Abstract
Crown gall disease in plants is caused by “Agrobacteria”, bacteria belonging to the Rhizobiaceae family, which carry a tumor-inducing (Ti) plasmid. Unexpectedly, we found evidence that a natural isolate from a rose crown gall, called NBC51/LBA8980, was a bacterium that did not belong to the Rhizobiaceae family. Whole-genome sequencing revealed that this bacterium contained three large DNA circles with rRNA and tRNA genes, representing one chromosome and two chromids, respectively, and two megaplasmids, including a Ti plasmid. Average nucleotide identity (ANIb, ANIm) and genome-to-genome distance (GGDC) values above the thresholds of 96% and 70%, respectively, showed that NBC51/LBA8980 belonged to the species Brucella (Ochrobactrum) pseudogrignonense. Its Ti plasmid was almost identical to certain succinamopine Ti plasmids previously identified in Agrobacterium strains, suggesting that this Ti plasmid may have been recently acquired by NBC51/LBA8980 in the tumor environment.
1. Introduction
Crown gall, an overgrowth that can occur on many plant species, is caused by the bacterium Agrobacterium tumefaciens. Because of its importance as a phytopathogen and for its use in plant biotechnology, the bacterium has been intensely studied over the last 50 years. The molecular mechanism by which the bacterium induces crown gall has been elucidated over the years. The results are briefly summarized in the following section, with references to reviews for further reading [1,2,3,4,5,6,7,8,9]. Crown galls are characterized by the presence of unusual compounds called opines, which are not found in normal plant cells or in Agrobacterium [1]. Remarkably, the bacterium was found to genetically modify plant cells at wound sites, turning them into tumor cells [2]. The bacterial DNA segment introduced into plant cells, called T-DNA, contains a number of genes that are expressed in plant cells, including various genes which encode enzymes for the production of the plant growth regulators indole acetic acid and isopentenyl-adenine. The T-DNA was also found to contain genes for the enzymes involved in the biosynthesis of opines. The T-DNA is derived from a large plasmid in the bacterium, the tumor-inducing (Ti) plasmid [2,3,4,5,6]. This plasmid also encodes some 20–30 virulence proteins involved in T-DNA transfer and transformation [4,5,6,7,8,9]. The T-DNA transfer system is evolutionarily derived from the bacterial conjugation system and delivers not only T-DNA (in single-stranded form) into the host cells, but also five virulence (effector) proteins [9]. In addition to a T-region and a virulence region, the Ti plasmid contains a repABC replicator, genes for conjugative transfer (between bacteria), and genes for the uptake and catabolism of the opines produced in the crown gall tumors [4,5,6,7,8,9]. A variable number of genes with unknown functions may also be present. Initially, two different classes of Agrobacterium strains were distinguished, octopine and nopaline strains. While both types induced crown gall tumors, octopine strains induced octopine-containing tumors and had the ability to degrade octopine, whereas nopaline strains could only degrade nopaline produced in the tumors they induced [1]. Over time, other opine types have been discovered. DNA sequencing has shown that Ti plasmids can be classified based on the opine synthases on their T-DNA [10]. Since the Ti plasmid determines the phytopathogenic properties of the bacterium, it was recognized that different types of tumor-inducing bacteria could actually harbor the Ti plasmid. These were initially classified as biotypes 1–3 on the basis of their growth characteristics [11]. These “agrobacteria” all belong to the family Rhizobiaceae, but according to current taxonomy, biotype 1 strains belong to different species within the genus Agrobacterium, biotype 2 strains to species within the genus Rhizobium, and biotype 3 to multiple species in the genus Allorhizobium [12].
2. Materials and Methods
2.1. Bacterial Culture, DNA Isolation, and Virulence Test
Strain NBC51/LBA8980 was obtained from NAKtuinbouw, Roelofarendsveen, the Netherlands (M. Ebskamp, E. Meekes). The bacterium was grown on TY medium (Difco tryptone 5 g/L, Difco yeast extract 3 g/L, CaCl2.6H2O 1.3 g/L) and tested for virulence by puncturing the plant stems with a sterile wooden toothpick that had been dipped in a colony of the bacterium. Genomic DNA was isolated using QIAGEN genomic-tip gravity-flow columns (QIAGEN Benelux, Venlo, The Netherlands).
2.2. Sequencing Methods
An initial genome assembly was obtained after Illumina sequencing at Baseclear (W. Pirovano, Leiden, The Netherlands), where Illumina paired-end sequence reads were generated using an Illumina HiSeq2500 system, providing 582,823,698 total bases and 4,631,674 quality filtered paired-end reads. The complete genomic sequence was obtained, but was distributed over 47 scaffolds. In order to obtain a high-quality genomic sequence, additional long-read Nanopore sequencing was performed in-house using Oxford Nanopore Technologies platforms. The Oxford Nanopore sequencing library was generated from 200 ng DNA using the SQK-RBK004 Rapid Barcoding Kit (Oxford Nanopore Technologies, Oxford, UK). The library was pooled with another library, followed by in-house sequencing on a MinION flow cell (version R9.4.1).
