Comprehensive Analysis of the Proteome of S. cerevisiae Wild-Type and pdr5Δ Cells in Response to Bisphenol A (BPA) Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. S. cerevisiae Strains, Growth Conditions, Cell Lysis, and Protein Extraction
2.3. Protein Digestion, TMT Labeling, and Sample Processing
2.4. Mass Spectrometry Data Acquisition and Processing
3. Results
3.1. BPA Inhibits S. cerevisiae Cell Growth
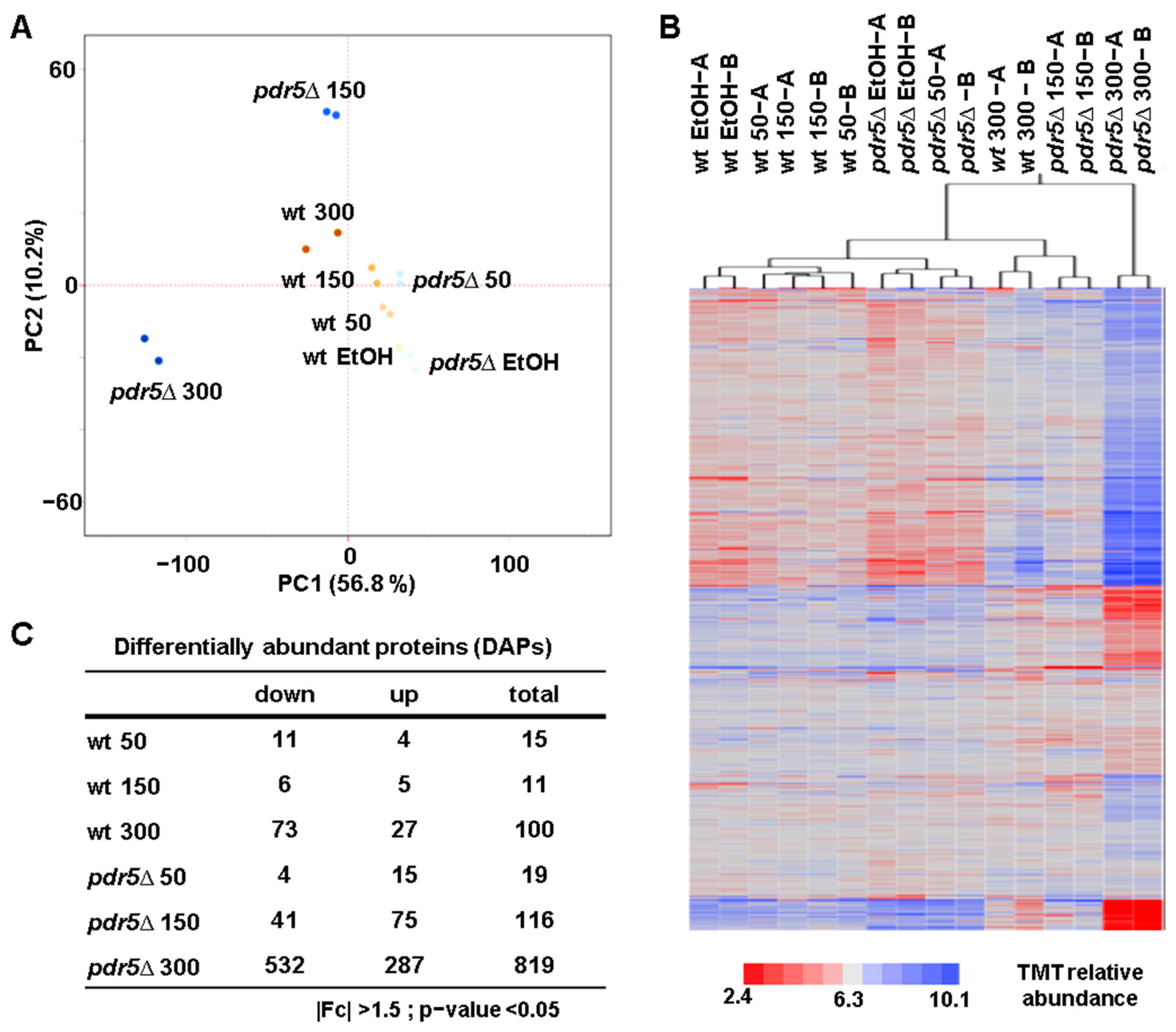
3.2. BPA Induces Proteome Changes in a Dose-Dependent Manner
3.3. BPA Exposure Affects the Levels of the Pleiotropic Drug Resistance (PDR) Proteins
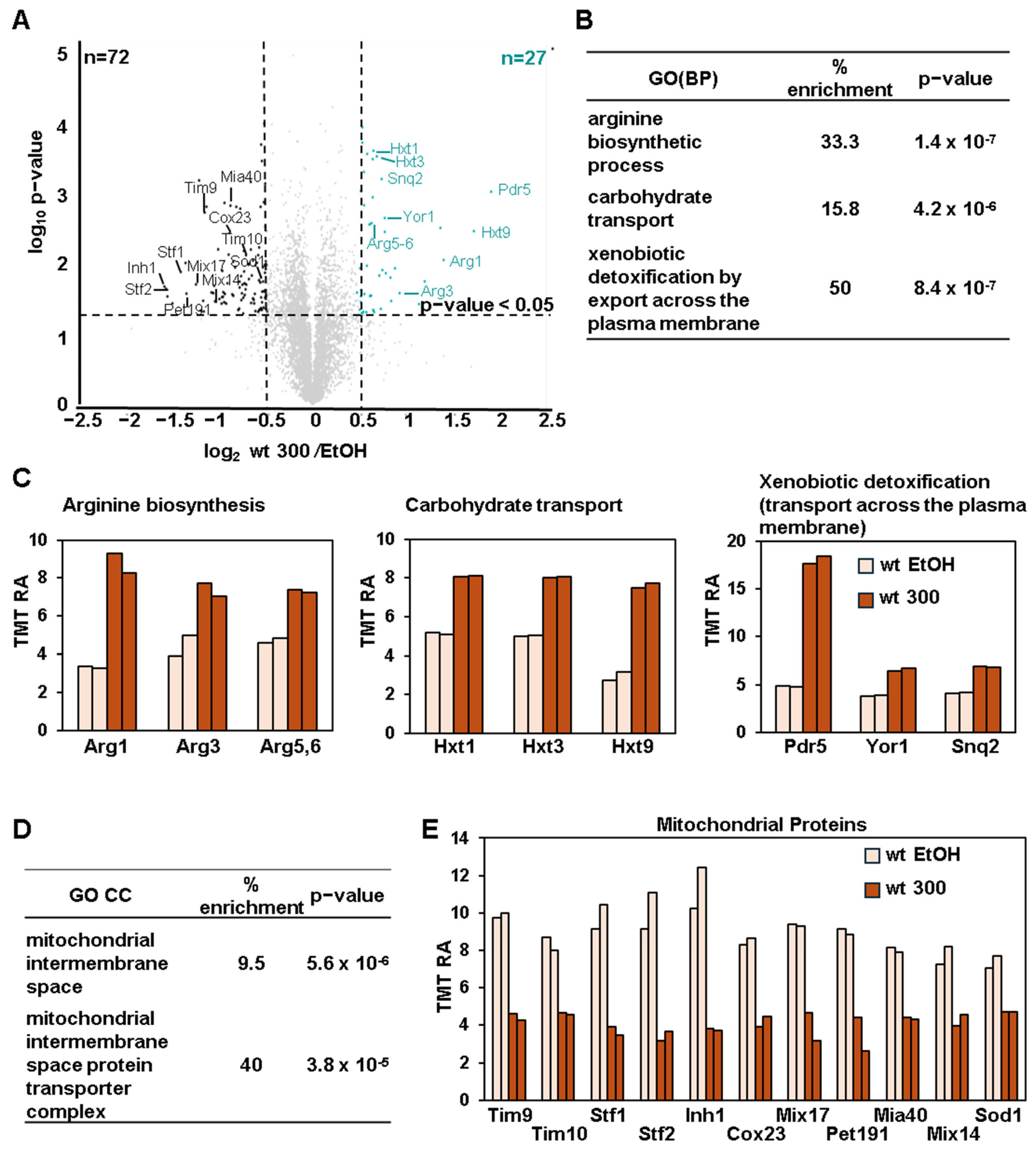
3.4. BPA Induces Arginine Biosynthesis and Glucose Transporters While Downregulating Mitochondrial Proteins in Wild-Type Cells
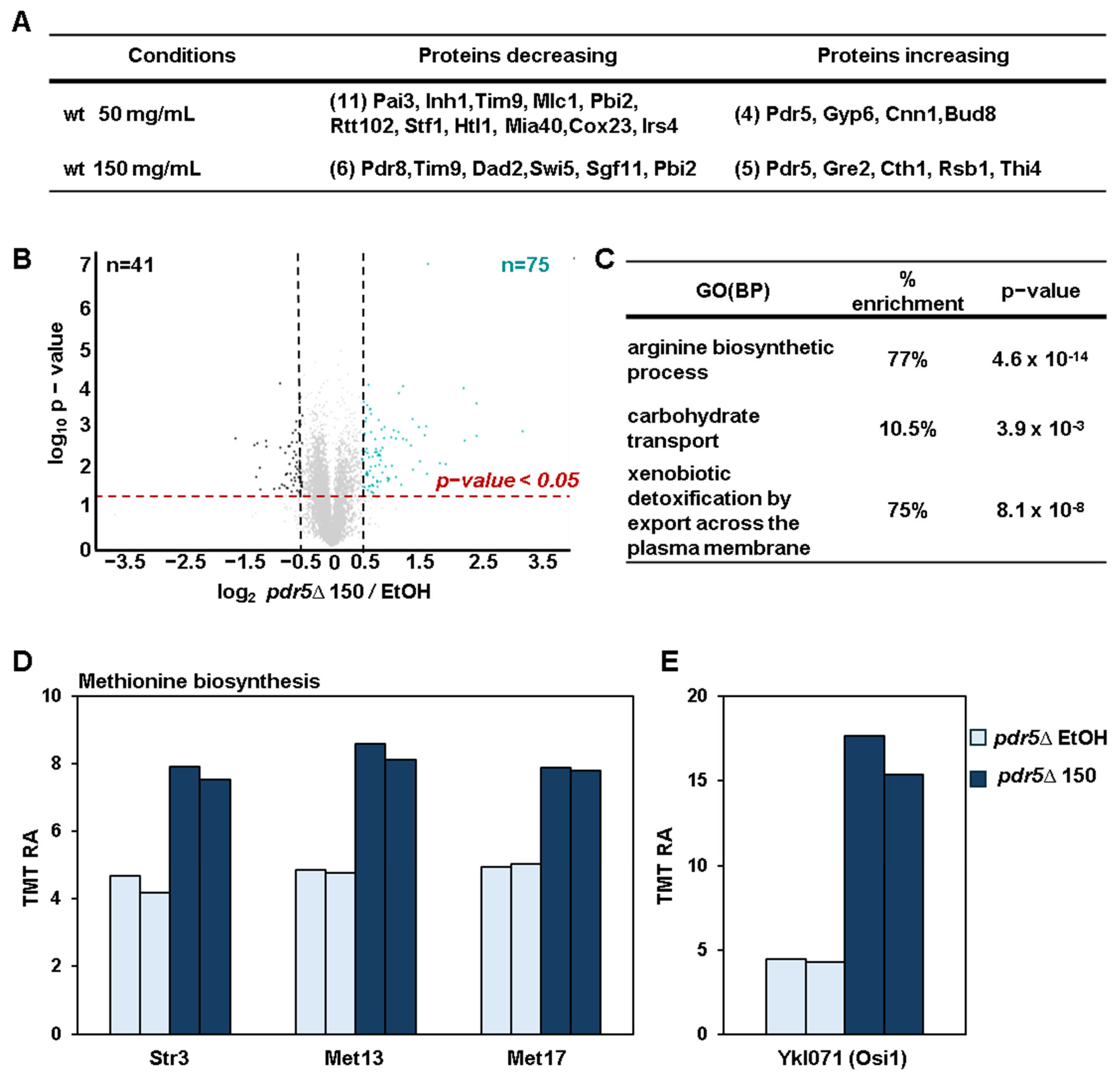
3.5. Proteomic Changes in pdr5Δ Cells Indicate Oxidative Stress
3.6. BPA-Induced Stress and Cellular Damage to S. cerevisiae Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L.; et al. A Review of Dietary and Non-Dietary Exposure to Bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, Toxicity and Biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.C.; Bottema, C.D.K.; Stathis, P.A.; Tokés, L.G.; Feldman, D. Unexpected Presence of Estrogens in Culture Medium Supplements: Subsequent Metabolism by the Yeast Sacchromyces Cerevisiae *. Endocrinology 1986, 119, 1362–1369. [Google Scholar] [CrossRef]
- Kubwabo, C.; Kosarac, I.; Stewart, B.; Gauthier, B.R.; Lalonde, K.; Lalonde, P.J. Migration of Bisphenol A from Plastic Baby Bottles, Baby Bottle Liners and Reusable Polycarbonate Drinking Bottles. Food Addit. Contam. 2009, 26, 928–937. [Google Scholar] [CrossRef]
- Chailurkit, L.; Srijaruskul, K.; Ongphiphadhanakul, B. Bisphenol A in Canned Carbonated Drinks and Plastic-Bottled Water from Supermarkets. Expo. Health 2017, 9, 243–248. [Google Scholar] [CrossRef]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef]
- Brede, C.; Fjeldal, P.; Skjevrak, I.; Herikstad, H. Increased Migration Levels of Bisphenol A from Polycarbonate Baby Bottles after Dishwashing, Boiling and Brushing. Food Addit. Contam. 2003, 20, 684–689. [Google Scholar] [CrossRef]
- Hunt, P.A.; Koehler, K.E.; Susiarjo, M.; Hodges, C.A.; Ilagan, A.; Voigt, R.C.; Thomas, S.; Thomas, B.F.; Hassold, T.J. Bisphenol a Exposure Causes Meiotic Aneuploidy in the Female Mouse. Curr. Biol. 2003, 13, 546–553. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human Exposure to Bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Lehmler, H.-J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef]
