Pseudomonas aeruginosa pqs Quorum Sensing Mediates Interaction with Mycobacterium abscessus In Vitro
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. P. aeruginosa Mutant Construction
2.3. Proteomics Analysis
2.4. Extraction and Purification of M. abscessus MVs
2.5. Characterization of MVs by Transmission Electron Microscopy (TEM) and Nanoparticle Tracking Analysis (NTA)
2.6. Scanning Electronic Microscopy (SEM)
2.7. qRT-PCT Analysis
2.8. Ethics Statement
2.9. Statistical Analysis
3. Results
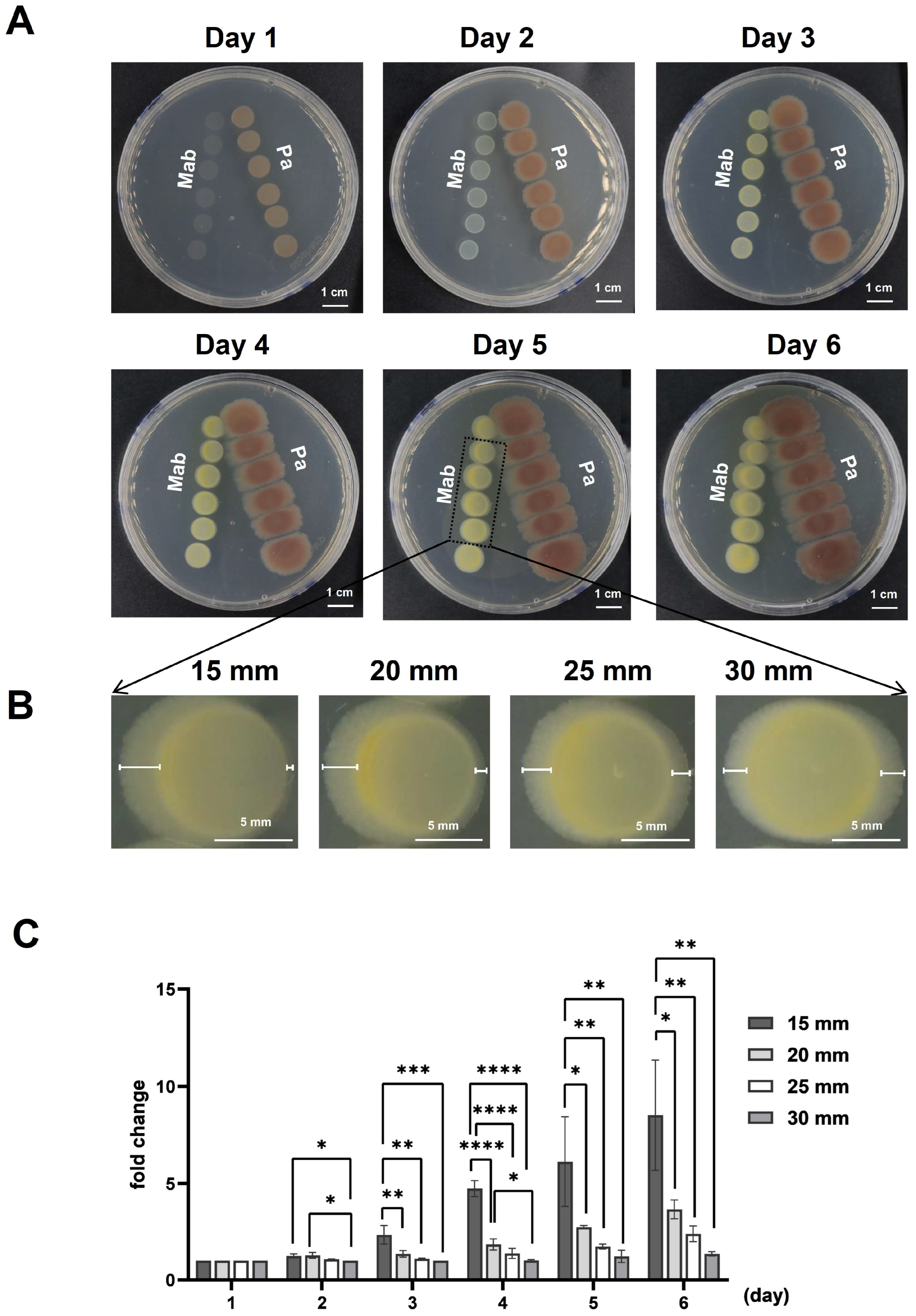
3.1. P. aeruginosa Inhibited the Growth of M. abscessus
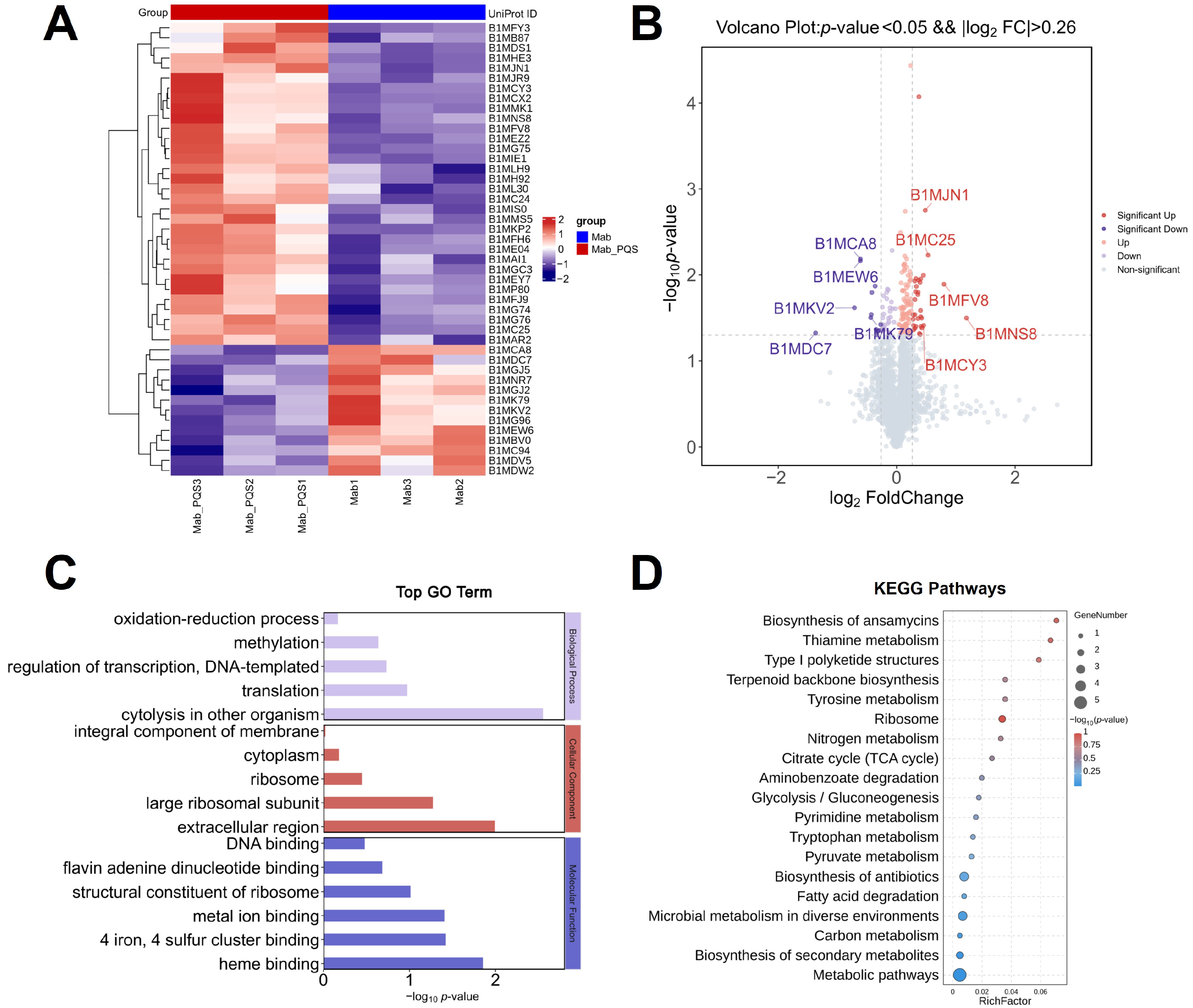
3.2. Global Impact of PQS on M. abscessus
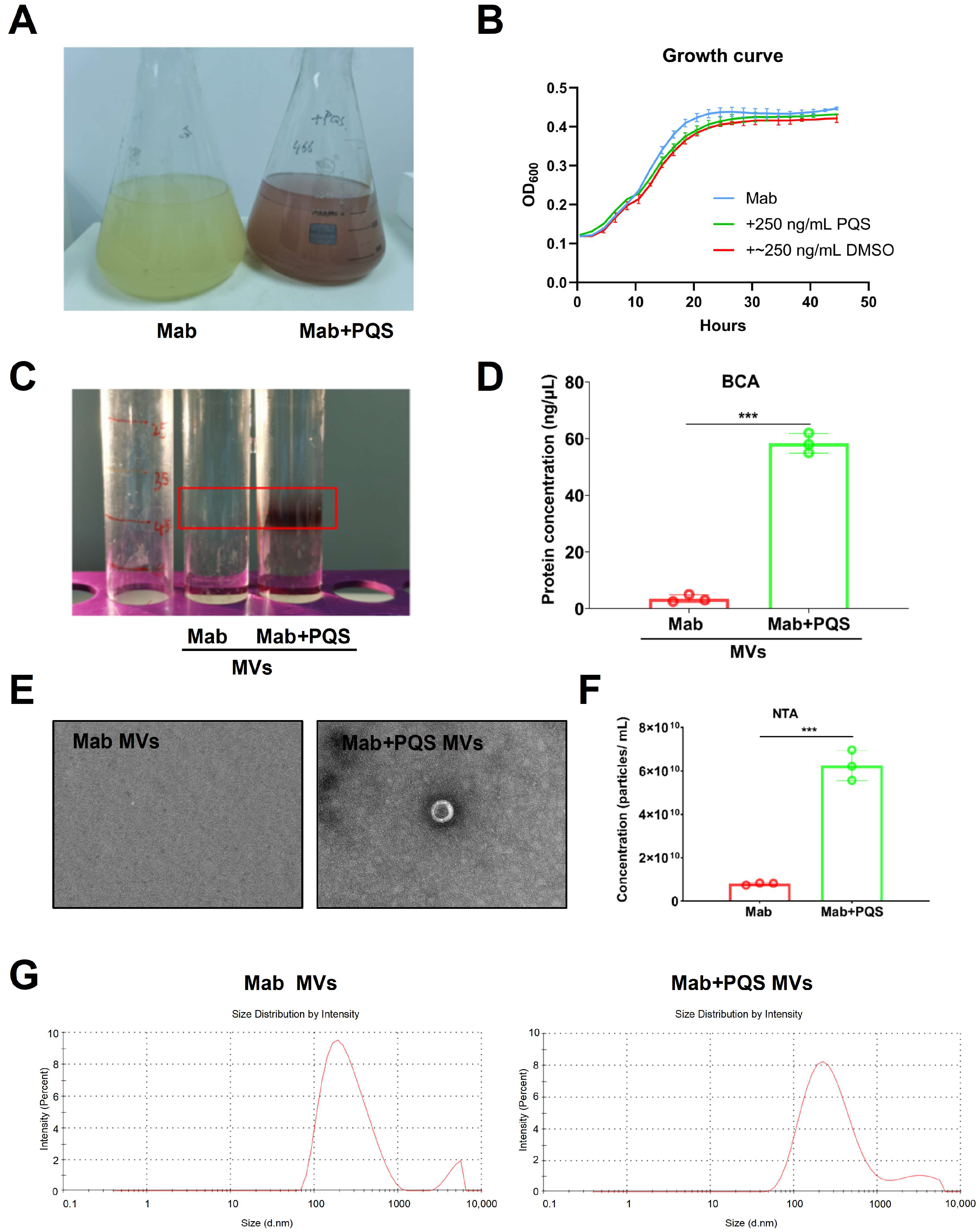
3.3. PQS Promotes the Production of MVs by M. abscessus
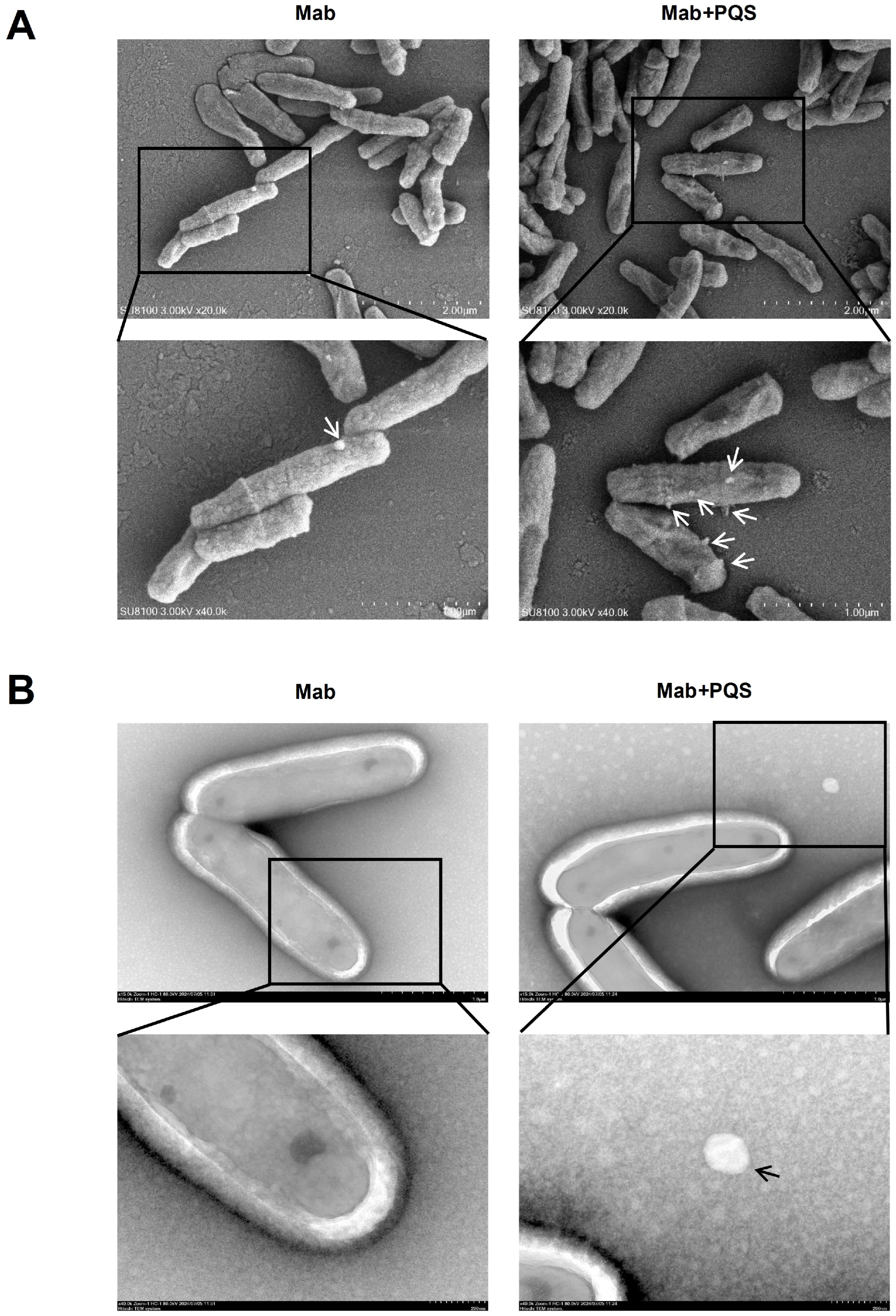
3.4. PQS Exposure Changes Cell Morphology of M. abscessus
3.5. Gene Expression Analysis upon PQS Exposure in M. abscessus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Michel-Briand, Y.; Baysse, C. The pyocins of Pseudomonas aeruginosa. Biochimie 2002, 84, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Soto-Aceves, M.P.; Smalley, N.E.; Schaefer, A.L.; Greenberg, E.P. The Relationship of pqs Gene Expression to Acylhomoserine Lactone Signaling in Pseudomonas aeruginosa. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Cheng, J.; Zeng, J.; Ma, X.; Lin, J. PQS and pyochelin in Pseudomonas aeruginosa share inner membrane transporters to mediate iron uptake. Microbiol. Spectr. 2024, 12, e0325623. [Google Scholar] [CrossRef]
- Mashburn, L.M.; Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 2005, 437, 422–425. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Abdel-Raouf, M.; Zayed, M.; Emara, M.A. Microbiological and molecular studies on a multidrug-resistant Pseudomonas aeruginosa from a liver transplant patient with urinary tract infection in Egypt. BMC Microbiol. 2024, 24, 184. [Google Scholar] [CrossRef]