2.3. Data Processing Methods
After basecalling with Albacore (version 2.3.4), the MinION reads were demultiplexed (with Epi2me). The total yield for NBC51/LBA8980 was 312,509 reads totaling 1.15 × 109 bp, with a mean read length of 3682 bp. The nanopore reads were end-trimmed and filtered for average quality (>Q10) and length (>5000 bp) with NanoFilt (64-fold coverage after filtering). A hybrid assembly (combining the filtered Nanopore and Illumina reads) was obtained using Unicycler version 0.4.7, which resulted in five contigs representing the chromosomes and the plasmids of NBC51/LBA8980. The assembly was annotated using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP, [13], see NCBI website), and using the RAST server [14], as shown in Table S1. Protein assignments to the Clusters of Orthologous Groups (COGs) functional categories were obtained with eggNOG-mapper v2 [15] using an online server (http://eggnog-mapper.embl.de, accessed on 21 November 2024). Average nucleotide identity (ANI) values were determined on the JSpeciesWS online server (https://www.ribocon.com/jspeciesws.html, accessed on 28 November 2024) using BLASTn (ANIb) and MUMmer (ANIm) against the GenomesDB reference database of 65,774 genomes, including those of 24,708 types of strains [16]. Digital DDH (dDDH) values of the genome-to-genome distances (GGDC, [17,18]) were obtained by a pairwise comparison of NBC51/LBA8980 with other genomes using GGDC 2.1 (identities/HSP length) online (https://ggdc.dsmz.de, accessed on 30 November 2024).
2.4. Data Availability
The complete genome sequence of strain NBC51/LBA8980 has been deposited in GenBank (BioSample SAMN45108879, Accession: PRJNA1192426; CP175669-CP175673).
3. Results
3.1. Crown Gall-Inducing Strain NBC51/LBA8980
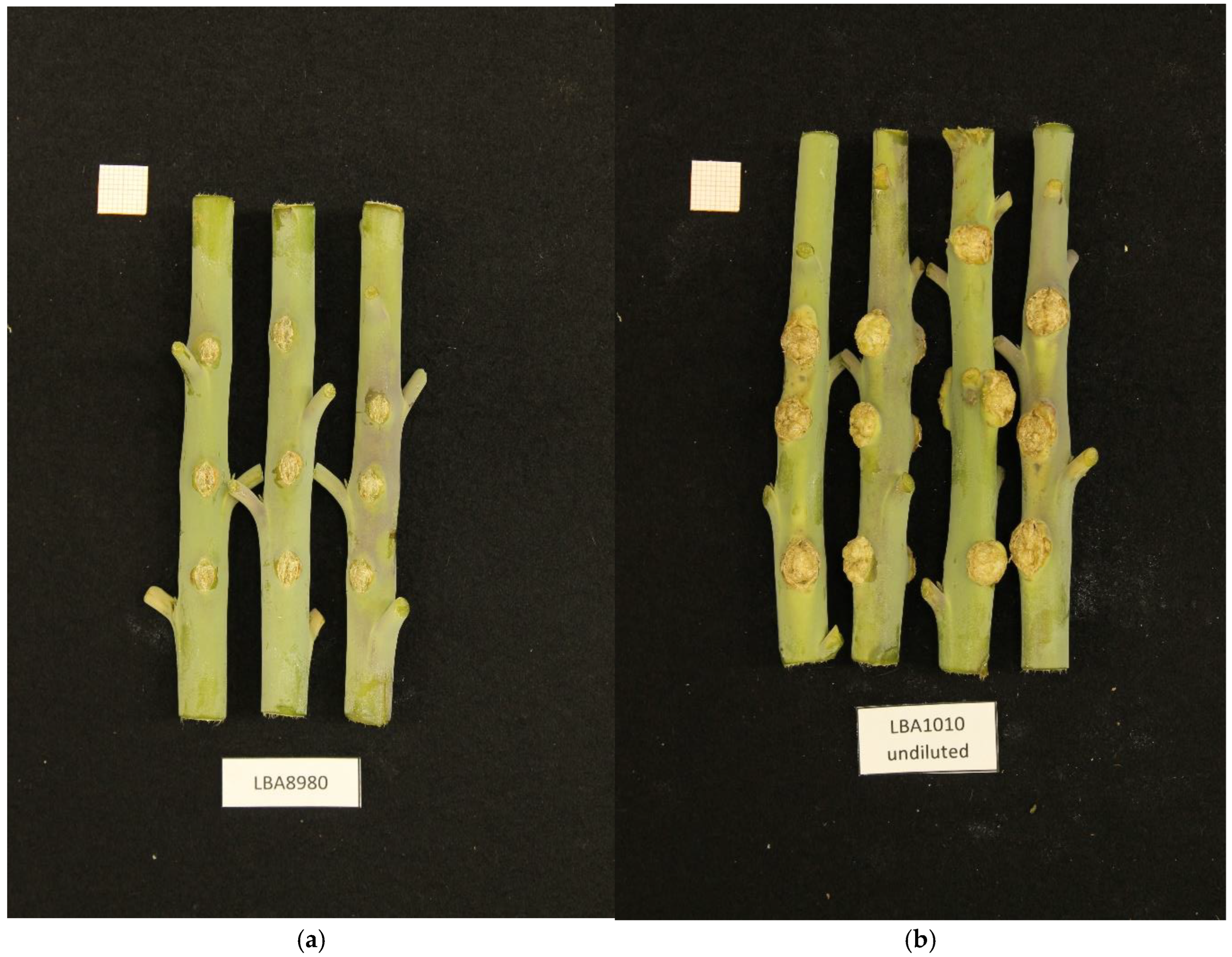
A number of bacterial strains were isolated from crown gall tumors in the Netherlands and characterized for growth characteristics and virulence by NAKtuinbouw (Roelofarendsveen, The Netherlands). A preliminary analysis of the genomic DNA by Illumina short-read sequencing revealed that one of these strains did not belong to any of the Agrobacterium or Rhizobium species known to cause crown gall. This strain, called NBC51 (LBA8980 in our collection), was isolated from a rose gall in 1997, and was able to induce crown gall on rose and tomato (Epskamp and Meekes, personal communication). We tested this isolate for crown gall formation on Kalanchoe tubiflora and Nicotiana glauca and found that the strain could indeed induce crown gall on these plants, although the tumors were small compared to those induced by commonly used laboratory Agrobacterium strains (Figure 1).