- Govarts, E.; Gilles, L.; Rodriguez Martin, L.; Santonen, T.; Apel, P.; Alvito, P.; Anastasi, E.; Andersen, H.R.; Andersson, A.-M.; Andryskova, L.; et al. Harmonized Human Biomonitoring in European Children, Teenagers and Adults: EU-Wide Exposure Data of 11 Chemical Substance Groups from the HBM4EU Aligned Studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 249, 114119. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Den Hond, E.; Geens, T.; Govarts, E.; Koppen, G.; Frederiksen, H.; Knudsen, L.E.; Mørck, T.A.; Gutleb, A.C.; Guignard, C.; et al. Urinary BPA Measurements in Children and Mothers from Six European Member States: Overall Results and Determinants of Exposure. Environ. Res. 2015, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-Tertiary-Octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Huang, R.-P.; Liu, Z.-H.; Yin, H.; Dang, Z.; Wu, P.-X.; Zhu, N.-W.; Lin, Z. Bisphenol A Concentrations in Human Urine, Human Intakes across Six Continents, and Annual Trends of Average Intakes in Adult and Child Populations Worldwide: A Thorough Literature Review. Sci. Total Environ. 2018, 626, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.J.; White, R.; Parker, M.G.; Sumpter, J.P. Differential Effects of Xenoestrogens on Coactivator Recruitment by Estrogen Receptor (ER) Alpha and ERbeta. J. Biol. Chem. 2000, 275, 35986–35993. [Google Scholar] [CrossRef]
- Matthews, J.B.; Twomey, K.; Zacharewski, T.R. In Vitro and in Vivo Interactions of Bisphenol A and Its Metabolite, Bisphenol A Glucuronide, with Estrogen Receptors Alpha and Beta. Chem. Res. Toxicol. 2001, 14, 149–157. [Google Scholar] [CrossRef]
- Husain, Q.; Qayyum, S. Biological and Enzymatic Treatment of Bisphenol A and Other Endocrine Disrupting Compounds: A Review. Crit. Rev. Biotechnol. 2013, 33, 260–292. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and Hormone-Associated Cancers: Current Progress and Perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- Keshavarz-Maleki, R.; Kaviani, A.; Omranipour, R.; Gholami, M.; Khoshayand, M.R.; Ostad, S.N.; Sabzevari, O. Bisphenol-A in Biological Samples of Breast Cancer Mastectomy and Mammoplasty Patients and Correlation with Levels Measured in Urine and Tissue. Sci. Rep. 2021, 11, 18411. [Google Scholar] [CrossRef]
