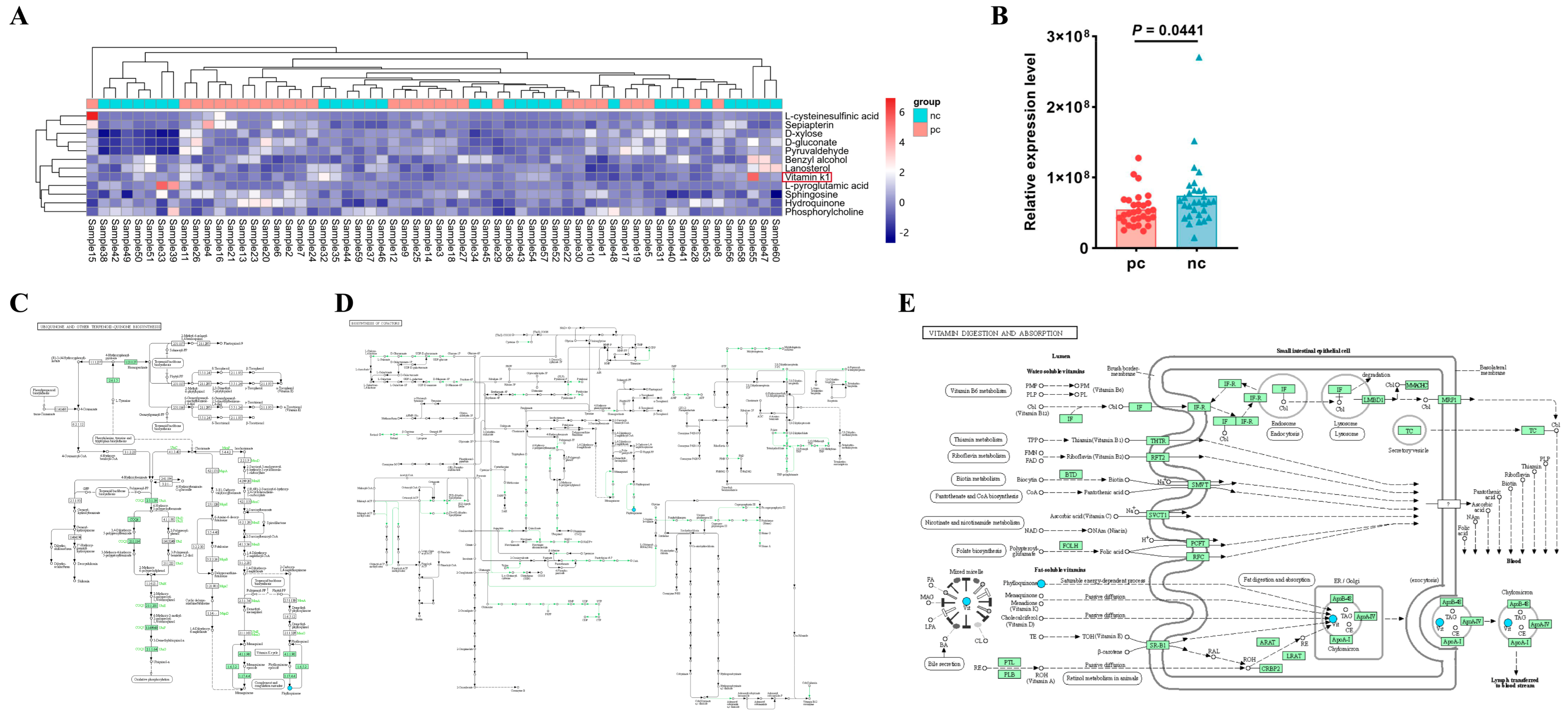
3.4. Vitamin K1 May Be a Potential Metabolic Biomarker for COVID-19 in HIV Patients
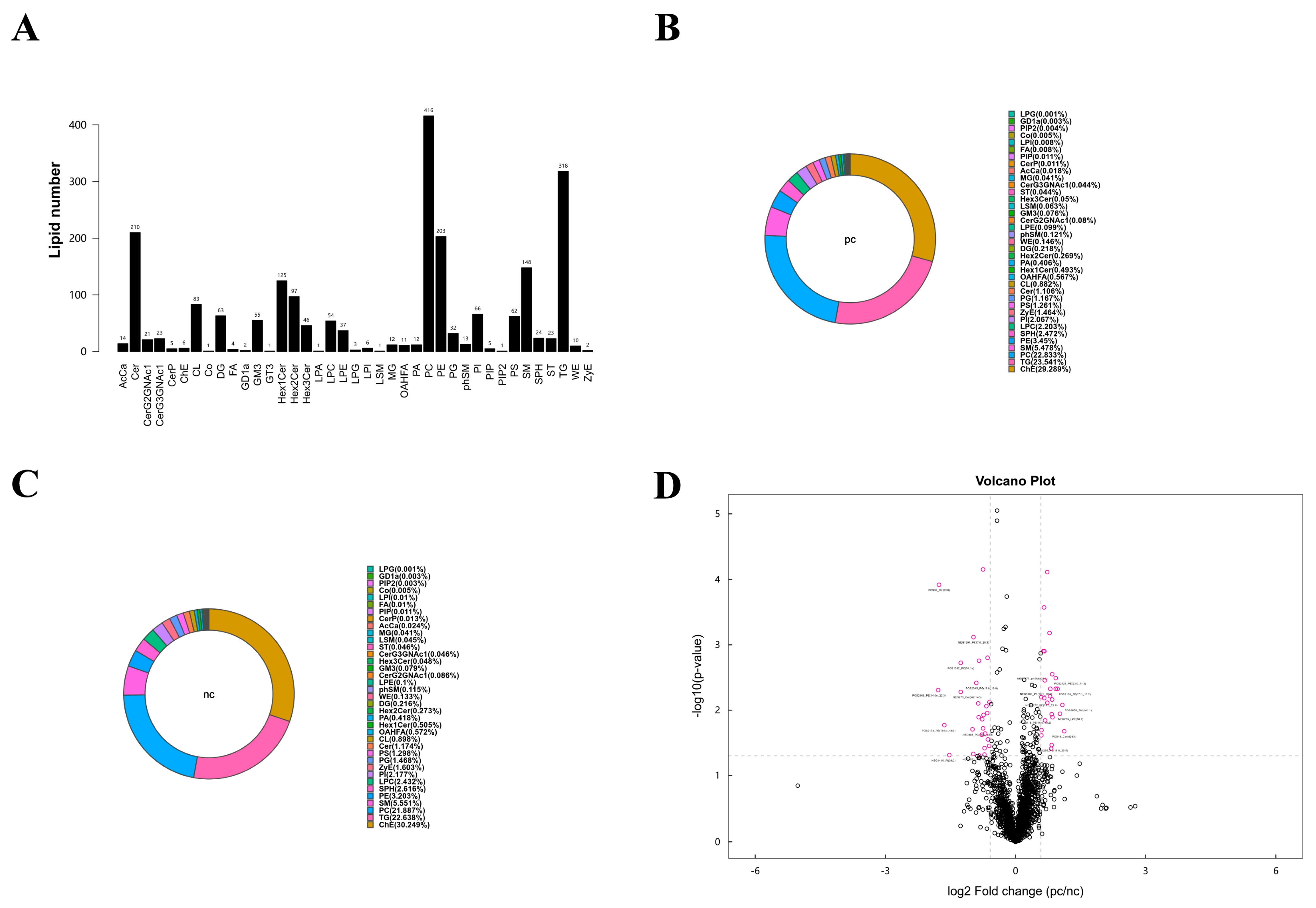
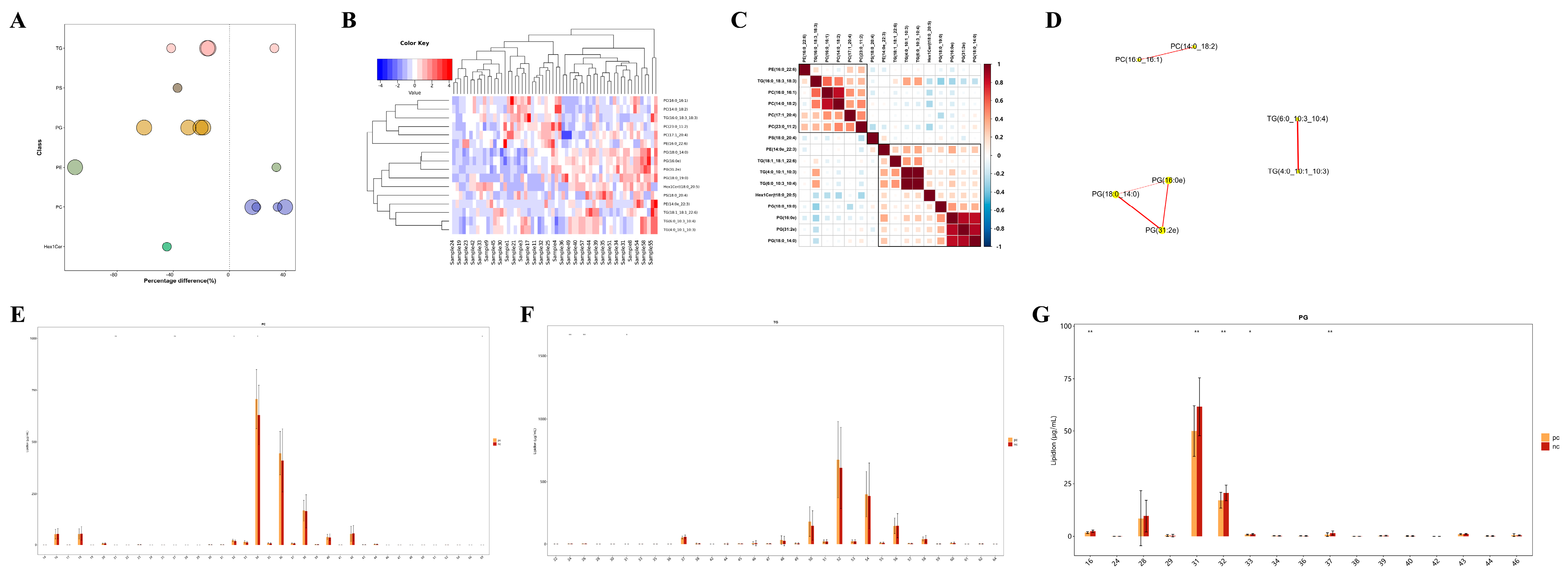
3.5. Lipid Metabolic Characteristics of Plasma Samples from Two Groups
3.6. Targeted Metabolomic Analysis of Lipid Metabolic Pathways: Focus on TG, PG, and PC Classes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- The unfinished agenda of communicable diseases among children and adolescents before the COVID-19 pandemic, 1990-2019: A systematic analysis of the Global Burden of Disease Study 2019. Lancet 2023, 402, 313–335. [CrossRef] [PubMed]
- Hoffmann, C.; Casado, J.L.; Härter, G.; Vizcarra, P.; Moreno, A.; Cattaneo, D.; Meraviglia, P.; Spinner, C.D.; Schabaz, F.; Grunwald, S.; et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021, 22, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Oyelade, T.; Alqahtani, J.S.; Hjazi, A.M.; Li, A.; Kamila, A.; Raya, R.P. Global and Regional Prevalence and Outcomes of COVID-19 in People Living with HIV: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Danwang, C.; Noubiap, J.J.; Robert, A.; Yombi, J.C. Outcomes of patients with HIV and COVID-19 co-infection: A systematic review and meta-analysis. AIDS Res. Ther. 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Kanwugu, O.N.; Adadi, P. HIV/SARS-CoV-2 coinfection: A global perspective. J. Med. Virol. 2021, 93, 726–732. [Google Scholar] [CrossRef]
- Geretti, A.M.; Stockdale, A.J.; Kelly, S.H.; Cevik, M.; Collins, S.; Waters, L.; Villa, G.; Docherty, A.; Harrison, E.M.; Turtle, L.; et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization Among People With Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin Infect Dis 2021, 73, e2095–e2106. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Hu, S.; Ai, W.; Tao, Y.; Tang, H.; Jing, F.; Tang, W. Systematic Review and Meta-Analyses of The Interaction Between HIV Infection And COVID-19: Two Years’ Evidence Summary. Front. Immunol. 2022, 13, 864838. [Google Scholar] [CrossRef]