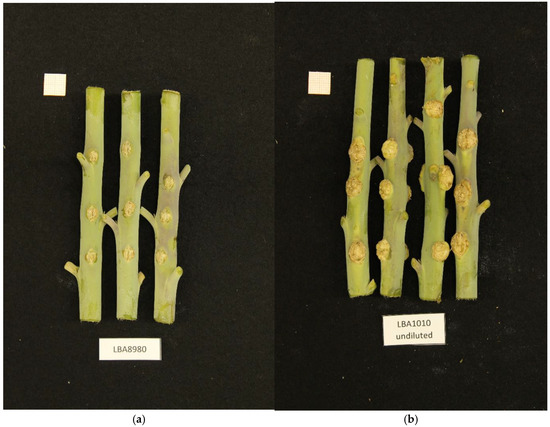
Figure 1.
(a). Small crown gall tumors induced in Nicotiana glauca by NBC51/LBA8980. (b). For comparison crown gall tumors induced by a common laboratory strain LBA1010 (C58 with pTiB6).
3.2. Assembly of a High Quality, Complete NBC51/LBA8980 Genome
To characterize its genomic DNA in detail, we isolated high-molecular-weight genomic DNA for long-read Nanopore sequencing and combined the reads with those previously obtained by Illumina short-read sequencing (using Illumina reads a preliminary genome sequence of 47 scaffolds was assembled earlier) for hybrid genome assembly using Unicycler. We obtained a complete, high-quality genome sequence. The NBC51/LBA8980 genome has a total size of 5,328,147 bp with 53.6% GC content, for which 5281 protein-coding genes are predicted, as well as sixty tRNA genes and five copies each of the 5S rRNA, 16S rRNA, and 23S rRNA genes. The chromosome, the largest DNA circle characterized by the presence of a dnaA replication initiation gene, has a size of 2,409,785 bp; it contains two copies of the rRNA operons and forty of the tRNA genes. In addition to the chromosome, four other DNA circles were identified with sizes of 1,731,835 bp, 876,982 bp, 180,077 bp, and 129,468 bp, respectively. These large DNA circles do not encode a DnaA replication initiation protein, but rely on a repABC system for replication, as do most plasmids in the bacterial order Hyphomicrobiales, to which the Rhizobiaceae belong. The two largest of these plasmids, like the chromosome, contain rRNA and tRNA genes, two copies of each of the rRNA genes and 17 tRNA genes in the largest plasmid, and one copy of the rRNA genes and three tRNA genes in the 876 kbp plasmid. These large plasmids appear to be developing into secondary chromosomes, chromids [19], and will be referred to hereafter as chromosome 2 and chromosome 3. They have a GC content of 53.1% (chromosome 2) and 54.7% (chromosome 3), which is slightly different from that of chromosome 1 (53.5% GC). The remaining DNA circles, plasmid4, which is the Ti plasmid pTi51 (see below for details), and the smallest circle, plasmid5 (now called plasmid pOp51), have a GC content that is more different from that of chromosome 1 with 56.1% GC for the Ti plasmid and 51.2% GC for pOp51.