- Hemmati, J.; Nazari, M.; Abolhasani, F.S.; Ahmadi, A.; Asghari, B. In vitro investigation of relationship between quorum-sensing system genes, biofilm forming ability, and drug resistance in clinical isolates of Pseudomonas aeruginosa. BMC Microbiol. 2024, 24, 99. [Google Scholar] [CrossRef]
- Rodriguez-Sevilla, G.; Crabbe, A.; Garcia-Coca, M.; Aguilera-Correa, J.J.; Esteban, J.; Perez-Jorge, C. Antimicrobial Treatment Provides a Competitive Advantage to Mycobacterium abscessus in a Dual-Species Biofilm with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Campo-Pérez, V.; Julián, E.; Torrents, E. Interplay of Mycobacterium abscessus and Pseudomonas aeruginosa in coinfection: Biofilm Dynamics and Host Immune Response. bioRxiv 2024. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Boo, Z.Z.; Chua, S.L.; Chong, K.H.C.; Long, Z.; Yang, R.; Zhou, Y.; Janela, B.; Chotirmall, S.H.; Ginhoux, F.; et al. A Bacterial Quorum Sensing Regulated Protease Inhibits Host Immune Responses by Cleaving Death Domains of Innate Immune Adaptors. Adv. Sci. 2023, 10, e2304891. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef]
- Moreau-Marquis, S.; Stanton, B.A.; O’Toole, G.A. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm. Pharmacol. Ther. 2008, 21, 595–599. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; de Lamo-Sevilla, C.; Cabezas-Fernandez, M.T.; Manzano-Agugliaro, F.; Martinez-Lirola, M. Global tuberculosis research and its future prospects. Tuberculosis 2020, 121, 101917. [Google Scholar] [CrossRef]
- Moon, W.K.; Im, J.G.; Yeon, K.M.; Han, M.C. Tuberculosis of the central airways: CT findings of active and fibrotic disease. AJR Am. J. Roentgenol. 1997, 169, 649–653. [Google Scholar] [CrossRef]
- Liang, Q.; Shang, Y.; Huo, F.; Xue, Y.; Li, Y.; Dong, L.; Li, S.; Pang, Y. Assessment of current diagnostic algorithm for detection of mixed infection with Mycobacterium tuberculosis and nontuberculous mycobacteria. J. Infect. Public Health 2020, 13, 1967–1971. [Google Scholar] [CrossRef]
- Lombard, J.E.; Patton, E.A.; Gibbons-Burgener, S.N.; Klos, R.F.; Tans-Kersten, J.L.; Carlson, B.W.; Keller, S.J.; Pritschet, D.J.; Rollo, S.; Dutcher, T.V.; et al. Human-to-Cattle Mycobacterium tuberculosis Complex Transmission in the United States. Front. Vet. Sci. 2021, 8, 691192. [Google Scholar] [CrossRef]