- Inciarte, A.; Gonzalez-Cordon, A.; Rojas, J.; Torres, B.; de Lazzari, E.; de la Mora, L.; Martinez-Rebollar, M.; Laguno, M.; Callau, P.; Gonzalez-Navarro, A.; et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: A single-center, prospective observational study. Aids 2020, 34, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Patel, R.C.; Zheng, Q.; Madhira, V.; Olex, A.L.; Islam, J.Y.; French, E.; Chiang, T.P.; Akselrod, H.; Moffitt, R.; et al. COVID-19 Disease Severity among People with HIV Infection or Solid Organ Transplant in the United States: A Nationally-representative, Multicenter, Observational Cohort Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Bachelard, A.; Sautereau, A.; Digumber, M.; Isernia, V.; Phung, B.; Lehur, A.C.; Gac, S.L.; Landman, R.; Yazdanpanah, Y.; Ghosn, J. Risk Factors Associated with Severe/Critical COVID-19 in People Living with HIV-1. Int. J. Infect. Dis. 2022, 122, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Illanes-Álvarez, F.; Márquez-Ruiz, D.; Márquez-Coello, M.; Cuesta-Sancho, S.; Girón-González, J.A. Similarities and differences between HIV and SARS-CoV-2. Int. J. Med. Sci. 2021, 18, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.; Giorgi, E.E.; Chakraborty, S.; Nguyen, K.; Bhattacharya, T.; Theiler, J.; Goloboff, P.A.; Yoon, H.; Abfalterer, W.; Foley, B.T.; et al. HIV-1 and SARS-CoV-2: Patterns in the evolution of two pandemic pathogens. Cell Host Microbe 2021, 29, 1093–1110. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J. Med. Virol. 2020, 92, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Mozzini, C.; Girelli, D. The role of Neutrophil Extracellular Traps in Covid-19: Only an hypothesis or a potential new field of research? Thromb. Res. 2020, 191, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Casari, I.; Manfredi, M.; Metharom, P.; Falasca, M. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 2021, 82, 101092. [Google Scholar] [CrossRef]