- Molina, L.; Figueroa, C.D.; Ehrenfeld, P.; Molina, L.; Figueroa, C.D.; Ehrenfeld, P. Interaction of Bisphenol A with G Protein: Coupled Receptors—New Paradigms in Breast Cancer. In Bisphenols; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Marotta, V.; Grumetto, L.; Neri, I.; Russo, G.; Tortora, A.; Izzo, G.; Panariello, I.; Rocco, D.; Pezzullo, L.; Vitale, M. Exposure to Bisphenol A Increases Malignancy Risk of Thyroid Nodules in Overweight/Obese Patients. Environ. Pollut. 2023, 316, 120478. [Google Scholar] [CrossRef]
- Dhimolea, E.; Wadia, P.R.; Murray, T.J.; Settles, M.L.; Treitman, J.D.; Sonnenschein, C.; Shioda, T.; Soto, A.M. Prenatal Exposure to BPA Alters the Epigenome of the Rat Mammary Gland and Increases the Propensity to Neoplastic Development. PLoS ONE 2014, 9, e99800. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, Y.; Zhang, X.; Zhang, G.; Jin, C.; Yang, J.; Wu, S.; Lu, X. Bisphenol A and Di(2-Ethylhexyl) Phthalate Promote Pulmonary Carcinoma in Female Rats via Estrogen Receptor Beta: In Vivo and in Silico Analysis. Ecotoxicol. Environ. Saf. 2023, 250, 114496. [Google Scholar] [CrossRef] [PubMed]
- Malloy, M.A.; Kochmanski, J.J.; Jones, T.R.; Colacino, J.A.; Goodrich, J.M.; Dolinoy, D.C.; Svoboda, L.K. Perinatal Bisphenol A Exposure and Reprogramming of Imprinted Gene Expression in the Adult Mouse Brain. Front. Genet. 2019, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-Specific Epigenetic Disruption and Behavioral Changes Following Low-Dose in Utero Bisphenol A Exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef]
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A Review on Immunomodulatory Effects of BPA Analogues. Arch. Toxicol. 2023, 97, 1831–1846. [Google Scholar] [CrossRef]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty Years of Microplastic Pollution Research-What Have We Learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; Grotta, R.L.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Li, S.; Keenan, J.I.; Shaw, I.C.; Frizelle, F.A. Could Microplastics Be a Driver for Early Onset Colorectal Cancer? Cancers 2023, 15, 3323. [Google Scholar] [CrossRef]
- Beal, M.A.; Coughlan, M.C.; Nunnikhoven, A.; Gagné, M.; Barton-Maclaren, T.S.; Bradford, L.M.; Rowan-Carroll, A.; Williams, A.; Meier, M.J. High-Throughput Transcriptomics Toxicity Assessment of Eleven Data-Poor Bisphenol A Alternatives. Environ. Pollut. 2024, 361, 124827. [Google Scholar] [CrossRef] [PubMed]