3.3. NBC51/LBA8980 Belongs to the Species Brucella (Ochrobactrum) pseudogrignonense
The availability of a high-quality genome sequence of strain NBC51/LBA8980 allowed the use of new genomics tools to classify the strain. We used the online server JSpeciesWS (https://jspecies.ribohost.com/jspeciesws/, accessed on 28 November 2024) to compare the genome of NBC51/LBA8980 with the reference database GenomesDB, which contains more than 60,000 genomes. The Tetra Correlation Search function (TCS) revealed that NBC51/LBA8980 was most closely related to Ochrobactrum sp. CDB2 and Brucella pseudogrignonense strains, including type strain CCUG 30717T (Table S1). This was confirmed by determining the average nucleotide identity (ANI) using BLASTn (ANIb) and MUMmer (ANIm) to perform alignments against the same reference database on the JSpeciesWS online server (Table 1). The high nucleotide identity of 97–98% found for these two bacteria (over approximately 87% of the genome) is above the recommended ANI limit for species discrimination of 95–96% [16,20]. In similar analyses, other Brucella species were found to share a much lower genomic identity over much smaller portions of the genome. Strain NBC51/LBA8980 thus appears to belong to the species Brucella (Ochrobactrum) pseudogrignonense, until recently named Ochrobactrum pseudogrignonense. We then used the Genome BLAST Distance Phylogeny (GBDP)-based digital DDH (dDDH) method online (https://ggdc.dsmz.de, accessed on 30 November 2024) to validate this result. This revealed a genome-to-genome distance (GGDC) value of over 80% between NBC51/LBA8980 and CCUG 30717T, the type strain of Brucella pseudogrignonense, which is well above the classical cut-off point of 70% DDH for species delimitation [17,18].

Table 1.
Pairwise comparison of the NBC51/LBA8980 genome with the most closely related Brucella/Ochrobactrum strains by ANIm using the JSpeciesWS online server [16].
3.4. Functions Encoded by the Chromosomes and the Plasmids
The genome was annotated with the RAST (Rapid Annotations using Subsystems Technology) server [14] as shown in Table S2, as well as with the NCBI Prokaryotic Genome Annotation Pipeline (PGAP [13]) at the web site. Chromosome 1 contains most of the rRNA and tRNA gene clusters, as well as the majority of the genes required for genome maintenance and replication for ribosomal proteins and translation. A complete set of genes for a gene transfer agent (GTA) are present in chromosome 1. Such genes were first identified in Rhodobacter capsulatus, but have since been identified in other alphaproteobacteria. They encode phage-like particles that facilitate gene transfer [21]. Chromosome 2, one of the chromids, also contains some rDNA and tRNA genes, as well as some genes involved in genome maintenance and repair, translation, and transcription, but also several metabolic genes and several genes encoding siderophores. Chromosome 3, the other chromid, has the fewest rRNA and tRNA genes of the three and is the most plasmid-like, carrying a full set of conjugation genes. It also contains some DNA maintenance and repair genes, including an imuABC cluster for SOS mutagenesis as well as Ku and LigC genes for non-homologous end-joining, and genes for metabolic and transport functions, again including siderophore gene clusters. Plasmid4 is the Ti plasmid discussed below. Plasmid5 is probably a conjugative plasmid as it contains a full set of conjugative genes. It contains many genes of unknown function and has very little homology to any plasmid or chromosomal DNA in the NCBI database.
3.5. Ti Plasmid pTi151
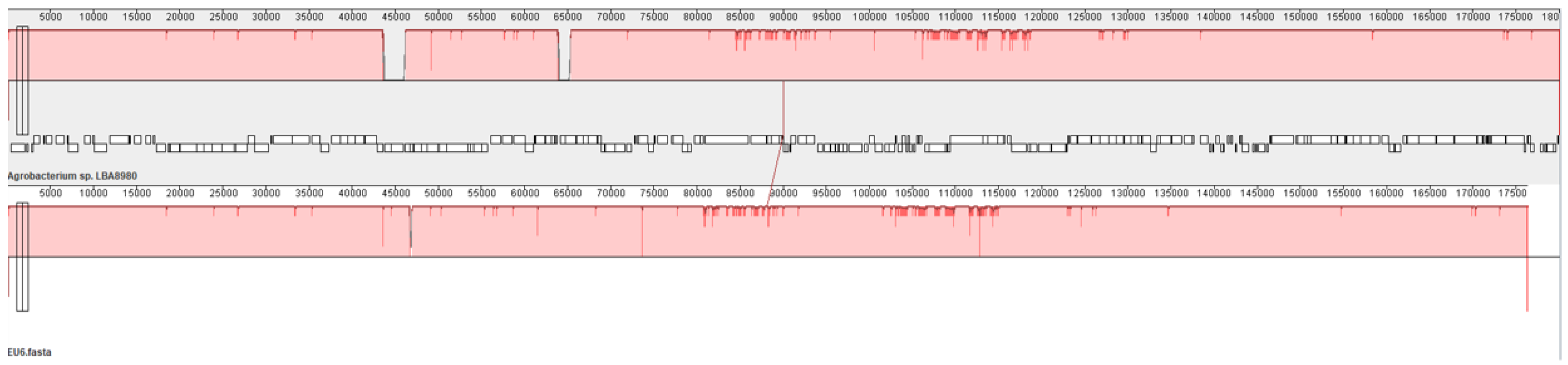
Plasmid4 is the Ti plasmid containing the genes essential for crown gall formation. Nucleotide Blast (NCBI) showed that pTi51, the name we propose for this plasmid, is almost identical to plasmid pTi186 identified in an Agrobacterium strain isolated from walnut [22] and also to plasmid pTi1D132 from an Agrobacterium strain isolated from a cherry gall [23]. Apart from a few single-nucleotide polymorphisms (3 snp’s with pTi186; 12 snp’s with pTi132), the only difference found was the insertion of a transposable element in the trbI gene in pTi151, which probably rendered this plasmid non-conjugative. These three plasmids are also very similar (99.7% nucleotide identity over almost the entire length of the plasmid) to plasmid pTiEU6, which has been analyzed in detail in [24]. Plasmid pTiEU6 is a succinamopine Ti plasmid with the susD gene in the T-DNA region for the production of D,L-succinamopine in plant tumors [24]. This susD gene and the genes for succinamopine catabolism are conserved in pTi51. Also, the virulence genes and the T-region are fully conserved. We can therefore conclude that pTi51 is a succinamopine Ti plasmid. A pairwise alignment of pTi51 and pTiEU6 is shown in Figure 2.