- Dubé, M.P.; Cadden, J.J. Lipid metabolism in treated HIV Infection. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 429–442. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gomes, M.T.; Mo, Y.; Prohaska, C.C.; Zhang, L.; Chelvanambi, S.; Clauss, M.A.; Zhang, D.; Machado, R.F.; Gao, M.; et al. Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation. Int. J. Mol. Sci. 2023, 24, 7472. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Minosse, C.; Del Porto, P. mRNA- and Adenovirus-Based Vaccines against SARS-CoV-2 in HIV-Positive People. Viruses 2022, 14, 748. [Google Scholar] [CrossRef] [PubMed]
- Chau, B.-A.; Chen, V.; Cochrane, A.W.; Parent, L.J.; Mouland, A.J. Liquid-liquid phase separation of nucleocapsid proteins during SARS-CoV-2 and HIV-1 replication. Cell Rep. 2023, 42, 111968. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, L.; Zhang, X.; Zhang, J.; Shang, H.; Liang, G. Neuropilin-1, a myeloid cell-specific protein, is an inhibitor of HIV-1 infectivity. Proc. Natl. Acad. Sci. USA 2022, 119, e2114884119. [Google Scholar] [CrossRef]
- Lu, J.; Chen, B.; Chen, T.; Guo, S.; Xue, X.; Chen, Q.; Zhao, M.; Xia, L.; Zhu, Z.; Zheng, L.; et al. Comprehensive metabolomics identified lipid peroxidation as a prominent feature in human plasma of patients with coronary heart diseases. Redox Biol. 2017, 12, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hou, Y.; He, H.; Chen, Y.; Zhou, R.; Wang, X.; Gong, T.; Jiang, W. Synthetic vitamin K analogs inhibit inflammation by targeting the NLRP3 inflammasome. Cell Mol. Immunol. 2021, 18, 2422–2430. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, J.; Jia, J.; Li, J.; Zhou, Y.; Song, Z.; Guan, F.; Li, X.; Liu, L. Vitamin K1 ameliorates lipopolysaccharide-triggered skeletal muscle damage revealed by faecal bacteria transplantation. J. Cachexia Sarcopenia Muscle 2024, 15, 81–97. [Google Scholar] [CrossRef]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced Vitamin K Status as a Potentially Modifiable Risk Factor of Severe Coronavirus Disease 2019. Clin. Infect Dis. 2021, 73, e4039–e4046. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.V. Chronic HIV disease and activation of the coagulation system. Thromb. Res. 2013, 132, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Yuan, S.; Cao, J.; Fung, K.; Lai, P.-M.; Yin, F.; Sze, K.-H.; Qin, Z.; Xie, Y.; Ye, Z.-W.; et al. Phosphatidic acid phosphatase 1 impairs SARS-CoV-2 replication by affecting the glycerophospholipid metabolism pathway. Int. J. Biol. Sci. 2022, 18, 4744–4755. [Google Scholar] [CrossRef]
- Haughey, N.J.; Tovar-y-Romo, L.B.; Bandaru, V.V.R. Roles for biological membranes in regulating human immunodeficiency virus replication and progress in the development of HIV therapeutics that target lipid metabolism. J. Neuroimmune Pharmacol. 2011, 6, 284–295. [Google Scholar] [CrossRef][Green Version]
- Whatley, R.E.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Lipid metabolism and signal transduction in endothelial cells. Prog. Lipid Res. 1990, 29, 45–63. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Crinelli, R.; Cesarini, E.; Canonico, B.; Guidi, L.; Zerbinati, C.; Di Sario, G.; Zamai, L.; Magnani, M.; Papa, S.; et al. Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox Biol. 2017, 13, 581–587. [Google Scholar] [CrossRef]
- Dufour, J.-F.; Marjot, T.; Becchetti, C.; Tilg, H. COVID-19 and liver disease. Gut 2022, 71, 2350–2362. [Google Scholar] [CrossRef] [PubMed]
- D’Avila, H.; Lima, C.N.R.; Rampinelli, P.G.; Mateus, L.C.O.; Sousa Silva, R.V.d.; Correa, J.R.; Almeida, P.E.d. Lipid Metabolism Modulation during SARS-CoV-2 Infection: A Spotlight on Extracellular Vesicles and Therapeutic Prospects. Int. J. Mol. Sci. 2024, 25, 640. [Google Scholar] [CrossRef] [PubMed]