- Ocepek, M.; Pate, M.; Zolnir-Dovc, M.; Poljak, M. Transmission of Mycobacterium tuberculosis from human to cattle. J. Clin. Microbiol. 2005, 43, 3555–3557. [Google Scholar] [CrossRef]
- Olea-Popelka, F.; Muwonge, A.; Perera, A.; Dean, A.S.; Mumford, E.; Erlacher-Vindel, E.; Forcella, S.; Silk, B.J.; Ditiu, L.; El Idrissi, A.; et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis-a call for action. Lancet Infect. Dis. 2017, 17, e21–e25. [Google Scholar] [CrossRef]
- Brugha, R.; Spencer, H. Mycobacterium abscessus in cystic fibrosis. Science 2021, 372, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Baena, A.; Martinez, L.R.; Luque-Garcia, J.; Kalscheuer, R.; Veeraraghavan, U.; Camara, C.; Nosanchuk, J.D.; Besra, G.S.; Chen, B.; et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Investig. 2011, 121, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Schweizer, H.P. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.J.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Pharmacol. 2007, 38, 3A. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Tashiro, Y.; Ichikawa, S.; Nakajima-Kambe, T.; Uchiyama, H.; Nomura, N. Pseudomonas quinolone signal affects membrane vesicle production in not only gram-negative but also gram-positive bacteria. Microbes Environ. 2010, 25, 120–125. [Google Scholar] [CrossRef]
- Li, A.; Schertzer, J.W.; Yong, X. Molecular conformation affects the interaction of the Pseudomonas quinolone signal with the bacterial outer membrane. J. Biol. Chem. 2019, 294, 1089–1094. [Google Scholar] [CrossRef]
- Bythrow, G.V.; Farhat, M.F.; Levendosky, K.; Mohandas, P.; Germain, G.A.; Yoo, B.; Quadri, L.E.N. Mycobacterium abscessus Mutants with a Compromised Functional Link between the Type VII ESX-3 System and an Iron Uptake Mechanism Reliant on an Unusual Mycobactin Siderophore. Pathogens 2022, 11, 953. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Esserman, D.A.; Gilligan, P.; Kerr, A.; Noone, P.G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 2010, 9, 117–123. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Lin, C.Y.; Wang, C.Y.; Fang, Y.F.; Lo, Y.L.; Lin, S.M.; Lin, H.C. Impact of concomitant nontuberculous mycobacteria and Pseudomonas aeruginosa isolates in non-cystic fibrosis bronchiectasis. Infect. Drug Resist. 2018, 11, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Simon, D.; Spiga, L.; Xiang, Y.; Takats, Z.; Williams, H. Laser desorption rapid evaporative ionization mass spectrometry (LD-REIMS) demonstrates a direct impact of hypochlorous acid stress on PQS-mediated quorum sensing in Pseudomonas aeruginosa. mSystems 2024, 9, e0116523. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.Y.; Zhang, X.Y.; Yang, M.H.; Huang, Y.J.; Lin, J.; Chen, W.M. 3-Hydroxypyridin-4(1H)-one Derivatives as pqs Quorum Sensing Inhibitors Attenuate Virulence and Reduce Antibiotic Resistance in Pseudomonas aeruginosa. J. Med. Chem. 2023, 66, 15823–15846. [Google Scholar] [CrossRef]
- Murray, E.J.; Dubern, J.F.; Chan, W.C.; Chhabra, S.R.; Williams, P. A Pseudomonas aeruginosa PQS quorum-sensing system inhibitor with anti-staphylococcal activity sensitizes polymicrobial biofilms to tobramycin. Cell Chem. Biol. 2022, 29, 1187–1199.e6. [Google Scholar] [CrossRef]
- Jia, T.; Liu, D.; Bi, X.; Li, M.; Cai, Z.; Fu, J.; Liu, Z.; Wu, P.; Ke, X.; Jia, A.; et al. The AhR ligand phthiocol and vitamin K analogs as Pseudomonas aeruginosa quorum sensing inhibitors. Front. Microbiol. 2022, 13, 896687. [Google Scholar] [CrossRef]
- Dehbashi, S.; Tahmasebi, H.; Alikhani, M.Y.; Vidal, J.E.; Seifalian, A.; Arabestani, M.R. The healing effect of Pseudomonas Quinolone Signal (PQS) with co-infection of Staphylococcus aureus and Pseudomonas aeruginosa: A preclinical animal co-infection model. J. Infect. Public Health 2024, 17, 329–338. [Google Scholar] [CrossRef]
- Shah, R.; Jankiewicz, O.; Johnson, C.; Livingston, B.; Dahl, J.U. Pseudomonas aeruginosa kills Staphylococcus aureus in a polyphosphate-dependent manner. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jia, T.; Bi, X.; Li, M.; Zhang, C.; Ren, A.; Li, S.; Zhou, T.; Zhang, Y.; Liu, Y.; Liu, X.; et al. Hfq-binding small RNA PqsS regulates Pseudomonas aeruginosa pqs quorum sensing system and virulence. NPJ Biofilms Microbiomes 2024, 10, 82. [Google Scholar] [CrossRef]