Figure 2.
Pairwise alignment of pTi51 (top LBA8980) and pTiEU6 (bottom EU6) by progressive MAUVE [25]. The large gap in the top (pTi51) represents the transposable element inserted in the trbI gene.
4. Discussion
Crown gall bacteria were originally divided into three biotypes [11]. These biotypes represent the genera Agrobacterium, Rhizobium, and Allorhizobium, all of which belong to the bacterial family Rhizobiaceae [12]. This is also the case for the Neorhizobium sp. strain carrying a Ti plasmid recently isolated from a crown gall in Taiwan [26]. However, we now add the genus Ochrobactrum/Brucella to the list of bacteria that can naturally carry a Ti plasmid and thus become pathogenic to plants. As the genus Ochrobactrum/Brucella does not belong to the bacterial family Rhizobiaceae, but to the bacterial family of the Bartonellaceae [27], strain NBC51/LBA8980 is the first natural isolate outside the Rhizobiaceae capable of inducing crown gall. This is not entirely unexpected, as laboratory experiments in the past have shown that transferring the Ti plasmid to other host bacteria can render them virulent. Initially, such experiments were restricted to different strains of the closely related bacterium Rhizobium leguminosarum [28], but later, more distant bacteria were also used as recipients, including Phyllobacterium myrsinacearum [29,30]. The latter host, which, like Ochrobactrum, becomes tumorigenic after receiving the Ti plasmid, does not belong to the Rhizobiaceae family. Transferring the Ti plasmid to bacteria outside the Alphaproteobacteria such as Escherichia coli could also be achieved in the form of a cointegrate with a broad host range R plasmid. However, such bacteria did not become plant pathogenic [31]. It may be relevant to mention here that the Ri plasmid, which is carried by hairy root-inducing Agrobacterium and Rhizobium strains, has previously been found in Ochrobactrum strains in greenhouse tomatoes and cucumbers affected by root mat disease [32]. Interestingly, it was also recently reported that the bacterium responsible for a tumor disease on the mushroom Flammulina velutipes was classified as Ochrobactrum pseudogrignonense [33]. It will be very interesting to identify the genes responsible for infection from this bacterium, as well as their origin.
Only small tumors are induced by strain NBC51/LBA8980. This may be due to the absence of certain chromosomal virulence factors. However, homologs of the known chromosomal virulence genes chvAB, chvGI, and acvB were identified in the genome, but whether these are functional remains to be tested. The formation of smaller tumors in novel bacterial backgrounds was previously observed when the Ti plasmid was introduced into novel bacterial hosts such as Rhizobium leguminosarum and Phyllobacterium myrsinacearum in the laboratory [29,30]. A Sinorhizobium meliloti strain receiving the Ti plasmid was even completely deficient in inducing crown gall in plants [34]. It may well be that the reduction or absence of tumorigenicity in these new host bacteria is due to a lack of co-evolution between the Ti plasmid and the host genome. In nature, new tumorigenic species may eventually evolve through adaptive changes in the Ti plasmid and the genome. Otherwise, the Ti plasmid may be lost from such new hosts or, if this is difficult, for example, due to the presence of toxin–antitoxin systems on the Ti plasmid, lead to degeneration of the Ti plasmid. The inactivation of the conjugation system by a transposable element in pTi151 may be the first step in this direction in NBC51/LBA8980. The presence of a Ti plasmid that had lost most of its T-region in a Rhizobium etli strain isolated from soil [6,35] also points in this direction. Conversely, specific Ti plasmids seem to have evolved in Rhizobium tumorigenes and R. rhododendri that allow tumor formation on blueberry and rhododendron [36], and this has also been described for Ti plasmids in Allorhizobium vitis that allow tumor formation on Vitis vinifera [37].