- Bhurwal, A.; Minacapelli, C.D.; Orosz, E.; Gupta, K.; Tait, C.; Dalal, I.; Zhang, C.; Zhao, E.; Rustgi, V.K. COVID-19 status quo: Emphasis on gastrointestinal and liver manifestations. World J. Gastroenterol. 2021, 27, 7969–7981. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Kandasamy, P.; Voelker, D.R. The anti-inflammatory and antiviral properties of anionic pulmonary surfactant phospholipids. Immunol. Rev. 2023, 317, 166–186. [Google Scholar] [CrossRef]
| Parameters | HIV/AIDS Patients Co-Infected with SARS-CoV-2 (n = 30) | HIV/AIDS Patients Without SARS-CoV-2 Infection (n = 30) | p Value |
|---|---|---|---|
| Male Gender (%) | 93.33 | 86.67 | 0.389 |
| Age (years) | 51.37 ± 17.19 | 45.00 ± 12.42 | 0.106 |
| HIV RNA (106 copies/mL) | 0.63 ± 1.38 | 0.58 ± 0.84 | 0.835 |
| On ART (%) | 56.67 | 36.67 | 0.121 |
| CD4+ T (cells/μL) | 81.27 ± 144.10 | 63.78 ± 104.50 | 0.621 |
| CD8+ T (cells/μL) | 493.20 ± 439.90 | 467.30 ± 287.90 | 0.921 |
| CD4+ T/CD8+ T cell count | 0.19 ± 0.29 | 0.18 ± 0.27 | 0.860 |
| WBC (109/L) | 5.89 ± 2.73 | 6.42 ± 3.19 | 0.491 |
| Hb (g/L) | 114.40 ± 25.18 | 114.2 ± 24.88 | 0.980 |
| N (109/L) | 4.37 ± 2.50 | 4.91 ± 3.07 | 0.459 |
| L (109/L) | 0.88 ± 0.62 | 0.86 ± 0.58 | 0.877 |
| M (109/L) | 0.49 ± 0.37 | 0.46 ± 0.28 | 0.649 |
| SaO2 (%) | 98.41 ± 2.46 | 97.30 ± 2.56 | 0.538 |
| Metabolites | VIP Value | Fold Change | p Value |
|---|---|---|---|
| Fenpropidin | 19.497 | 1.135 | <0.001 |
| L-carnitine | 17.529 | 1.271 | 0.017 |
| Pro-ile | 11.623 | 2.443 | 0.036 |
| 2,4,6-tri-tert-butylaniline | 8.859 | 1.144 | <0.001 |
| Picrotin | 8.251 | 76.152 | 0.006 |
| Phytosphingosine | 5.745 | 1.262 | <0.001 |
| Lauryldimethylamine oxide | 4.605 | 1.189 | <0.001 |
| Ng,ng-dimethyl-l-arginine | 4.136 | 1.429 | 0.030 |
| Daunorubicin | 3.513 | 3.499 | 0.003 |
| Myristamine oxide | 3.329 | 1.248 | <0.001 |
| Heptadecasphinganine | 3.115 | 0.329 | 0.001 |