- Mhaouty-Kodja, S.; Zalko, D.; Tait, S.; Testai, E.; Viguié, C.; Corsini, E.; Grova, N.; Buratti, F.M.; Cabaton, N.J.; Coppola, L.; et al. A Critical Review to Identify Data Gaps and Improve Risk Assessment of Bisphenol A Alternatives for Human Health. Crit. Rev. Toxicol. 2024, 54, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; He, H.; Wan, H.; Shen, N.; Li, J.; Zhang, S.; Zeng, Q.; Chang, J.; Lu, Q.; Zhong, R.; et al. Bisphenol A Exposure, Interaction with Genetic Variants and Colorectal Cancer via Mediating Oxidative Stress Biomarkers. Environ. Pollut. 2021, 287, 117630. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative Stress and BPA Toxicity: An Antioxidant Approach for Male and Female Reproductive Dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Goyal, S.; Tiwari, S.; Seth, B.; Tandon, A.; Shankar, J.; Sinha, M.; Singh, S.J.; Priya, S.; Chaturvedi, R.K. Bisphenol-A Inhibits Mitochondrial Biogenesis via Impairment of GFER Mediated Mitochondrial Protein Import in the Rat Brain Hippocampus. Neurotoxicology 2021, 85, 18–32. [Google Scholar] [CrossRef]
- Nayak, D.; Adiga, D.; Khan, N.G.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Gassman, N.R.; Kabekkodu, S.P. Impact of Bisphenol A on Structure and Function of Mitochondria: A Critical Review. Rev. Env. Contam. (Former. Residue Rev.) 2022, 260, 10. [Google Scholar] [CrossRef]
- Khan, N.G.; Tungekar, B.; Adiga, D.; Chakrabarty, S.; Rai, P.S.; Kabekkodu, S.P. Alterations Induced by Bisphenol A on Cellular Organelles and Potential Relevance on Human Health. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2023, 1870, 119505. [Google Scholar] [CrossRef]
- Kim, S.; Gwon, D.; Kim, J.A.; Choi, H.; Jang, C.-Y. Bisphenol A Disrupts Mitotic Progression via Disturbing Spindle Attachment to Kinetochore and Centriole Duplication in Cancer Cell Lines. Toxicol. In Vitro 2019, 59, 115–125. [Google Scholar] [CrossRef]
- Ďurovcová, I.; Goffa, E.; Šestáková, Z.; Mániková, D.; Gaplovská-Kyselá, K.; Chovanec, M.; Ševčovičová, A. Acute Exposure to Bisphenol A Causes Oxidative Stress Induction with Mitochondrial Origin in Saccharomyces Cerevisiae Cells. J. Fungi 2021, 7, 543. [Google Scholar] [CrossRef]
- Kumar, R.; Oke, A.; Rockmill, B.; de Cruz, M.; Verduzco, R.; Shodhan, A.; Woodruff-Madeira, X.; Abrahamsson, D.P.; Varshavsky, J.; Lam, J.; et al. Rapid Identification of Reproductive Toxicants among Environmental Chemicals Using an in Vivo Evaluation of Gametogenesis in Budding Yeast Saccharomyces Cerevisiae. Reprod. Toxicol. 2024, 128, 108630. [Google Scholar] [CrossRef] [PubMed]