Recently, disarmed Ti plasmids have been introduced into several different host bacteria and tested for their ability to transfer selectable genes present on the T-region of a binary vector into plants [38,39]. Several of these species, including Mesorhizobium loti [38] and Ensifer adhaerans [39], were found to be capable of transferring genes into plants. Remarkably, S. meliloti was one of the species able to transfer genes into plants [38]. Whether this positive result was due to the use of a different strain of S. meliloti than that used to transfer the natural Ti plasmid, or to the use of a different assay system to detect transfer (growth of transformed plant cells on selective medium instead of crown gall tumor formation) requires further study. Interestingly, one of the bacteria that was selected for use as a plant vector in an even more recent experiment was a bacterium that was called Ochrobactrum haywardense H1 [40]. It is therefore possible that the Ochrobactrum/Brucella isolate described here may have similar useful properties to a plant gene vector. To test this, the Ti plasmid present in strain LBA8980 needs to be disarmed or replaced by one of the existing disarmed helper Ti plasmids.
For the practical application of such a disarmed strain, it would be important to verify that the strain lacks human/animal pathogenicity factors that are prevalent in pathogenic Brucella strains. This is likely to be the case as pairwise comparisons of hundreds of genomes revealed a clear separation of the pathogenic Brucella strains from the environmental strains previously referred to as Ochrobactrum strains [41]. This is also consistent with genomic studies showing that major genomic rearrangements have occurred in the Brucella genome over time, with dozens of genes present in chromosome 1 of Ochrobactrum moving to chromosome 2 [42]. At the same time, certain metabolic genes were inactivated by mutation, resulting in the evolution of zoonotic Brucella species towards the selective use of the pentose phosphate pathway for glucose catabolism [43]. All of this adds weight to the criticism of the decision to include the environmental Ochrobactrum strains in the genus Brucella, which traditionally contains zoonotic species [44,45]. Strain NBC51/LBA8980 belongs to the group of environmental strains (Ochrobactrum) because it still has the above-mentioned genes on chromosome 1 and lacks the mutations that restrict glucose catabolism through the pentose phosphate pathway. Nevertheless, Ochrobactrum has also been found as an opportunistic human pathogen in hospitals [46]. In this respect it is no different from Agrobacterium [47].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13010102/s1, Table S1: List of genomes most closely related to strain NBC51/LBA8980 found in the GenomesDB database; Table S2: RAST annotation of the Brucella (Ochrobactrum) pseudogrignonense NBC51/LBA8980 genome.
Author Contributions
Conceptualization, M.J.G.H. and P.J.J.H.; methodology, M.J.G.H.; investigation, M.J.G.H.; writing—original draft preparation, P.J.J.H.; writing—review and editing, M.J.G.H.; visualization, M.J.G.H. and P.J.J.H.; supervision, P.J.J.H.; funding acquisition, P.J.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
By the fellowship associated with the appointment of P.H. as Academy Professor by the Royal Netherlands Academy of Sciences.
Data Availability Statement
DNA sequencing data are available at GenBank.
Acknowledgments
We thank Michel Ebskamp and Ellis Meekes (NAKtuinbouw, Roelofarendsveen) for providing the strain, Barbara Gravendeel (Naturalis, Leiden) for initiating a collaborative project in which several Agrobacterium strains were sequenced, Baseclear (Leiden, The Netherlands) for providing the Illumina sequence data, and Christiaan Henkel for bioinformatics support in the initial phase of the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dessaux, Y.; Petit, A.; Tempé, J. Chemistry and biochemistry of opines, chemical mediators of parasitism. Phytochemistry 1993, 34, 31–38. [Google Scholar] [CrossRef]
- Bevan, M.W.; Chilton, M.D. T-DNA of the Agrobacterium Ti and Ri plasmids. Ann. Rev. Genet. 1982, 16, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Van Montagu, M.; Schell, J. The Ti plasmids of Agrobacterium. Curr. Top. Microbiol. Immunol. 1982, 96, 237–254. [Google Scholar] [PubMed]