- Yu, Y.J.; Wang, X.H.; Fan, G.C. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol. Sin. 2018, 39, 514–533. [Google Scholar] [CrossRef]
- Weng, Y.W.; Huang, C.K.; Sy, C.L.; Wu, K.S.; Tsai, H.C.; Lee, S.S. Treatment for Mycobacterium abscessus complex-lung disease. J. Formos. Med. Assoc. 2020, 119 (Suppl. 1), S58–S66. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Voskuil, M.I.; Gold, B.; Schoolnik, G.K.; Smith, I. ideR, An essential gene in mycobacterium tuberculosis: Role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 2002, 70, 3371–3381. [Google Scholar] [CrossRef] [PubMed]
- Foreman, M.; Kolodkin-Gal, I.; Barkan, D. A Pivotal Role for Mycobactin/mbtE in Growth and Adaptation of Mycobacterium abscessus. Microbiol. Spectr. 2022, 10, e0262322. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Smith, I. Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J. Bacteriol. 2006, 188, 424–430. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, R.; Cuthbert, B.J.; Klevorn, T.; Chao, A.; Mendoza, J.; Arbing, M.; Sieminski, P.J.; Papavinasasundaram, K.; Abdul-Hafiz, S.; Chan, S.; et al. Differentiating the roles of Mycobacterium tuberculosis substrate binding proteins, FecB and FecB2, in iron uptake. PLoS Pathog. 2023, 19, e1011650. [Google Scholar] [CrossRef]
- Birmes, F.S.; Wolf, T.; Kohl, T.A.; Ruger, K.; Bange, F.; Kalinowski, J.; Fetzner, S. Mycobacterium abscessus subsp. abscessus Is Capable of Degrading Pseudomonas aeruginosa Quinolone Signals. Front. Microbiol. 2017, 8, 339. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.Y.; Luo, Z.Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 2017, 8, 14888. [Google Scholar] [CrossRef]
- Newton-Foot, M.; Warren, R.M.; Sampson, S.L.; van Helden, P.D.; Gey van Pittius, N.C. The plasmid-mediated evolution of the mycobacterial ESX (Type VII) secretion systems. BMC Evol. Biol. 2016, 16, 62. [Google Scholar] [CrossRef]