- Alhoch, B.; Chen, A.; Chan, E.; Elkabti, A.; Fariña, S.; Gilbert, C.; Kang, J.; King, B.; Leung, K.; Levy, J.; et al. Comparative Genomic Screen in Two Yeasts Reveals Conserved Pathways in the Response Network to Phenol Stress. G3 2019, 9, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Bereketoglu, C.; Arga, K.Y.; Eraslan, S.; Mertoglu, B. Analysis of Transcriptional Profiles of Saccharomyces Cerevisiae Exposed to Bisphenol A. Curr. Genet. 2017, 63, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Rossio, V.; Liu, X.; Paulo, J.A. Comparative Proteomic Analysis of Two Commonly Used Laboratory Yeast Strains: W303 and BY4742. Proteomes 2023, 11, 30. [Google Scholar] [CrossRef]
- Rossio, V.; Paulo, J.A. Comparison of the Proteomes and Phosphoproteomes of S. Cerevisiae Cells Harvested with Different Strategies. Proteomes 2023, 11, 28. [Google Scholar] [CrossRef]
- Liu, X.; Rossio, V.; Paulo, J.A. Spin Column-Based Peptide Fractionation Alternatives for Streamlined Tandem Mass Tag (SL-TMT) Sample Processing. J. Proteom. 2023, 276, 104839. [Google Scholar] [CrossRef]
- Liu, X.; Rossio, V.; Gygi, S.P.; Paulo, J.A. Enriching Cysteine-Containing Peptides Using a Sulfhydryl-Reactive Alkylating Reagent with a Phosphonic Acid Group and Immobilized Metal Affinity Chromatography. J. Proteome Res. 2023, 22, 1270–1279. [Google Scholar] [CrossRef]
- Rossio, V.; Paulo, J.A.; Liu, X.; Gygi, S.P.; King, R.W. Specificity Profiling of Deubiquitylases against Endogenously Generated Ubiquitin-Protein Conjugates. Cell Chem. Biol. 2024, 31, 1349–1362.e5. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, X.; Rossio, V.; Dawson, S.L.; Gygi, S.P.; Paulo, J.A. Enhancing Proteome Coverage by Using Strong Anion-Exchange in Tandem with Basic-pH Reversed-Phase Chromatography for Sample Multiplexing-Based Proteomics. J. Proteome Res. 2023, 23, 2870–2881. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Beausoleil, S.A.; Villén, J.; Gerber, S.A.; Rush, J.; Gygi, S.P. A Probability-Based Approach for High-Throughput Protein Phosphorylation Analysis and Site Localization. Nat. Biotechnol. 2006, 24, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Jedrychowski, M.P.; Elias, J.E.; Goswami, T.; Rad, R.; Beausoleil, S.A.; Villén, J.; Haas, W.; Sowa, M.E.; Gygi, S.P. A Tissue-Specific Atlas of Mouse Protein Phosphorylation and Expression. Cell 2010, 143, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.E.; Gygi, S.P. Target-Decoy Search Strategy for Mass Spectrometry-Based Proteomics. In Proteome Bioinformatics; Hubbard, S.J., Jones, A.R., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 55–71. [Google Scholar] [CrossRef]