- Zhu, J.; Oger, P.M.; Schrammeijer, B.; Hooykaas, P.J.; Farrand, S.K.; Winans, S.C. The bases of crown gall tumorigenesis. J. Bacteriol. 2000, 182, 3885–3895. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef]
- Lacroix, B.; Citovsky, V. Pathways of DNA transfer to plants from Agrobacterium tumefaciens and related bacterial species. Ann. Rev. Phytopathol. 2019, 57, 231–251. [Google Scholar] [CrossRef]
- Weisberg, A.J.; Wu, Y.; Chang, J.H.; Lai, E.M.; Kuo, C.H. Virulence and ecology of Agrobacteria in the context of evolutionary genomics. Ann. Rev. Phytopathol. 2023, 61, 1–23. [Google Scholar] [CrossRef]
- Brown, P.J.B.; Chang, J.H.; Fuqua, C. Agrobacterium tumefaciens: A transformative agent for fundamental insights into host-microbe interactions. J. Bacteriol. 2023, 205, e0000523. [Google Scholar] [CrossRef]
- Hooykaas, P.J.J. The Ti plasmid, driver of Agrobacterium pathogenesis. Phytopathology 2023, 113, 594–604. [Google Scholar] [CrossRef]
- Nabi, N.; Ben Hafsa, A.; Gaillard, V.; Nesme, X.; Chaouachi, M.; Vial, L. Evolutionary classification of tumor- and root-inducing plasmids based on T-DNAs and virulence regions. Mol. Phylogenet. Evol. 2022, 169, 107388. [Google Scholar] [CrossRef]
- Kerr, A.; Panagopoulos, C.G. Biotypes of Agrobacterium radiobacter var. tumefaciens and their biological control. Phytopath. Z. 1977, 90, 172–179. [Google Scholar]
- Mousavi, S.A.; Willems, A.; Nesme, X.; de Lajudie, P.; Lindstrom, K. Revised phylogeny of Rhizobiaceae: Proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst. Appl. Microbiol. 2015, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2, Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Glöckner, F.O.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Sardà Carbasse, J.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef]
- Harrison, P.W.; Lower, R.P.; Kim, N.K.; Young, J.P. Introducing the bacterial ‘chromid’: Not a chromosome, not a plasmid. Trends Microbiol. 2010, 18, 141–148. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Lang, A.S.; Zhaxybayeva, O.; Beatty, J.T. Gene transfer agents: Phage-like elements of genetic exchange. Nature reviews. Microbiology 2012, 10, 472–482. [Google Scholar] [PubMed]
- Poret-Peterson, A.T.; Bhatnagar, S.; McClean, A.E.; Kluepfel, D.A. Draft genome sequence of Agrobacterium tumefaciens biovar 1 strain 186, Isolated from Walnut. Genome Announc. 2017, 5, e01232-17. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Santos, M.N.M.; Cho, S.T.; Chang, H.H.; Tsai, Y.M.; Smith, D.A.; Kuo, C.H.; Chang, J.H.; Lai, E.M. Plant-pathogenic Agrobacterium tumefaciens strains have diverse Type VI effector-immunity pairs and vary in in-planta competitiveness. Mol. Plant-Microbe Interact. 2019, 32, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; van Heusden, G.P.H.; Hooykaas, P.J.J. Complete sequence of succinamopine Ti-plasmid pTiEU6 reveals its evolutionary relatedness with nopaline-type Ti-plasmids. Genome Biol. Evol. 2019, 11, 2480–2491. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Haryono, M.; Tsai, Y.M.; Lin, C.T.; Huang, F.C.; Ye, Y.C.; Deng, W.L.; Hwang, H.H.; Kuo, C.H. Presence of an Agrobacterium-Type Tumor-Inducing plasmid in Neorhizobium sp. NCHU2750 and the link to phytopathogenicity. Genome Biol. Evol. 2018, 10, 3188–3195. [Google Scholar] [CrossRef]
- diCenzo, G.C.; Yang, Y.; Young, J.P.W.; Kuzmanović, N. Refining the taxonomy of the order Hyphomicrobiales (Rhizobiales) based on whole genome comparisons of over 130 type strains. Int. J. System. Evol. Microbiol. 2024, 74, 006328. [Google Scholar] [CrossRef]
- Hooykaas, P.J.J.; Klapwijk, P.M.; Nuti, M.P.; Schilperoort, R.A.; Rorsch, A. Transfer of the Agrobacterium tumefaciens TI plasmid to avirulent Agrobacteria and to Rhizobium ex planta. J. Gen. Microbiol. 1977, 98, 477–484. [Google Scholar] [CrossRef]
- van Veen, R.J.M.; den Dulk-Ras, H.; Bisseling, T.; Schilperoort, R.A.; Hooykaas, P.J.J. Crown gall tumor and root nodule formation by the bacterium Phyllobacterium myrsinacearum after the introduction of an Agrobacterium Ti plasmid or a Rhizobium Sym plasmid. Mol. Plant Microbe Interact. 1988, 1, 231–234. [Google Scholar] [CrossRef]
- Teyssier-Cuvelle, S.; Oger, P.; Mougel, C.; Groud, K.; Farrand, S.K.; Nesme, X. A highly selectable and highly transferable Ti plasmid to study conjugal host range and Ti plasmid dissemination in complex ecosystems. Microb. Ecol. 2004, 48, 10–18. [Google Scholar] [CrossRef]