- Lagune, M.; Le Moigne, V.; Johansen, M.D.; Vasquez Sotomayor, F.; Daher, W.; Petit, C.; Cosentino, G.; Paulowski, L.; Gutsmann, T.; Wilmanns, M.; et al. The ESX-4 substrates, EsxU and EsxT, modulate Mycobacterium abscessus fitness. PLoS Pathog. 2022, 18, e1010771. [Google Scholar] [CrossRef]
- Clark, R.R.; Judd, J.; Lasek-Nesselquist, E.; Montgomery, S.A.; Hoffmann, J.G.; Derbyshire, K.M.; Gray, T.A. Direct cell-cell contact activates SigM to express the ESX-4 secretion system in Mycobacterium smegmatis. Proc. Natl. Acad. Sci. USA 2018, 115, E6595–E6603. [Google Scholar] [CrossRef]
- Aldriwesh, M.G.; Alaqeel, R.A.; Mashraqi, A.M.; Mashraqi, M.M.; Albdah, B.A.; Alharbi, A.S. Coinfection of pulmonary tuberculosis with other lower respiratory tract infections: A retrospective cross-sectional study. Ann. Thorac. Med. 2022, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Pi, J.; Xu, J.F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front. Immunol. 2021, 12, 628973. [Google Scholar] [CrossRef] [PubMed]
- Biadglegne, F.; Schmidt, J.R.; Engel, K.M.; Lehmann, J.; Lehmann, R.T.; Reinert, A.; Konig, B.; Schiller, J.; Kalkhof, S.; Sack, U. Mycobacterium tuberculosis Affects Protein and Lipid Content of Circulating Exosomes in Infected Patients Depending on Tuberculosis Disease State. Biomedicines 2022, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gonzalez, P.; Cervera-Hernandez, M.E.; Martinez-Gamboa, A.; Garcia-Garcia, L.; Cruz-Hervert, L.P.; Bobadilla-Del Valle, M.; Ponce-de Leon, A.; Sifuentes-Osornio, J. Human tuberculosis caused by Mycobacterium bovis: A retrospective comparison with Mycobacterium tuberculosis in a Mexican tertiary care centre, 2000–2015. BMC Infect Dis 2016, 16, 657. [Google Scholar] [CrossRef]
- Chen, Y.; Chao, Y.; Deng, Q.; Liu, T.; Xiang, J.; Chen, J.; Zhou, J.; Zhan, Z.; Kuang, Y.; Cai, H.; et al. Potential challenges to the Stop TB Plan for humans in China; cattle maintain M. bovis and M. tuberculosis. Tuberculosis 2009, 89, 95–100. [Google Scholar] [CrossRef]