| Prolintane | 3.096 | 1.115 | <0.001 |
| C17-sphinganine | 3.026 | 0.333 | 0.002 |
| Arachidoyl ethanolamide | 2.964 | 1.098 | <0.001 |
| (r)-(+)-arachidonyl-1′-hydroxy-2′-propylamide | 2.848 | 2.720 | <0.001 |
| Thioetheramide-PC | 2.770 | 0.792 | 0.050 |
| Oxaprozin | 2.765 | 11.136 | 0.008 |
| D-erythro-imidazolylglycerol phosphate | 2.559 | 0.771 | 0.043 |
| Myristoyl ethanolamide | 2.283 | 1.123 | <0.001 |
| Phosphorylcholine | 2.215 | 0.787 | 0.038 |
| Hydroquinone | 2.166 | 1.339 | 0.033 |
| Palmitamide | 1.976 | 1.155 | <0.001 |
| Triclabendazole | 1.890 | 1.327 | 0.040 |
| N,n-dimethyldodecylamine | 1.857 | 1.367 | 0.004 |
| 1-stearoyl-2-arachidonyl-sn-glycero-3-phosphocholine | 1.657 | 0.722 | 0.038 |
| N-stearoylsphinganine | 1.626 | 0.930 | 0.001 |
| Ddao | 1.608 | 1.204 | <0.001 |
| Gardenin b | 1.604 | 1.307 | 0.007 |
| Thr-Ala-Arg | 1.591 | 1.334 | 0.014 |
| N,n-dimethyltetradecylamine | 1.531 | 1.251 | 0.004 |
| Doxorubicin | 1.519 | 4.255 | 0.003 |
| Benzyl alcohol | 1.437 | 0.757 | 0.044 |
| Diethanolamine | 1.425 | 1.586 | 0.006 |
| (1r,5s)-9-methyl-9-azabicyclo[3.3.1]nonan-3-amine | 1.346 | 2.131 | 0.029 |
| Ethosuximide | 1.253 | 1.289 | 0.027 |
| Hydralazine | 1.228 | 0.740 | 0.006 |
| Sphingosine | 1.190 | 1.099 | 0.001 |
| N-octanoylsphingosine | 1.165 | 2.461 | 0.001 |
| N-myristoylsphinganine | 1.164 | 1.172 | <0.001 |
| Isoeugenyl acetate | 1.163 | 1.341 | 0.036 |
| Pro-hyp | 1.133 | 1.518 | 0.036 |
| 4′-hydroxychalcone | 1.091 | 0.711 | 0.038 |
| Ethylmorphine | 1.038 | 3.202 | 0.001 |
| 6h-dibenzo[b,d]pyran,3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-, (6ar,10ar)- | 1.025 | 1.476 | <0.001 |
| N-methyldodecylamine | 1.013 | 1.184 | 0.003 |
| 5-androstene-3.beta.,17.beta.-diol | 1.012 | 1.497 | <0.001 |
| 2-imidazolidinethione | 1.012 | 1.221 | 0.035 |
| Metabolites | VIP Value | Fold Change | p Value |
|---|---|---|---|
| Gly-His-Lys | 8.783 | 1.182 | 0.001 |