- McAlister, G.C.; Huttlin, E.L.; Haas, W.; Ting, L.; Jedrychowski, M.P.; Rogers, J.C.; Kuhn, K.; Pike, I.; Grothe, R.A.; Blethrow, J.D.; et al. Increasing the Multiplexing Capacity of TMTs Using Reporter Ion Isotopologues with Isobaric Masses. Anal. Chem. 2012, 84, 7469–7478. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Schäfer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Johnstone, R.; Mohammed, A.K.A.; Hamon, C. Tandem Mass Tags: A Novel Quantification Strategy for Comparative Analysis of Complex Protein Mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Perea, J.; Liu, X.; Rad, R.; Gygi, J.P.; Gygi, S.P.; Paulo, J.A. Assessing Interference in Isobaric Tag-Based Sample Multiplexing Using an 18-Plex Interference Standard. Proteomics 2022, 22, e2100317. [Google Scholar] [CrossRef]
- Liu, X.; Rossio, V.; Thakurta, S.G.; Flora, A.; Foster, L.; Bomgarden, R.D.; Gygi, S.P.; Paulo, J.A. Fe3+-NTA Magnetic Beads as an Alternative to Spin Column-Based Phosphopeptide Enrichment. J. Proteom. 2022, 260, 104561. [Google Scholar] [CrossRef]
- Golin, J.; Ambudkar, S.V.; May, L. The Yeast Pdr5p Multidrug Transporter: How Does It Recognize so Many Substrates? Biochem. Biophys. Res. Commun. 2007, 356, 1–5. [Google Scholar] [CrossRef]
- Paulo, J.A.; O’Connell, J.D.; Gygi, S.P. A Triple Knockout (TKO) Proteomics Standard for Diagnosing Ion Interference in Isobaric Labeling Experiments. J. Am. Soc. Mass. Spectrom. 2016, 27, 1620–1625. [Google Scholar] [CrossRef]
- Balzi, E.; Goffeau, A. Yeast Multidrug Resistance: The PDR Network. J. Bioenerg. Biomembr. 1995, 27, 71–76. [Google Scholar] [CrossRef]
- Kihara, A.; Igarashi, Y. Cross Talk between Sphingolipids and Glycerophospholipids in the Establishment of Plasma Membrane Asymmetry. Mol. Biol. Cell 2004, 15, 4949–4959. [Google Scholar] [CrossRef]
- Garay-Arroyo, A.; Covarrubias, A.A. Three Genes Whose Expression Is Induced by Stress in Saccharomyces Cerevisiae. Yeast 1999, 15, 879–892. [Google Scholar] [CrossRef]
- Ayers, M.C.; Sherman, Z.N.; Gallagher, J.E.G. Oxidative Stress Responses and Nutrient Starvation in MCHM Treated Saccharomyces Cerevisiae. G3 2020, 10, 4665–4678. [Google Scholar] [CrossRef] [PubMed]