- Colombo, E.; Borgiani, B.; Verderio, C.; Furlan, R. Microvesicles: Novel biomarkers for neurological disorders. Front. Physiol. 2012, 3, 63. [Google Scholar] [CrossRef]
- Tegl, G.; Schiffer, D.; Sigl, E.; Heinzle, A.; Guebitz, G.M. Biomarkers for infection: Enzymes, microbes, and metabolites. Appl. Microbiol. Biotechnol. 2015, 99, 4595–4614. [Google Scholar] [CrossRef]
- Dauros-Singorenko, P.; Blenkiron, C.; Phillips, A.; Swift, S. The functional RNA cargo of bacterial membrane vesicles. FEMS Microbiol. Lett. 2018, 365, fny023. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Guo, M.; Tan, Q.; Zhou, E.; Deng, J.; Li, M.; Chen, J.; Yang, Z.; Jin, Y. Metabolomics of Extracellular Vesicles: A Future Promise of Multiple Clinical Applications. Int. J. Nanomed. 2022, 17, 6113–6129. [Google Scholar] [CrossRef]

| Targeted Gene | Genome Identifier | Primer Sequence (5′-3′) |
|---|---|---|
| sigA | MAB_3009-F | AGCGTGAGCTGCTACAGGAC |
| MAB_3009-R | TGGATTTCCAGCACCTTCTC | |
| ideR | MAB_3029-F | CTGGTCGACATCATCGGGTT |
| MAB_3029-R | GATTGTTGAGCACCGTGAGC | |
| bfrB | MAB_0127c-F | GGGATCGTGATGGCTACGTT |
| MAB_0127c-R | TGCACGATCAAATCCGCAAC | |
| mbtE | MAB_2248c-F | CCGATTCGTCGGTACGTCAT |
| MAB_2248c-R | GCCATATCGCTTGCAGTTGG | |
| fecB | MAB_4390-F | TCCGCACATACCGGTTTCAA |
| MAB_4390-R | CCCAAGTCCGGAGAGGAAAC | |
| irtA | MAB_2262c-F | TGAGTTTCTTGACCGACCCG |
| MAB_2262c-R | TTCGTCTTTCTGGTGCTGCT | |
| aqdC | MAB_0303-F | ATTGACGAGGCCTACCGAAC |
| MAB_0303-R | CCCCGATCTTGGGATGTGAG | |
| MAB_3747c | MAB_3747c-F | ACTTCGCGTAATCCGCTTCT |
| MAB_3747c-R | ATTGTCTTTCGGTGGGGCAT | |
| MAB_4316 | MAB_4316-F | AGTCAGTCCTGAGTTGCTGC |
| MAB_4316-R | TCGTTCTCAACGTGCTTGGA | |
| esxT | MAB_3753c-F | CGCTGGTTGCTGATGTCAAG |
| MAB_3753c-R | GTACTCCTGGTACGCAGTGG | |
| esxU | MAB_3754c-F | CATCTCCTGGTCGAAGCGAG |
| MAB_3754c-R | TTGCTCGATTCCACTGCCAA | |
| esxH | MAB_2228c-F | AAGGTGTTGGTTTCGTGGGT |
| MAB_2228c-R | CGGTGATACCTCGATGAGCC | |
| esxG | MAB_2229c-F | GTCGAACGCATCAACGCC |
| MAB_2229c-R | CTTGACGCACACATTCCCG | |
| sigM | MAB_4938-F | GCTGTTTCGCCGTCATCATC |
| MAB_4938-R | TCACCACGATGCGATAGAGC |
| Uniprot ID | Protein Name | Gene Name | Log2 (FoldChang) | p-Value | |