| Hydroquinidine | 8.553 | 1.159 | 0.003 |
| Thymol-beta-d-glucoside | 7.115 | 1.143 | 0.011 |
| Dl-lactate | 5.896 | 0.774 | 0.028 |
| L-pyroglutamic acid | 4.410 | 0.723 | 0.045 |
| Ostruthin | 4.106 | 1.174 | 0.001 |
| Embelin | 3.971 | 1.226 | 0.012 |
| D-xylose | 3.463 | 1.288 | 0.025 |
| 3,4-dihydroxymandelic acid | 3.403 | 1.333 | 0.025 |
| Kendomycin | 3.316 | 3.534 | <0.001 |
| D-glucosamine 1-phosphate | 3.093 | 3.964 | 0.002 |
| Rauwolscine | 2.731 | 1.200 | 0.038 |
| Methylphosphonic acid | 2.611 | 1.236 | 0.025 |
| Amastatin | 2.143 | 1.213 | 0.001 |
| Sepiapterin | 2.075 | 1.717 | <0.001 |
| L-cysteinesulfinic acid | 2.024 | 3.714 | 0.006 |
| 5-heptenoic acid, 7-[(1r,2r,3s,5s)-2-[(1e,3s)-3-(2,3-dihydro-1h-inden-2-yl)-3-hydroxy-1-propen-1-yl]-3-fluoro-5-hydroxycyclopentyl]-, (5z)- | 2.007 | 1.238 | 0.019 |
| Aminocyclopyrachlor | 1.846 | 0.611 | 0.015 |
| D-gluconate | 1.762 | 1.532 | 0.003 |
| 3-[(2,6-dihydroxy-3,4-dimethylphenyl)methyl]-2,4-dihydroxy-6-methylbenzaldehyde | 1.677 | 0.625 | <0.001 |
| Pyruvaldehyde | 1.495 | 1.530 | <0.001 |
| Dehydrotumulosic acid | 1.486 | 0.723 | 0.019 |
| 17,20-dimethylprostaglandin f1.alpha. | 1.449 | 0.689 | 0.003 |
| 1-naphthol | 1.337 | 1.566 | 0.025 |
| Vitamin k1 | 1.231 | 0.736 | 0.043 |
| Lanosterol | 1.207 | 0.696 | 0.010 |
| 2-arachidonoyl-1-stearoyl-sn-glycero-3-phosphoethanolamine | 1.199 | 0.739 | 0.022 |
| 3-furancarboxylic acid, tetrahydro-4-methylene-2-octyl-5-oxo-, (2r,3s)-rel- | 1.186 | 0.781 | <0.001 |
| Beta-elemonic acid | 1.157 | 0.654 | 0.002 |
| 5′-o-acetyladenosine | 1.029 | 4.974 | 0.013 |
| Lipid | Class | VIP Value | Fold Change | p Value |
|---|---|---|---|---|
| PC (23:0_11:2) +H | PC | 6.967 | 1.178 | 0.007 |
| PG (31:2e) +NH4 | PG | 6.323 | 0.813 | 0.001 |
| PG (18:0_14:0) +H | PG | 3.560 | 0.829 | 0.001 |
| PC (16:0_16:1) +HCOO | PC | 2.871 | 1.494 | 0.001 |
| PS (18:0_20:4)-H | PS | 2.644 | 0.688 | 0.044 |