- Holsters, M.; Silva, B.; Van Vliet, F.; Hernalsteens, J.P.; Genetello, C.; Van Montagu, M.; Schell, J. In vivo transfer of the ti-plasmid of Agrobacterium tumefaciens to Escherichia coli. Mol. Gen. Genet. 1978, 163, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.A.; Stead, D.E.; Young, J.P. Acquisition of an Agrobacterium Ri plasmid and pathogenicity by other alpha-Proteobacteria in cucumber and tomato crops affected by root mat. Appl. Environ. Microbiol. 2004, 70, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Peng, W.; He, X.; Wang, B.; Gan, B.; Zhang, X. Mushroom tumor: A new disease on Flammulina velutipes caused by Ochrobactrum pseudogrignonense. FEMS Microbiol. Lett. 2016, 363, fnv226. [Google Scholar] [CrossRef] [PubMed]
- van Veen, R.J.M.; den Dulk-Ras, H.; Schilperoort, R.A.; Hooykaas, P.J.J. Ti plasmid containing Rhizobium meliloti are non-tumorigenic on plants, despite proper virulence gene induction and T-strand formation. Arch. Microbiol. 1989, 153, 85–89. [Google Scholar] [CrossRef]
- Hooykaas, M.J.G.; Hooykaas, P.J.J. Complete genomic sequence and phylogenomics analysis of Agrobacterium strain AB2/73, a new Rhizobium species with a unique mega-Ti plasmid. BMC Microbiol. 2021, 21, 295. [Google Scholar] [CrossRef]
- Kuzmanović, N.; diCenzo, G.C.; Bunk, B.; Spröer, C.; Frühling, A.; Neumann-Schaal, M.; Overmann, J.; Smalla, K. Genomics of the “tumorigenes” clade of the family Rhizobiaceae and description of Rhizobium rhododendri sp. nov. MicrobiologyOpen 2023, 12, e1352. [Google Scholar] [CrossRef]
- Otten, L.; Canaday, J.; Gérard, J.C.; Fournier, P.; Crouzet, P.; Paulus, F. Evolution of agrobacteria and their Ti plasmids—A review. Mol. Plant-Microbe Interact. 1992, 5, 279–287. [Google Scholar] [CrossRef]
- Broothaerts, W.; Mitchell, H.J.; Weir, B.; Kaines, S.; Smith, L.M.; Yang, W.; Mayer, J.E.; Roa-Rodríguez, C.; Jefferson, R.A. Gene transfer to plants by diverse species of bacteria. Nature 2005, 433, 629–633. [Google Scholar] [CrossRef]
- Rudder, S.; Doohan, F.; Creevey, C.J.; Wendt, T.; Mullins, E. Genome sequence of Ensifer adhaerens OV14 provides insights into its ability as a novel vector for the genetic transformation of plant genomes. BMC Genom. 2014, 15, 268. [Google Scholar] [CrossRef]
- Cho, H.J.; Moy, Y.; Rudnick, N.A.; Klein, T.M.; Yin, J.; Bolar, J.; Hendrick, C.; Beatty, M.; Castañeda, L.; Kinney, A.J.; et al. Development of an efficient marker-free soybean transformation method using the novel bacterium Ochrobactrum haywardense H1. Plant Biotechnol. J. 2022, 20, 977–990. [Google Scholar] [CrossRef]
- Yang, Z.; Chai, Z.; Wang, X.; Zhang, Z.; Zhang, F.; Kang, F.; Liu, W.; Ren, H.; Jin, Y.; Yue, J. Comparative genomic analysis provides insights into the genetic diversity and pathogenicity of the genus Brucella. Front. Microbiol. 2024, 15, 1389859. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.C.; Goldman, B.S.; Goodner, B.; Setubal, J.C.; Farrand, S.K.; Nester, E.W.; Burr, T.J.; Banta, L.; Dickerman, A.W.; Paulsen, I.; et al. Genome sequences of three Agrobacterium biovars help elucidate the evolution of multichromosome genomes in bacteria. J. Bacteriol. 2009, 191, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Machelart, A.; Willemart, K.; Zúñiga-Ripa, A.; Godard, T.; Plovier, H.; Wittmann, C.; Moriyón, I.; De Bolle, X.; Van Schaftingen, E.; Letesson, J.J.; et al. Convergent evolution of zoonotic Brucella species toward the selective use of the pentose phosphate pathway. Proc. Natl. Acad. Sci. USA 2020, 117, 26374–26381. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Blasco, J.M.; Letesson, J.J.; Gorvel, J.P.; Moriyón, I. Pathogenicity and its implications in taxonomy: The Brucella and Ochrobactrum case. Pathogens 2022, 11, 377. [Google Scholar] [CrossRef]
- Moreno, E.; Middlebrook, E.A.; Altamirano-Silva, P.; Al Dahouk, S.; Araj, G.F.; Arce-Gorvel, V.; Arenas-Gamboa, Á.; Ariza, J.; Barquero-Calvo, E.; Battelli, G.; et al. If you’re not confused, you’re not paying attention: Ochrobactrum is not Brucella. J. Clin. Microbiol. 2023, 61, e0043823. [Google Scholar] [CrossRef]
- Li, S.Y.; Huang, Y.E.; Chen, J.Y.; Lai, C.H.; Mao, Y.C.; Huang, Y.T.; Liu, P.Y. Genomics of Ochrobactrum pseudogrignonense (newly named Brucella pseudogrignonensis) reveals a new blaOXA subgroup. Microb. Genom. 2021, 7, 000626. [Google Scholar]
- Alnor, D.; Frimodt-Møller, N.; Espersen, F.; Frederiksen, W. Infections with the unusual human pathogens Agrobacterium species and Ochrobactrum anthropi. Clin. Infect. Dis. 1994, 18, 914–920. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).