|---|---|---|---|---|---|
| 1 | B1MC25 | CBS domain-containing protein | MAB_2717c | 0.530 | 5.892 × 10−3 |
| 2 | B1MCY3 | DUF3039 domain-containing protein | MAB_3026c | 0.454 | 3.868 × 10−2 |
| 3 | B1MFV8 | Transcriptional regulator WhiB | whiB | 0.797 | 1.284 × 10−2 |
| 4 | B1MJN1 | WXG100 family type VII secretion target | MAB_4316 | 0.482 | 1.776 × 10−3 |
| 5 | B1MNS8 | Uncharacterized protein | MAB_1896c | 1.178 | 3.176 × 10−2 |
| 6 | B1MCA8 | MspA protein | MAB_2800 | −0.613 | 6.532× 10−3 |
| 7 | B1MDC7 | 1-deoxy-D-xylulose 5-phosphate reductoisomerase | dxr | −1.370 | 4.739 × 10−2 |
| 8 | B1MEW6 | Uncharacterized protein | MAB_3496 | −0.615 | 6.869 × 10−3 |
| 9 | B1MK79 | Hypothetical porin | MAB_1081 | −0.438 | 3.143 × 10−2 |
| 10 | B1MKV2 | Haemophore haem-binding domain-containing protein | MAB_4528c | −0.713 | 2.410 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Li, Z.; Li, M.; Lu, P.; Deng, Y.; Wu, P.; Li, X.; Qin, G.; Huang, J.; Gao, W.; et al. Pseudomonas aeruginosa pqs Quorum Sensing Mediates Interaction with Mycobacterium abscessus In Vitro. Microorganisms 2025, 13, 116. https://doi.org/10.3390/microorganisms13010116
Long Y, Li Z, Li M, Lu P, Deng Y, Wu P, Li X, Qin G, Huang J, Gao W, et al. Pseudomonas aeruginosa pqs Quorum Sensing Mediates Interaction with Mycobacterium abscessus In Vitro. Microorganisms. 2025; 13(1):116. https://doi.org/10.3390/microorganisms13010116
Chicago/Turabian StyleLong, Yun, Zhi Li, Menglu Li, Peiyi Lu, Yujia Deng, Pengyao Wu, Xue Li, Gangjian Qin, Jiamin Huang, Wenying Gao, and et al. 2025. "Pseudomonas aeruginosa pqs Quorum Sensing Mediates Interaction with Mycobacterium abscessus In Vitro" Microorganisms 13, no. 1: 116. https://doi.org/10.3390/microorganisms13010116
APA StyleLong, Y., Li, Z., Li, M., Lu, P., Deng, Y., Wu, P., Li, X., Qin, G., Huang, J., Gao, W., Li, G., Jia, T., & Yang, L. (2025). Pseudomonas aeruginosa pqs Quorum Sensing Mediates Interaction with Mycobacterium abscessus In Vitro. Microorganisms, 13(1), 116. https://doi.org/10.3390/microorganisms13010116