| TG (16:0_18:3_18:3) +H | TG | 1.871 | 1.380 | 0.028 |
| PC (14:0_18:2) +HCOO | PC | 1.765 | 1.413 | 0.028 |
| TG (6:0_10:3_10:4) +NH4 | TG | 1.493 | 0.853 | 0.001 |
| TG (18:1_18:1_22:6) +NH4 | TG | 1.448 | 0.656 | 0.035 |
| PG (16:0e) +NH4 | PG | 1.390 | 0.744 | 0.000 |
| PE (14:0e_22:3) +Na | PE | 1.356 | 0.290 | 0.005 |
| TG (4:0_10:1_10:3) +Na | TG | 1.274 | 0.860 | 0.001 |
| PE (16:0_22:6)-H | PE | 1.127 | 1.401 | 0.019 |
| PC (17:1_20:4)-CH3 | PC | 1.112 | 1.212 | 0.033 |
| Hex1Cer(t18:0_20:5) +HCOO | Hex1Cer | 1.035 | 0.636 | 0.011 |
| PG (18:0_19:0) +H | PG | 1.018 | 0.534 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zhang, X.; Song, W.; Qi, T.; Wang, Z.; Tang, Y.; Sun, J.; Xu, S.; Yang, J.; Wang, J.; et al. Metabolomic Profiling Reveals Potential Biomarkers and Prominent Features in HIV/AIDS Patients Co-Infected with SARS-CoV-2. Microorganisms 2025, 13, 144. https://doi.org/10.3390/microorganisms13010144
Yan X, Zhang X, Song W, Qi T, Wang Z, Tang Y, Sun J, Xu S, Yang J, Wang J, et al. Metabolomic Profiling Reveals Potential Biomarkers and Prominent Features in HIV/AIDS Patients Co-Infected with SARS-CoV-2. Microorganisms. 2025; 13(1):144. https://doi.org/10.3390/microorganisms13010144
Chicago/Turabian StyleYan, Xuan, Xinyu Zhang, Wei Song, Tangkai Qi, Zhenyan Wang, Yang Tang, Jianjun Sun, Shuibao Xu, Junyang Yang, Jiangrong Wang, and et al. 2025. "Metabolomic Profiling Reveals Potential Biomarkers and Prominent Features in HIV/AIDS Patients Co-Infected with SARS-CoV-2" Microorganisms 13, no. 1: 144. https://doi.org/10.3390/microorganisms13010144
APA StyleYan, X., Zhang, X., Song, W., Qi, T., Wang, Z., Tang, Y., Sun, J., Xu, S., Yang, J., Wang, J., Chen, J., Zhang, R., Liu, L., & Shen, Y. (2025). Metabolomic Profiling Reveals Potential Biomarkers and Prominent Features in HIV/AIDS Patients Co-Infected with SARS-CoV-2. Microorganisms, 13(1), 144. https://doi.org/10.3390/microorganisms13010144