- Chelstowska, A.; Liu, Z.; Jia, Y.; Amberg, D.; Butow, R.A. Signalling between Mitochondria and the Nucleus Regulates the Expression of a New D-Lactate Dehydrogenase Activity in Yeast. Yeast 1999, 15, 1377–1391. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Véronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; André, B.; et al. Functional Profiling of the Saccharomyces Cerevisiae Genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef]
- Moldavski, O.; Amen, T.; Levin-Zaidman, S.; Eisenstein, M.; Rogachev, I.; Brandis, A.; Kaganovich, D.; Schuldiner, M. Lipid Droplets Are Essential for Efficient Clearance of Cytosolic Inclusion Bodies. Dev. Cell 2015, 33, 603–610. [Google Scholar] [CrossRef]
- Mazur, C.S.; Marchitti, S.A.; Dimova, M.; Kenneke, J.F.; Lumen, A.; Fisher, J. Human and Rat ABC Transporter Efflux of Bisphenol A and Bisphenol A Glucuronide: Interspecies Comparison and Implications for Pharmacokinetic Assessment. Toxicol. Sci. 2012, 128, 317–325. [Google Scholar] [CrossRef][Green Version]
- Reifenberger, E.; Boles, E.; Ciriacy, M. Kinetic Characterization of Individual Hexose Transporters of Saccharomyces Cerevisiae and Their Relation to the Triggering Mechanisms of Glucose Repression. Eur. J. Biochem. 1997, 245, 324–333. [Google Scholar] [CrossRef]
- Ermini, L.; Nuzzo, A.M.; Ietta, F.; Romagnoli, R.; Moretti, L.; Masturzo, B.; Paulesu, L.; Rolfo, A. Placental Glucose Transporters and Response to Bisphenol A in Pregnancies from of Normal and Overweight Mothers. Int. J. Mol. Sci. 2021, 22, 6625. [Google Scholar] [CrossRef]
- Mullainadhan, V.; Viswanathan, M.P.; Karundevi, B. Effect of Bisphenol-A (BPA) on Insulin Signal Transduction and GLUT4 Translocation in Gastrocnemius Muscle of Adult Male Albino Rat. Int. J. Biochem. Cell Biol. 2017, 90, 38–47. [Google Scholar] [CrossRef]
- Campbell, K.; Vowinckel, J.; Keller, M.A.; Ralser, M. Methionine Metabolism Alters Oxidative Stress Resistance via the Pentose Phosphate Pathway. Antioxid. Redox Signal. 2016, 24, 543. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, Z.; Zhu, H.; Guo, X.; He, X. Protective Effects of Arginine on Saccharomyces Cerevisiae Against Ethanol Stress. Sci. Rep. 2016, 6, 31311. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.U.G.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the Central Regulator of the S. Cerevisiae Oxidative Stress Response, Is Activated by Allicin, a Natural Oxidant and Defence Substance of Garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Mendoza, M.; Gómez de León, C.T.; García-Becerra, R.; Ambrosio, J.; Nava-Castro, K.E.; Morales-Montor, J. The Chemical Environmental Pollutants BPA and BPS Induce Alterations of the Proteomic Profile of Different Phenotypes of Human Breast Cancer Cells: A Proposed Interactome. Environ. Res. 2020, 191, 109960. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Zhang, Y.; Pan, Y.; Wang, T.; Wang, Z.; Li, Z.; Zeng, Q.; Qian, Y.; Qiu, J.; et al. Developmental Effects and Lipid Disturbances of Zebrafish Embryos Exposed to Three Newly Recognized Bisphenol A Analogues. Environ. Int. 2024, 189, 108795. [Google Scholar] [CrossRef]
- Yadav, S.K.; Kumar, A.; Yadav, B.G.; Bijalwan, V.; Yadav, S.; Patil, G.P.; Sarkar, K.; Palkhade, R.; Das, S.; Singh, D.P. Sub-acute bisphenol A exposure induces proteomic alterations and impairs male reproductive health in mice-Yadav-2024. J. Biochem. Mol. Toxicol. 2024, 38, e23862. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Marques Dos Santos, M.; Li, C.; Fang, M.; Sureshkumar, M.; Snyder, S.A. Analogy or Fallacy, Unsafe Chemical Alternatives: Mechanistic Insights into Energy Metabolism Dysfunction Induced by Bisphenol Analogs in HepG2 Cells. Environ. Int. 2023, 175, 107942. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Dong, X.; Zhang, Z.; Yang, M.; Xu, H. Proteomics Analysis of Zebrafish Brain Following Chronically Exposed to Bisphenol A. Toxicol. Environ. Chem. 2017, 99, 469–481. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, R.; Shi, W.; Zhou, X.; Sun, S. The Association between Bisphenol A Exposure and Oxidative Damage in Rats/Mice: A Systematic Review and Meta-Analysis. Environ. Pollut. 2022, 292, 118444. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossio, V.; Paulo, J.A. Comprehensive Analysis of the Proteome of S. cerevisiae Wild-Type and pdr5Δ Cells in Response to Bisphenol A (BPA) Exposure. Microorganisms 2025, 13, 114. https://doi.org/10.3390/microorganisms13010114
Rossio V, Paulo JA. Comprehensive Analysis of the Proteome of S. cerevisiae Wild-Type and pdr5Δ Cells in Response to Bisphenol A (BPA) Exposure. Microorganisms. 2025; 13(1):114. https://doi.org/10.3390/microorganisms13010114
Chicago/Turabian StyleRossio, Valentina, and Joao A. Paulo. 2025. "Comprehensive Analysis of the Proteome of S. cerevisiae Wild-Type and pdr5Δ Cells in Response to Bisphenol A (BPA) Exposure" Microorganisms 13, no. 1: 114. https://doi.org/10.3390/microorganisms13010114
APA StyleRossio, V., & Paulo, J. A. (2025). Comprehensive Analysis of the Proteome of S. cerevisiae Wild-Type and pdr5Δ Cells in Response to Bisphenol A (BPA) Exposure. Microorganisms, 13(1), 114. https://doi.org/10.3390/microorganisms13010114






